Long-Term Variations in Retinal Parameters after Uncomplicated Cataract Surgery
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Best Corrected Visual Acuity
3.2. Central Retinal Thickness
3.3. Macular Volume
3.4. Average Central Retinal Thickness
3.5. Vessel Density Central
3.6. Vessel Density Full
3.7. Vessel Perfusion Central
3.8. Vessel Perfusion Full
4. Discussion
4.1. Summary of the Results
4.2. SD-OCT and OCTA Measurements
4.3. EPT Time and Results after the Surgery
4.4. Other Studies
4.5. The Pathophysiological Concepts
4.6. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tu, K.L.; Kaye, S.B.; Sidaras, G.; Taylor, W.; Shenkin, A. Effect of intraocular surgery and ketamine on aqueous and serum cytokines. Mol. Vis. 2007, 13, 1130–1137. [Google Scholar] [PubMed]
- Favuzza, E.; Becatti, M.; Gori, A.M.; Mencucci, R. Cytokines, chemokines, and flare in the anterior chamber after femtosecond laser–assisted cataract surgery. J. Cataract Refract. Surg. 2019, 45, 910–914. [Google Scholar] [CrossRef] [PubMed]
- Shingleton, B.J.; Gamell, L.S.; O’Donoghue, M.W.; Baylus, S.L.; King, R. Long-term changes in intraocular pressure after clear corneal phacoemulsification: Normal patients versus glaucoma suspect and glaucoma patients. J. Cataract Refract. Surg. 1999, 25, 885–890. [Google Scholar] [CrossRef]
- Shingleton, B.J.; Pasternack, J.J.; Hung, J.W.; O’Donoghue, M.W. Three and five year changes in intraocular pressures after clear corneal phacoemulsification in open angle glaucoma patients, glaucoma suspects, and normal patients. J. Glaucoma 2006, 15, 494–498. [Google Scholar] [CrossRef] [Green Version]
- Kur, J.; Newman, E.A.; Chan-Ling, T. Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Prog. Retin. Eye Res. 2012, 31, 377–406. [Google Scholar] [CrossRef] [Green Version]
- Noonan, J.E.; Lamoureux, E.L.; Sarossy, M. Neuronal activity-dependent regulation of retinal blood flow. Clin. Exp. Ophthalmol. 2015, 43, 673–682. [Google Scholar] [CrossRef]
- Von Jagow, B.; Ohrloff, C.; Kohnen, T. Macular thickness after uneventful cataract surgery determined by optical coherence tomography. Graefes Arch. Clin. Exp. Ophthalmol. 2007, 245, 1765–1771. [Google Scholar] [CrossRef]
- Cagini, C.; Fiore, T.; Iaccheri, B.; Piccinelli, F.; Ricci, M.A.; Fruttini, D. Macular thickness measured by optical coherence tomography in a healthy population before and after uncomplicated cataract phacoemulsification surgery. Curr. Eye Res. 2009, 34, 1036–1041. [Google Scholar] [CrossRef]
- Cheng, B.; Liu, Y.; Liu, X.; Ge, J.; Ling, Y.; Zheng, X. Macular image changes of optical coherence tomography after phacoemulsi-fication. Zhonghua Yan Ke Za Zhi 2002, 38, 265–267. [Google Scholar]
- Perente, I.; Utine, C.A.; Ozturker, C.; Cakir, M.; Kaya, V.; Eren, H.; Kapran, Z.; Yilmaz, O.F. Evaluation of macular changes after uncomplicated phacoemulsification surgery by optical coherence tomography. Curr. Eye Res. 2007, 32, 241–247. [Google Scholar] [CrossRef]
- Gudauskiene, G.; Matuleviciute, I.; Mockute, R.; Maciulaityte, E.; Zaliuniene, D. Changes in subfoveal choroidal thickness after uncomplicated cataract surgery. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech Repub. 2019, 163, 179–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurt, A.; Kılıç, R. The effects of uncomplicated cataract surgery on retinal layer thickness. J. Ophthalmol. 2018, 2018, 7218639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Li, Z.; Sun, R.; Liu, D.; Liu, N. Subfoveal choroidal and macular thickness changes after phacoemulsification using enhanced depth imaging optical coherence tomography. Ophthalmic Res. 2018, 60, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Pašová, P.; Skorkovská, K. The effect of cataract surgery on the reproducibility and outcome of optical coherence tomography measurements of macular and retinal nerve fibre layer thickness. Czech Slovak Ophthalmol. 2016, 72, 20–26. [Google Scholar]
