Comparison of the Hemocompatibility of an Axial and a Centrifugal Left Ventricular Assist Device in an In Vitro Test Circuit
Abstract
:1. Introduction
2. Materials and Methods
2.1. Technical Features of Pumps under Investigation
2.2. Test Circuit Design
2.3. Circulation and Hemodynamics
2.4. Blood Donation and Experimental Groups
2.5. Blood Sampling and Analysis
2.6. Fluorescence-Activated Cell Sorting
2.7. Isolation of Extracellular Vesicles
2.8. Western Blot Analysis
2.9. Nanoparticle Tracking Analysis (NTA)
2.10. MiRNA Isolation and Quantification
2.11. Transmission Electron Microscopy (TEM)
2.12. Statistical Analysis
3. Results
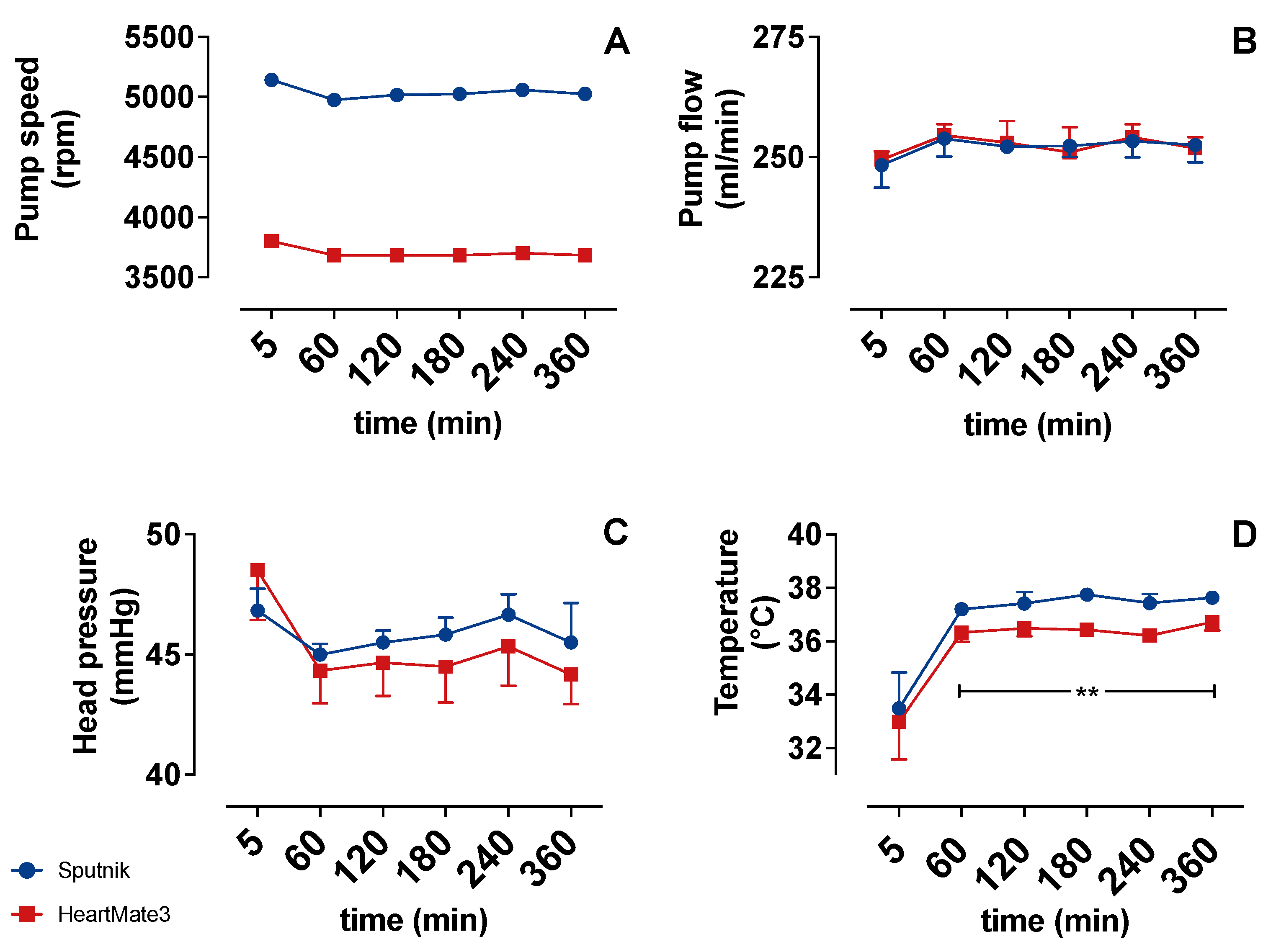
3.1. Operating Parameters