- Yılmaz, T.; Karci, A.A.; Yilmaz, I.; Yılmaz, A.; Yıldırım, Y.; Sakalar, Y.B. Long-term changes in subfoveal choroidal thickness after cataract surgery. Med. Sci. Monit. 2016, 22, 1566–1570. [Google Scholar] [CrossRef] [Green Version]
- Gołebiewska, J.; Kęcik, D.; Turczyńska, M.; Moneta-Wielgoś, J.; Kopacz, D.; Pihowicz-Bakoń, K. Evaluation of macular thickness after uneventful phacoemulsification in selected patient populations using optical coherence tomography. Klin. Ocz. 2014, 116, 242–247. [Google Scholar]
- Gharbiya, M.; Cruciani, F.; Cuozzo, G.; Parisi, F.; Russo, P.; Abdolrahimzadeh, S. Macular thickness changes evaluated with spectral domain optical coherence tomography after uncomplicated phacoemulsification. Eye 2013, 27, 605–611. [Google Scholar] [CrossRef] [Green Version]
- Giansanti, F.; Bitossi, A.; Giacomelli, G.; Virgili, G.; Pieretti, G.; Giuntoli, M.; Abbruzzese, G.; Menchini, U. Evaluation of macular thickness after uncomplicated cataract surgery using optical coherence tomography. Eur. J. Ophthalmol. 2013, 23, 751–756. [Google Scholar] [CrossRef]
- Tsilimbaris, M.; Diakonis, V.F.; Kymionis, G.D.; Eleftheriadou, M.I.; Fragkiskou, S.; Moschandreas, J.; Frueh, B.E.; Epstein, D.; Pallikaris, A.I. Prospective study of foveal thickness alterations after cataract surgery assessed by optical coherence tomography. Ophthalmologica 2012, 228, 53–58. [Google Scholar] [CrossRef]
- Križanović, A.; Bjeloš, M.; Bušić, M.; Elabjer, B.K.; Rak, B.; Vukojević, N. Macular perfusion analysed by optical coherence tomography angiography after uncomplicated phacoemulsification: Benefits beyond restoring vision. BMC Ophthalmol. 2021, 21, 71. [Google Scholar] [CrossRef]
- Pilotto, E.; Leonardi, F.; Stefanon, G.; Longhin, E.; Torresin, T.; Deganello, D.; Cavarzeran, F.; Miglionico, G.; Parrozzani, R.; Midena, E. Early retinal and choroidal OCT and OCT angiography signs of inflammation after uncomplicated cataract surgery. Br. J. Ophthalmol. 2019, 103, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wen, W.; Jiang, C.; Lu, Y. Changes in macular vasculature after uncomplicated phacoemulsification surgery: Optical coherence tomography angiography study. J. Cataract Refract. Surg. 2018, 44, 453–458. [Google Scholar] [CrossRef]
- Li, T.; Guadie, A.; Feng, L.; Fan, J.; Jiang, Z.; Liu, F. Influence of cataract surgery on macular vascular density in patients with myopia using optical coherence tomography angiography. Exp. Ther. Med. 2020, 20, 258. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Azhati, G.; Li, T.; Liu, F. Macular vascular density changes following cataract surgery in diabetic patients: An optical coherence tomography angiography study. J. Ophthalmol. 2021, 2021, 6641944. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, M.; Wang, Y.; Ben, S.; Gao, M.; Zhang, S.; Liu, H.; Sun, X. Short-term changes in retinal vasculature and layer thickness after phacoemulsification surgery. Curr. Eye Res. 2020, 45, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Flitcroft, D.I.; He, M.; Jonas, J.B.; Jong, M.; Naidoo, K.; Ohno-Matsui, K.; Rahi, J.; Resnikoff, S.; Vitale, S.; Yannuzzi, L. IMI—Defining and Classifying Myopia: A Proposed Set of Standards for Clinical and Epidemiologic Studies. Investig. Ophthalmol. Vis. Sci. 2019, 60, M20–M30. [Google Scholar] [CrossRef] [Green Version]
- Tideman, J.W.L.; Polling, J.R.; Vingerling, J.R.; Jaddoe, V.W.V.; Williams, C.; Guggenheim, J.A.; Klaver, C.C.W. Axial length growth and the risk of developing myopia in European children. Acta Ophthalmol. 2018, 96, 301–309. [Google Scholar] [CrossRef]
- Isik, M.U.; Akay, F.; Akmaz, B.; Guven, Y.Z.; Isik, I.G. Normative Data of Superficial Retinal Vascular Plexus and the Relationship to Retinal Layers. Beyoglu Eye J. 2021, 6, 37–42. [Google Scholar] [CrossRef]
- How Do I Acquire a High-Quality Scan on My CIRRUS HD-OCT? (Part 3 of 3—OCT Alignment) ZEISS Quick Help: CIRRUS™ HD-OCT. Available online: https://www.zeiss.com/content/dam/Meditec/us/download/QuickHelp/how-do-i-acquire-quality-scan-cirrus-d3c.pdf (accessed on 16 April 2022).