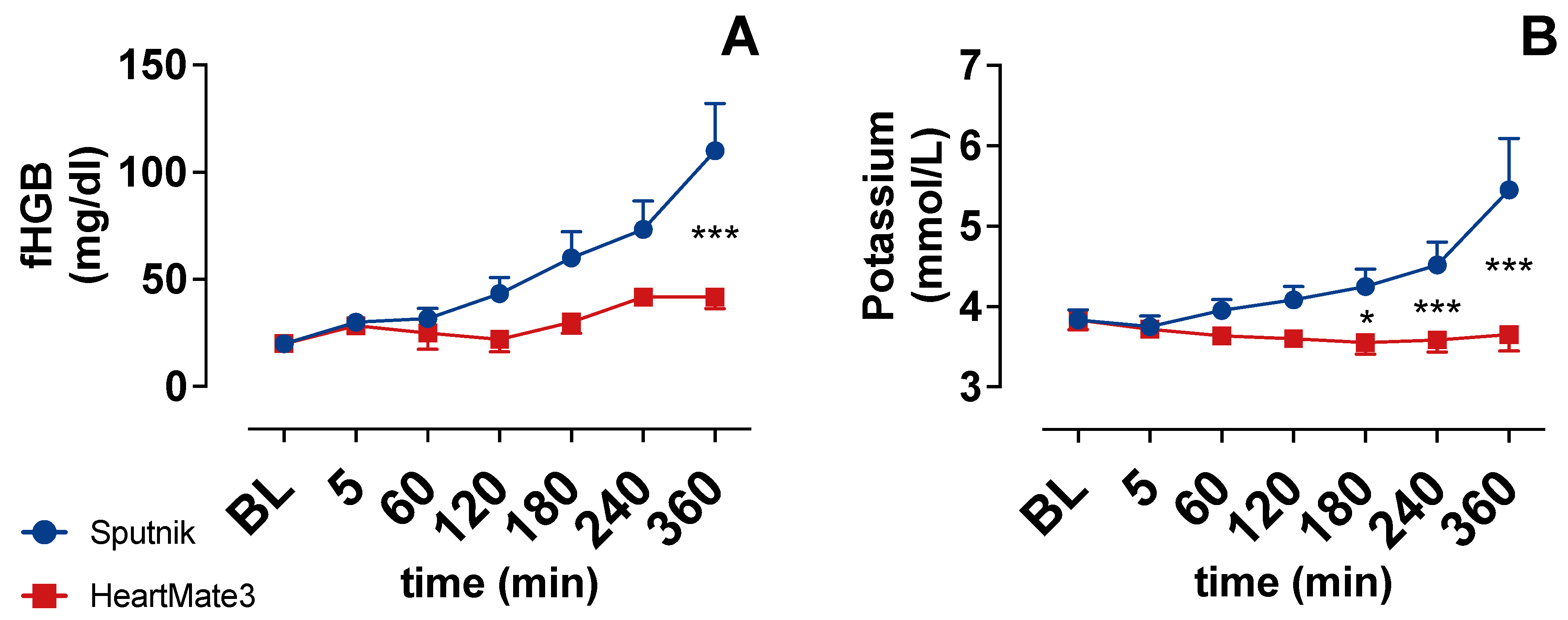
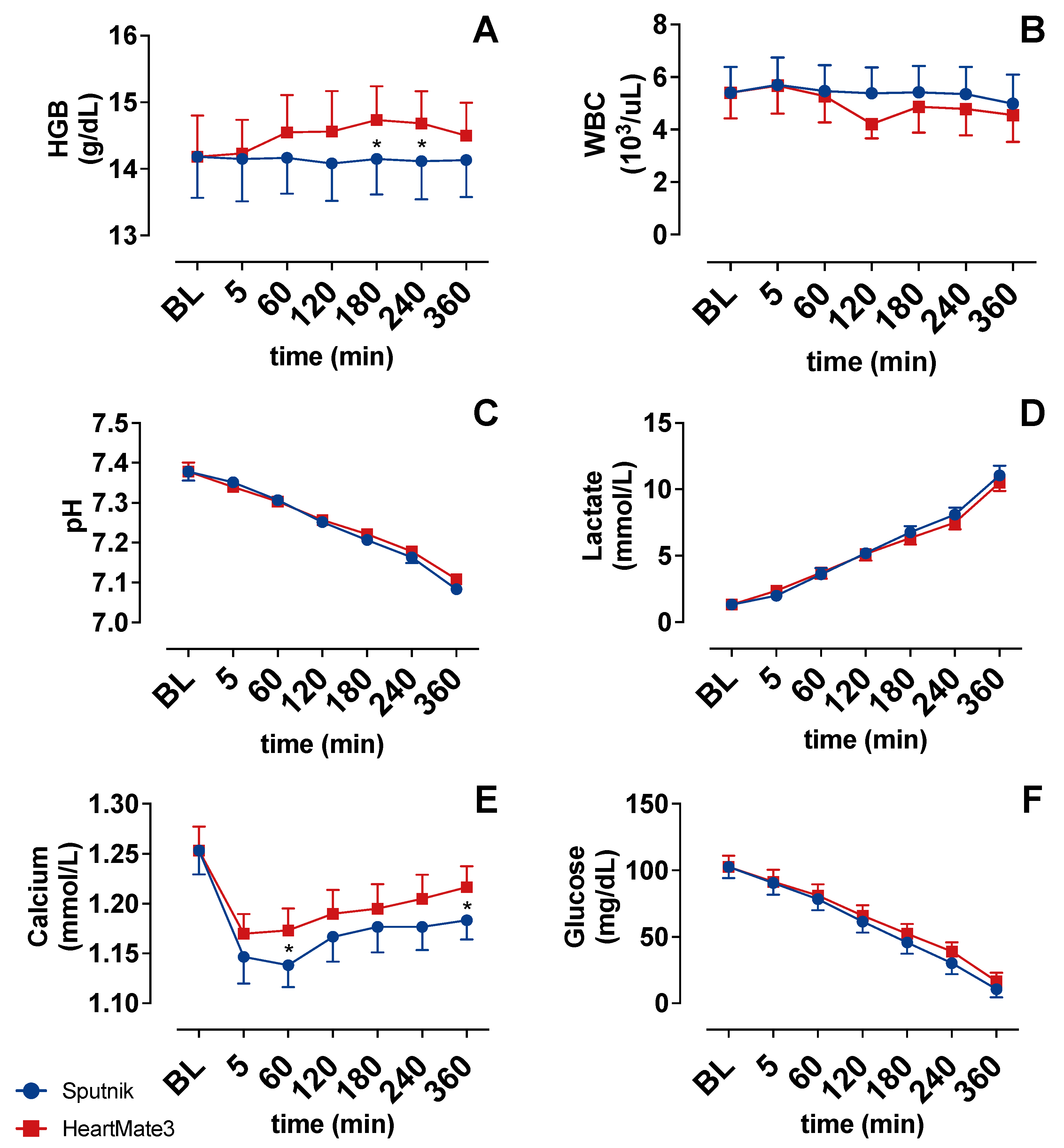
3.2. Indicators of Hemolysis
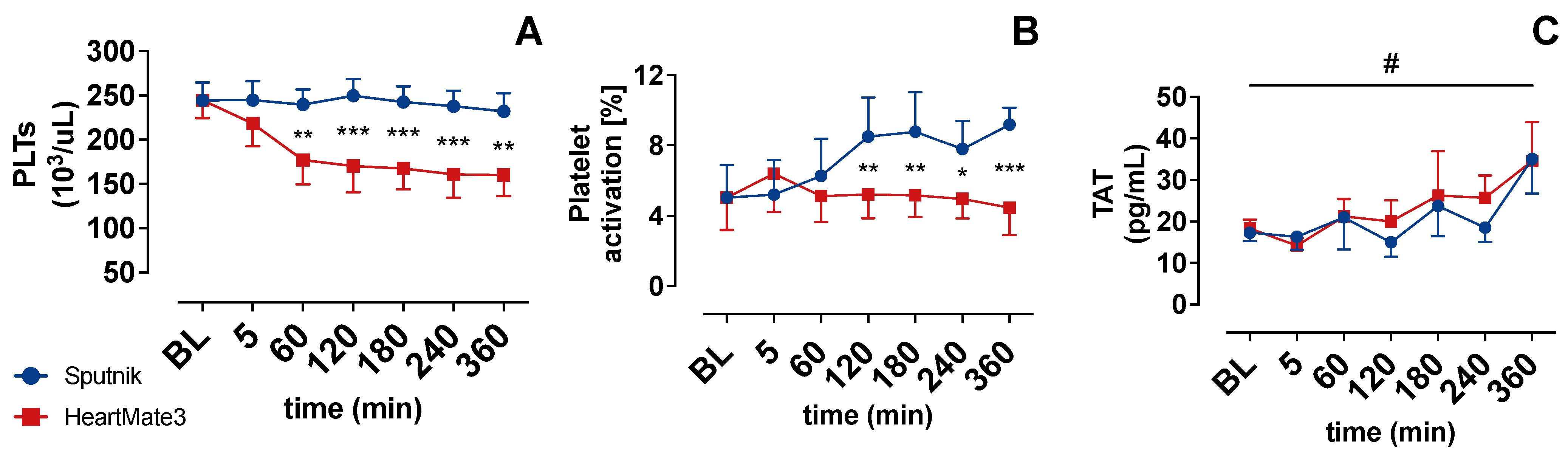
3.3. Platelet Count and Activation
3.4. Coagulation Activation
3.5. Cell Counter
3.6. Blood Gas Analysis
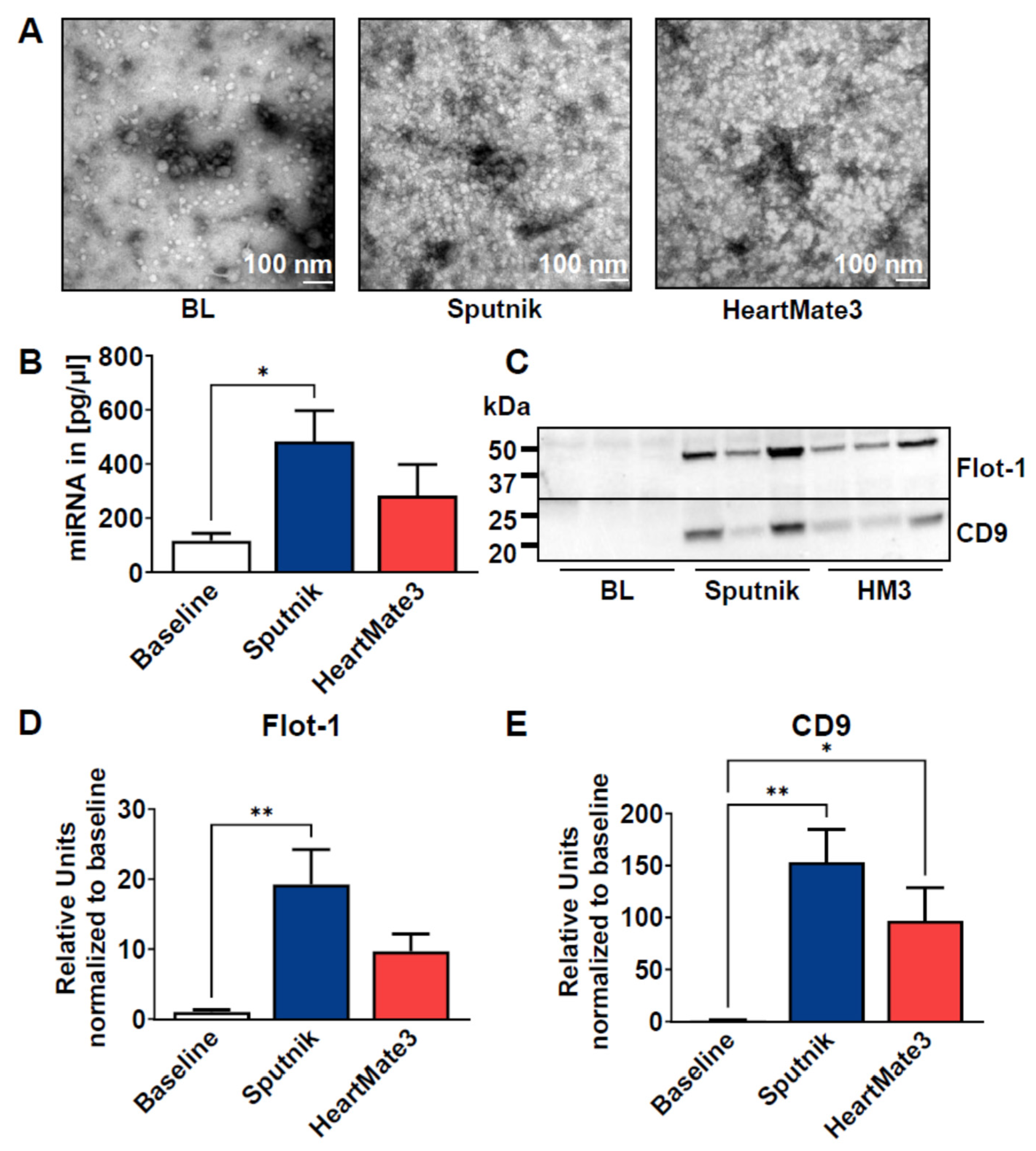
3.7. Release of Extracellular Vesicles
4. Discussion
4.1. Hemolysis
4.2. Platelet Count and Activation
4.3. Coagulation Activation
4.4. Release of Extracellular Vesicles
4.5. Limitations, Outlook and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miller, L.; Birks, E.; Guglin, M.; Lamba, H.; Frazier, O.H. Use of Ventricular Assist Devices and Heart Transplantation for Advanced Heart Failure. Circ. Res. 2019, 124, 1658–1678. [Google Scholar] [CrossRef] [PubMed]