- Sander, B.; Al-Abiji, H.A.; Kofod, M.; Jørgensen, T.M. Do different spectral domain OCT hardwares measure the same? Comparison of retinal thickness using third-party software. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 1915–1921. [Google Scholar] [CrossRef] [Green Version]
- Rao, H.L.; Addepalli, U.K.; Yadav, R.K.; Senthil, S.; Choudhari, N.S.; Garudadri, C.S. Effect of scan quality on diagnostic accuracy of spectral-domain optical coherence tomography in glaucoma. Am. J. Ophthalmol. 2014, 157, 719–727.e1. [Google Scholar] [CrossRef]
- Hardin, J.; Taibbi, G.; Nelson, S.C.; Chao, D.; Vizzeri, G. Factors affecting cirrus-HD OCT optic disc scan quality: A review with case examples. J. Ophthalmol. 2015, 2015, 746150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Na, J.H.; Sung, K.R.; Lee, Y. Factors associated with the signal strengths obtained by spectral domain optical coherence tomography. Korean J. Ophthalmol. 2012, 26, 169–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.J.; Camino, A.; Liu, L.; Zhang, X.; Wang, J.; Gao, S.S.; Jia, Y.; Huang, D. Signal strength reduction effects in OCT angiography. Ophthalmol. Retin. 