- ASTM F1841-19; Standard Practice for Assessment of Hemolysis in Continuous Flow Blood Pumps. ASTM International: West Conshohocken, PA, USA, 2019.
- Mehra, M.R. The Burden of Haemocompatibility with Left Ventricular Assist Systems: A Complex Weave. Eur. Heart J. 2019, 40, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Fraser, K.H.; Zhang, T.; Taskin, M.E.; Griffith, B.P.; Wu, Z.J. A Quantitative Comparison of Mechanical Blood Damage Parameters in Rotary Ventricular Assist Devices: Shear Stress, Exposure Time and Hemolysis Index. J. Biomech. Eng. 2012, 134, 0810021–08100211. [Google Scholar] [CrossRef] [PubMed]
- Moazami, N.; Fukamachi, K.; Kobayashi, M.; Smedira, N.G.; Hoercher, K.J.; Massiello, A.; Lee, S.; Horvath, D.J.; Starling, R.C. Axial and Centrifugal Continuous-Flow Rotary Pumps: A Translation from Pump Mechanics to Clinical Practice. J. Heart Lung Transplant. 2013, 32, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nalezinková, M. In Vitro Hemocompatibility Testing of Medical Devices. Thromb. Res. 2020, 195, 146–150. [Google Scholar] [CrossRef]
- Papanastasiou, C.A.; Kyriakoulis, K.G.; Theochari, C.A.; Kokkinidis, D.G.; Karamitsos, T.D.; Palaiodimos, L. Comprehensive Review of Hemolysis in Ventricular Assist Devices. World J. Cardiol. 2020, 12, 334–341. [Google Scholar] [CrossRef]
- Ravichandran, A.K.; Parker, J.; Novak, E.; Joseph, S.M.; Schilling, J.D.; Ewald, G.A.; Silvestry, S. Hemolysis in Left Ventricular Assist Device: A Retrospective Analysis of Outcomes. J. Heart Lung Transplant. 2014, 33, 44–50. [Google Scholar] [CrossRef]
- Burke, E.J.; Parker, C. Novel HeartMate Cardiac Assist Systems (Thoratec). In Mechanical Circulatory Support in End-Stage Heart Failure: A Practical Manual; Montalto, A., Loforte, A., Musumeci, F., Krabatsch, T., Slaughter, M.S., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 557–563. ISBN 978-3-319-43383-7. [Google Scholar]
- DIN EN ISO 10993-4:2017-12; Biologische Beurteilung von Medizinprodukten_-Teil_4: Auswahl von Prüfungen Zur Wechselwirkung Mit Blut (ISO_10993-4:2017). Deutsche Fassung EN_ISO_10993-4:2017. Beuth Verlag GmbH: Berlin, Germany, 2017.