2019, 3, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Lujan, B.J.; Calhoun, C.T.; Glassman, A.R.; Googe, J.M.; Jampol, L.M.; Melia, M.; Schlossman, D.K.; Sun, J.K.; DRCR retina network. Optical coherence tomography angiography quality across three multicenter clinical studies of diabetic retinopathy. Transl. Vis. Sci. Technol. 2021, 10, 2. [Google Scholar] [CrossRef]
- Czakó, C.; István, L.; Ecsedy, M.; Récsán, Z.; Sándor, G.; Benyó, F.; Horváth, H.; Papp, A.; Resch, M.; Borbándy, Á.; et al. The effect of image quality on the reliability of OCT angiography measurements in patients with diabetes. Int. J. Retin. Vitr. 2019, 5, 46. [Google Scholar] [CrossRef] [Green Version]
- Bin Lim, H.; Kim, Y.W.; Kim, J.M.; Jo, Y.J.; Kim, J.Y. The importance of signal strength in quantitative assessment of retinal vessel density using optical coherence tomography angiography. Sci. Rep. 2018, 8, 12897. [Google Scholar] [CrossRef]
- Hida, W.T.; de Medeiros, A.L.; Rolim, A.G.D.A.; Motta, A.F.P.; Kniggendorf, D.V.; de Queiroz, R.L.F.; Chaves, M.A.P.D.; Carricondo, P.C.; Nakano, C.T.; Nosé, W. Prospective randomized comparative study between venturi and peristaltic pumps in WhiteStar Signature® phacoemulsification machine. Clin. Ophthalmol. 2018, 13, 49–52. [Google Scholar] [CrossRef] [Green Version]