- Krishnamoorthy, P.; Naranjo, M.; Koshkelashvili, N.; Gopalakrishnan, A.; Tuluca, A.; Rangaswami, J.; Figueredo, V.; Anderson, M.; Morris, D. White Blood Cell Count to Platelet Ratio: A Novel Biomarker of Outcomes in Patients with Circulatory Support on Left Ventricular Impella Assist Devices. J. Am. Coll. Cardiol. 2017, 69, 1252. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, J.; Li, T.; Tran, D.; Griffith, B.P.; Wu, Z.J. The Impact of Shear Stress on Device-Induced Platelet Hemostatic Dysfunction Relevant to Thrombosis and Bleeding in Mechanically Assisted Circulation. Artif. Organs 2020, 44, E201–E213. [Google Scholar] [CrossRef]
- Scott-Burden, T.; Tock, C.L.; Bosely, J.P.; Clubb, F.J.; Parnis, S.M.; Schwarz, J.J.; Engler, D.A.; Frazier, O.H.; Casscells, S.W. Nonthrombogenic, Adhesive Cellular Lining for Left Ventricular Assist Devices. Circulation 1998, 98, II339–II345. [Google Scholar]
- Menconi, M.J.; Pockwinse, S.; Owen, T.A.; Dasse, K.A.; Stein, G.S.; Lian, J.B. Properties of Blood-Contacting Surfaces of Clinically Implanted Cardiac Assist Devices: Gene Expression, Matrix Composition, and Ultrastructural Characterization of Cellular Linings. J. Cell. Biochem. 1995, 57, 557–573. [Google Scholar] [CrossRef] [PubMed]
- Spanier, T.; Oz, M.; Levin, H.; Weinberg, A.; Stamatis, K.; Stern, D.; Rose, E.; Schmidt, A.M. Activation of Coagulation and Fibrinolytic Pathways in Patients with Left Ventricular Assist Devices. J. Thorac. Cardiovasc. Surg. 1996, 112, 1090–1097. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Griffith, B.P.; Wu, Z.J. Device-Induced Hemostatic Disorders in Mechanically Assisted Circulation. Clin. Appl. Thromb. Off. J. Int. Acad. Clin. Appl. Thromb. 2021, 27, 1076029620982374. [Google Scholar] [CrossRef] [PubMed]
- Kramser, N.; Oehler, D.; Saeed, D.; Aubin, H.; Akhyari, P.; Kelm, M.; Westenfeld, R.; Horn, P. Thromboembolic Events in Patients With Left Ventricular Assist Devices Are Related to Microparticle-Induced Coagulation. ASAIO J. Am. Soc. Artif. Intern. Organs 1992 2021, 67, 59–66. [Google Scholar] [CrossRef]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic Comparison Defines Novel Markers to Characterize Heterogeneous Populations of Extracellular Vesicle Subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Li, S.; Li, L.; Li, M.; Guo, C.; Yao, J.; Mi, S. Exosome and Exosomal MicroRNA: Trafficking, Sorting, and Function. Genom. Proteom. Bioinform. 2015, 13, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Diehl, P.; Aleker, M.; Helbing, T.; Sossong, V.; Beyersdorf, F.; Olschewski, M.; Bode, C.; Moser, M. Enhanced Microparticles in Ventricular Assist Device Patients Predict Platelet, Leukocyte and Endothelial Cell Activation. Interact. Cardiovasc. Thorac. Surg. 2010, 11, 133–137. [Google Scholar] [CrossRef]
- Nascimbene, A.; Hernandez, R.; George, J.K.; Parker, A.; Bergeron, A.L.; Pradhan, S.; Vijayan, K.V.; Civitello, A.; Simpson, L.; Nawrot, M.; et al. Association between Cell-Derived Microparticles and Adverse Events in Patients with Nonpulsatile Left Ventricular Assist Devices. J. Heart Lung Transplant. 2014, 33, 470–477. [Google Scholar] [CrossRef] [Green Version]
- Romanova, A.N.; Pugovkin, A.A.; Denisov, M.V.; Ephimov, I.A.; Gusev, D.V.; Walter, M.; Groth, T.; Bockeria, O.L.; Le, T.G.; Satyukova, A.S.; et al. Hemolytic Performance in Two Generations of the Sputnik Left Ventricular Assist Device: A Combined Numerical and Experimental Study. J. Funct. Biomater. 2022, 13, 7. [Google Scholar] [CrossRef]
- About the HeartMate II LVAD | Abbott. Available online: https://www.cardiovascular.abbott/us/en/hcp/products/heart-failure/left-ventricular-assist-devices/heartmate-2/about.html (accessed on 24 January 2022).