- Karagüzel, H.; Karalezlı, A.; Aslan, B.S.; Karalezli, A. Comparison of peristaltic and Venturi pumps in bimanual microincisional cataract surgery. Int. Ophthalmol. 2009, 29, 471–475. [Google Scholar] [CrossRef]
- Wielders, L.H.; Schouten, J.S.; Winkens, B.; Biggelaar, F.J.V.D.; Veldhuizen, C.A.; Findl, O.; Murta, J.; Goslings, W.R.; Tassignon, M.-J.; Joosse, M.V.; et al. European multicenter trial of the prevention of cystoid macular edema after cataract surgery in nondiabetics: ESCRS PREMED study report 1. J. Cataract Refract. Surg. 2018, 44, 429–439. [Google Scholar] [CrossRef]
- Kessel, L.; Tendal, B.; Jørgensen, K.J.; Erngaard, D.; Flesner, P.; Andresen, J.L.; Hjortdal, J. Post-cataract prevention of inflammation and macular edema by steroid and nonsteroidal anti-inflammatory eye drops: A systematic review. Ophthalmology 2014, 121, 1915–1924. [Google Scholar] [CrossRef] [Green Version]
- Taravati, P.; Lam, D.L.; Leveque, T.; Van Gelder, R.N. Postcataract surgical inflammation. Curr. Opin. Ophthalmol. 2012, 23, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Melancia, D.; Pinto, L.A.; Marques-Neves, C. Cataract Surgery and Intraocular Pressure. Ophthalmic Res. 2015, 53, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Berdahl, J.P. Cataract surgery to lower intraocular pressure. Middle East Afr. J. Ophthalmol. 2009, 16, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Hilton, E.J.R.; Hosking, S.L.; Gherghel, D.; Embleton, S.; Cunliffe, I.A. Beneficial effects of small-incision cataract surgery in patients demonstrating reduced ocular blood flow characteristics. Eye 2005, 19, 670–675. [Google Scholar] [CrossRef]
- Attwell, D.; Buchan, A.M.; Charpak, S.; Lauritzen, M.J.; MacVicar, B.A.; Newman, E.A. Glial and neuronal control of brain blood flow. Nature 2010, 468, 232–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
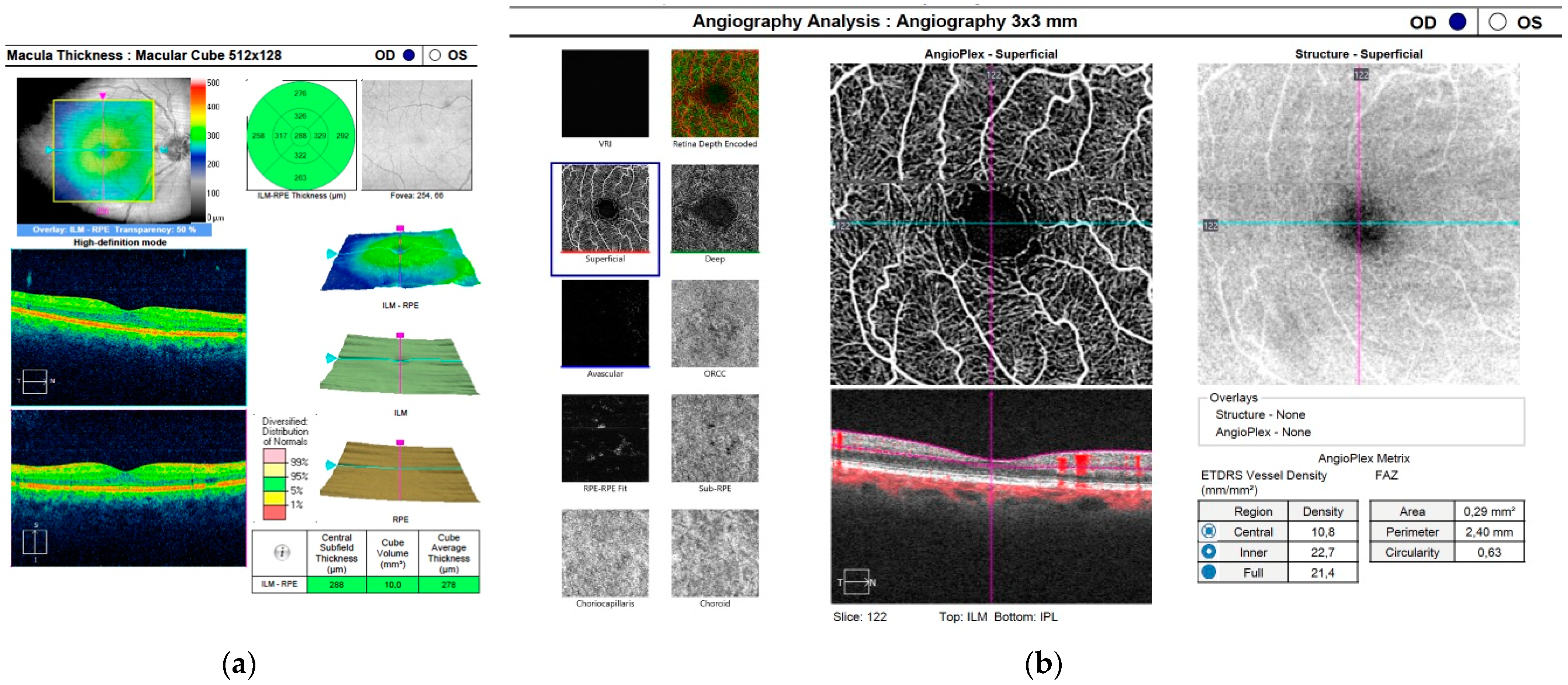



| Parameter | Mean | Median | SD | p |
|---|---|---|---|---|
| Phacoemulsification time | ||||
| effective (EPT) | 2.95 | 2.35 | 3.44 | <0.001 |
| At baseline | ||||
| best corrected visual acuity (logMAR) | 0.57 | 0.50 | 0.34 | 0.001 |
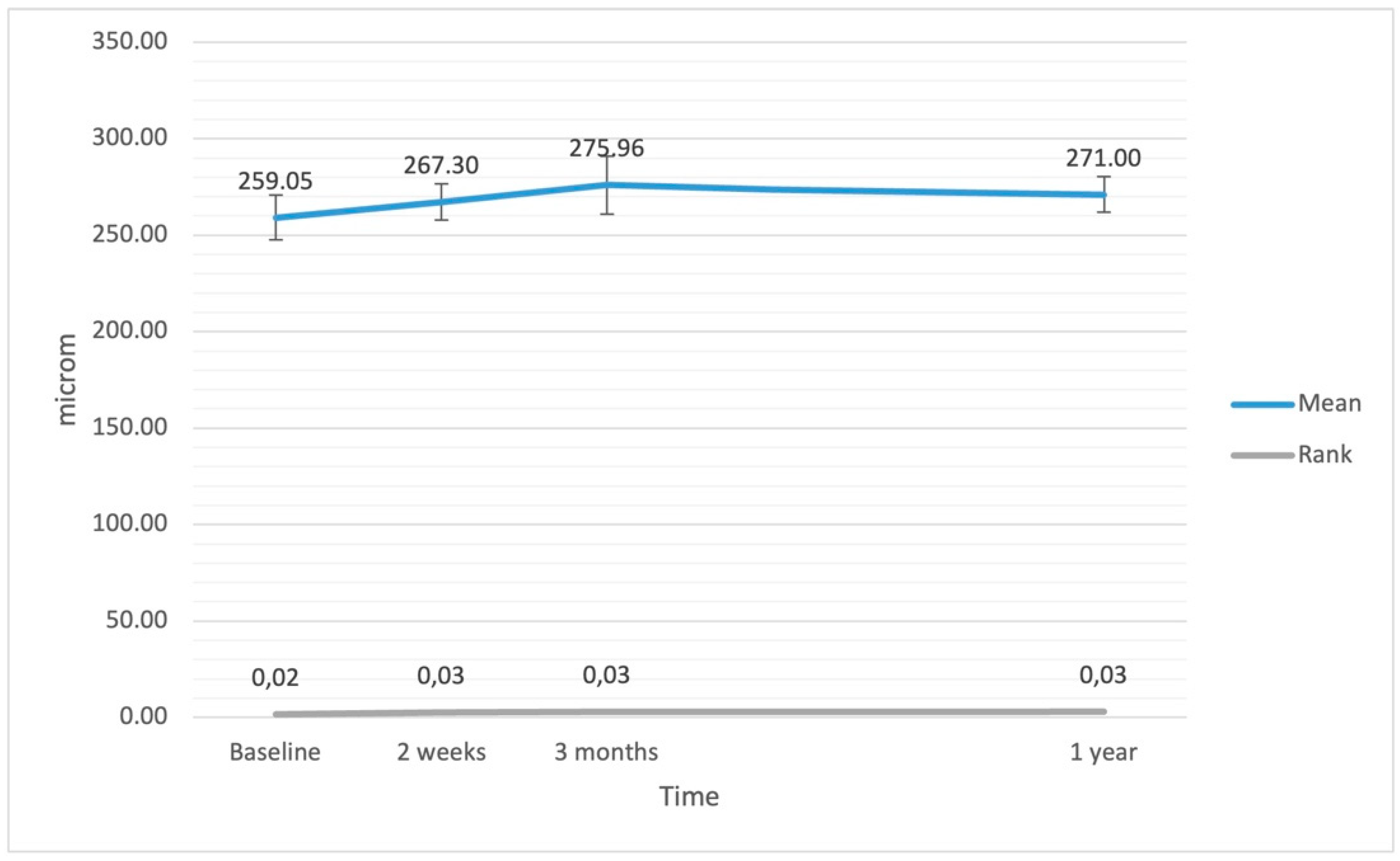
| central retinal thickness (μm) | 259.05 | 262.00 | 38.94 | <0.001 |
| central volume (mm3) | 9.39 | 9.65 | 1.07 | <0.001 |
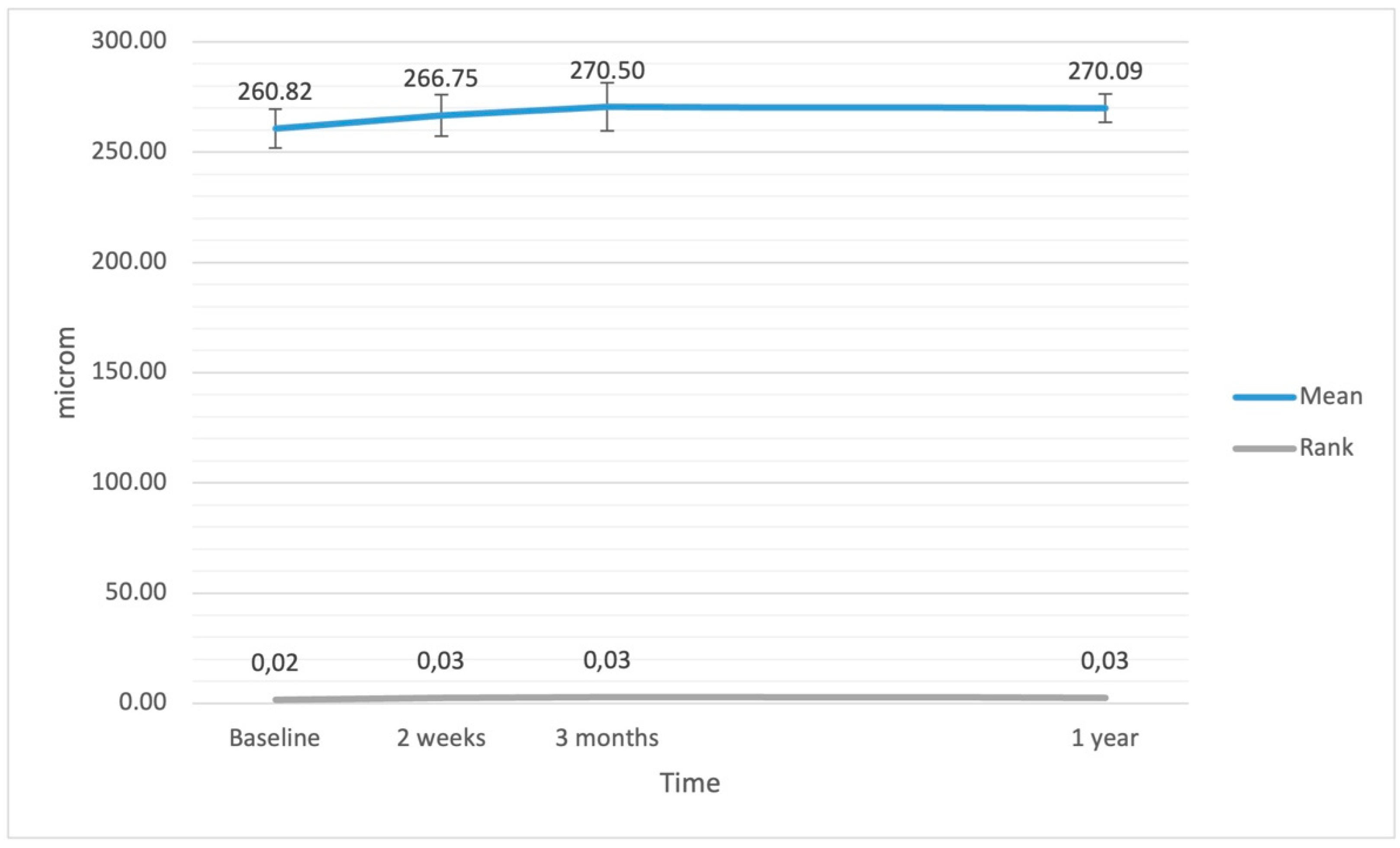
| average retinal thickness (μm) | 260.82 | 267.50 | 29.73 | <0.001 |
| vascular density central (mm/mm2) | 7.22 | 5.1 | 5.29 | 0.071 |
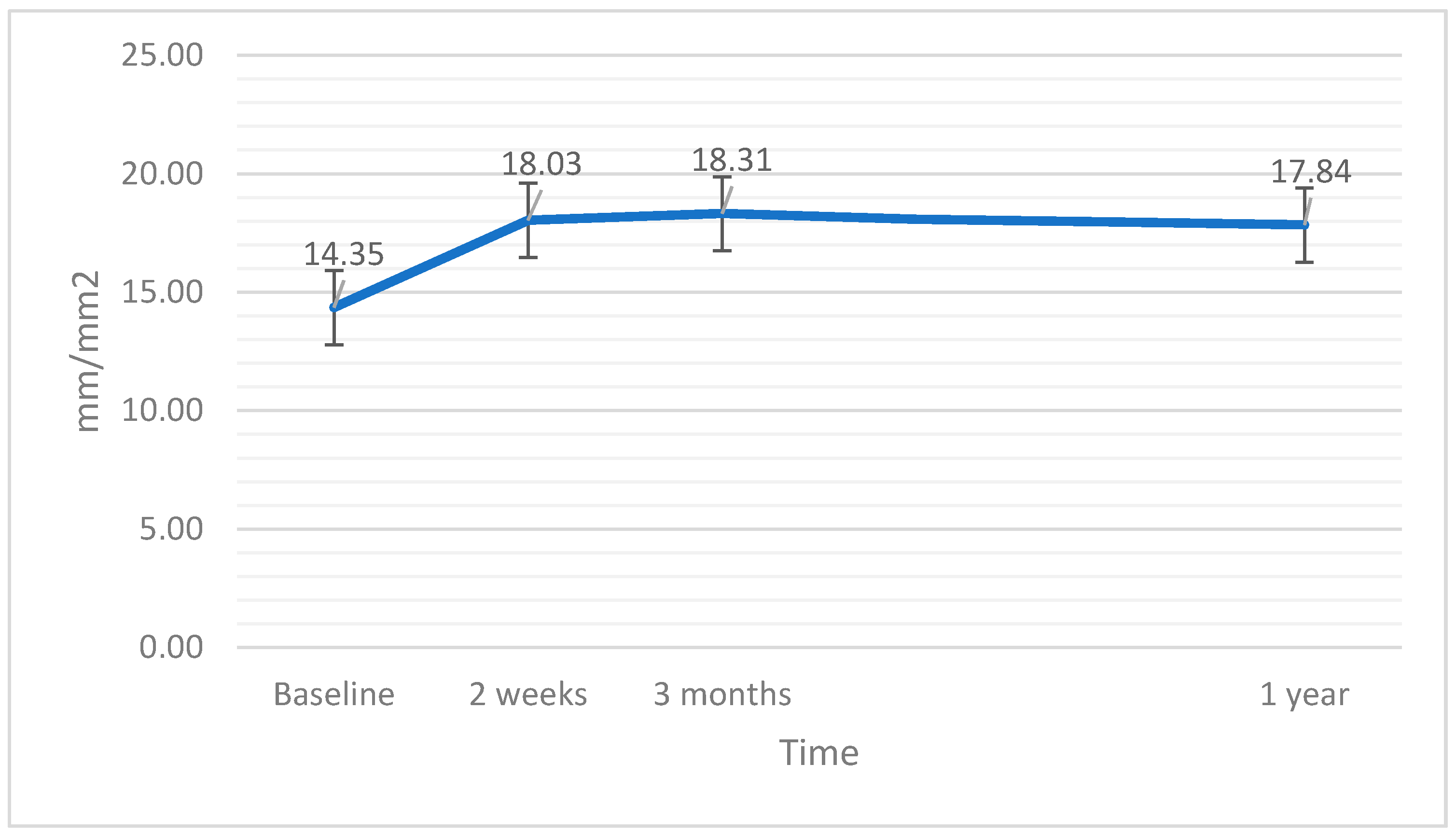
| vascular density full (mm/mm2) | 14.35 | 13.90 | 4.05 | 0.969 |
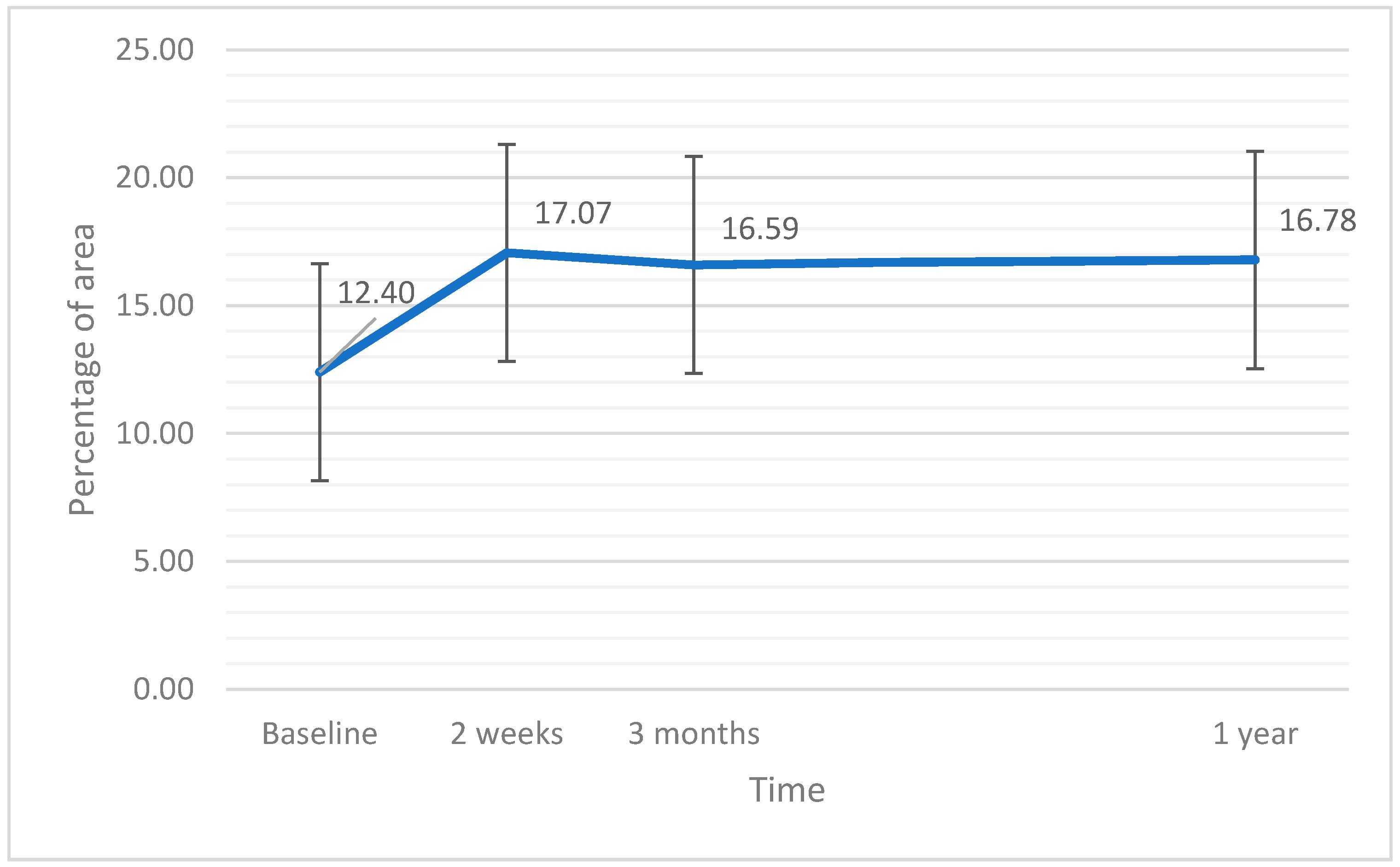
| vascular perfusion central (%) | 12.40 | 9.30 | 9.34 | 0.038 |
| vascular perfusion full (%) | 26.58 | 25.80 | 8.16 | 0.556 |
| 2 weeks after the surgery | ||||
| best corrected visual acuity (logMAR) | 0.10 | 0.00 | 0.13 | <0.001 |
| central retinal thickness (μm) | 267.30 | 259.00 | 31.80 | 0.010 |
| central volume (mm3) | 9.61 | 9.90 | 1.12 | <0.001 |
| average retinal thickness (μm) | 266.75 | 275.50 | 31.28 | <0.001 |
| vascular density central (mm/mm2) | 9.45 | 8.80 | 4.77 | <0.001 |
| vascular density full (mm/mm2) | 18.03 | 18.70 | 2.66 | <0.001 |
| vascular perfusion central (%) | 17.06 | 15.60 | 8.83 | 0.003 |
| vascular perfusion full (%) | 33.55 | 33.70 | 4.69 | <0.001 |
| 3 months after the surgery | ||||
| best corrected visual acuity (logMAR) | 0.02 | 0.00 | 0.06 | <0.001 |
| central retinal thickness (μm) | 275.95 | 271.00 | 50.97 | <0.001 |
| central volume (mm3) | 9.94 | 10.00 | 0.72 | 0.753 |
| average retinal thickness (μm) | 270.50 | 273.00 | 36.32 | <0.001 |
| vascular density central (mm/mm2) | 9.21 | 8.30 | 4.61 | 0.218 |
| vascular density full (mm/mm2) | 18.31 | 19.40 | 3.38 | 0.007 |
| vascular perfusion central (%) | 16.59 | 14.60 | 8.33 | 0.107 |
| vascular perfusion full (%) | 33.97 | 35.50 | 5.63 | 0.025 |
| 1 year after the surgery | ||||
| best corrected visual acuity (logMAR) | 0.01 | 0.00 | 0.03 | <0.001 |
| central retinal thickness (μm) | 271.00 | 272.00 | 31.26 | 0.198 |
| central volume (mm3) | 9.70 | 9.80 | 0.79 | 0.011 |
| average retinal thickness (μm) | 270.09 | 273.00 | 21.79 | 0.017 |
| vascular density central (mm/mm2) | 8.40 | 8.40 | 4.68 | 0.418 |
| vascular density full (mm/mm2) | 17.84 | 18.40 | 3.25 | 0.209 |
| vascular perfusion central (%) | 16.78 | 14.70 | 8.53 | 0.240 |
| vascular perfusion full (%) | 33.43 | 35.30 | 5.37 | 0.142 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gawęcki, M.; Prądzyńska, N.; Karska-Basta, I. Long-Term Variations in Retinal Parameters after Uncomplicated Cataract Surgery. J. Clin. Med. 2022, 11, 3426. https://doi.org/10.3390/jcm11123426
Gawęcki M, Prądzyńska N, Karska-Basta I. Long-Term Variations in Retinal Parameters after Uncomplicated Cataract Surgery. Journal of Clinical Medicine. 2022; 11(12):3426. https://doi.org/10.3390/jcm11123426
Chicago/Turabian StyleGawęcki, Maciej, Natalia Prądzyńska, and Izabella Karska-Basta. 2022. "Long-Term Variations in Retinal Parameters after Uncomplicated Cataract Surgery" Journal of Clinical Medicine 11, no. 12: 3426. https://doi.org/10.3390/jcm11123426