- Tinnemans, A. Linksventrikuläres Herzunterstützungssystem (LVAD) HeartMate 3. Available online: https://dam.abbott.com/de-de/documents/Mediathek/2021-Abbott-Factsheets-LVAD.pdf (accessed on 12 June 2022).
- Mehra, M.R.; Goldstein, D.J.; Uriel, N.; Cleveland, J.C.; Yuzefpolskaya, M.; Salerno, C.; Walsh, M.N.; Milano, C.A.; Patel, C.B.; Ewald, G.A.; et al. Two-Year Outcomes with a Magnetically Levitated Cardiac Pump in Heart Failure. N. Engl. J. Med. 2018, 378, 1386–1395. [Google Scholar] [CrossRef]
- Chatterjee, A.; Feldmann, C.; Hanke, J.S.; Ricklefs, M.; Shrestha, M.; Dogan, G.; Haverich, A.; Schmitto, J.D. The Momentum of HeartMate 3: A Novel Active Magnetically Levitated Centrifugal Left Ventricular Assist Device (LVAD). J. Thorac. Dis. 2018, 10, S1790–S1793. [Google Scholar] [CrossRef] [PubMed]
- Mehra, M.R.; Cleveland Jr, J.C.; Uriel, N.; Cowger, J.A.; Hall, S.; Horstmanshof, D.; Naka, Y.; Salerno, C.T.; Chuang, J.; Williams, C.; et al. Primary Results of Long-Term Outcomes in the MOMENTUM 3 Pivotal Trial and Continued Access Protocol Study Phase: A Study of 2200 HeartMate 3 Left Ventricular Assist Device Implants. Eur. J. Heart Fail. 2021, 23, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, M.K.; Pagani, F.D.; Mehra, M.R.; Estep, J.D.; Pinney, S.P.; Silvestry, S.C.; Uriel, N.; Goldstein, D.J.; Long, J.; Cleveland, J.C.; et al. Center Variability in Patient Outcomes Following HeartMate 3 Implantation: An Analysis of the MOMENTUM 3 Trial. J. Card. Fail. 2022. [Google Scholar] [CrossRef] [PubMed]
- Sivathasan, C.; Lim, C.P.; Kerk, K.L.; Sim, D.K.L.; Mehra, M.R. Mechanical Circulatory Support and Heart Transplantation in the Asia Pacific Region. J. Heart Lung Transplant. 2017, 36, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Telyshev, D.; Selishchev, S. Ventricular Assist Device Sputnik: Description, Technical Features and Characteristics. Trends Biomater. Artif. Organs 2015, 29, 207–210. [Google Scholar]
- Selishchev, S.; Telyshev, D. Optimisation of the Sputnik-VAD Design. Int. J. Artif. Organs 2016, 39, 407–414. [Google Scholar] [CrossRef]
- Mehra, M.R.; Naka, Y.; Uriel, N.; Goldstein, D.J.; Cleveland, J.C.; Colombo, P.C.; Walsh, M.N.; Milano, C.A.; Patel, C.B.; Jorde, U.P.; et al. A Fully Magnetically Levitated Circulatory Pump for Advanced Heart Failure. N. Engl. J. Med. 2017, 376, 440–450. [Google Scholar] [CrossRef]
- Our Heritage | about Abbott | Abbott U.S. Available online: https://www.abbott.com/about-abbott/our-heritage.html (accessed on 10 April 2022).
- Hosseinipour, M.; Gupta, R.; Bonnell, M.; Elahinia, M. Rotary Mechanical Circulatory Support Systems. J. Rehabil. Assist. Technol. Eng. 2017, 4, 2055668317725994. [Google Scholar] [CrossRef] [Green Version]
- Gautier, S.V.; Shevchenko, A.O.; Itkin, G.P.; Zakharevich, V.M.; Poptsov, V.N.; Drobyshev, A.A.; Telyshev, D.V. Artificial Heart in Russia: Past, Present, and Future. Artif. Organs 2021, 45, 111–114. [Google Scholar] [CrossRef]
- Heatley, G.; Sood, P.; Goldstein, D.; Uriel, N.; Cleveland, J.; Middlebrook, D.; Mehra, M.R. Clinical Trial Design and Rationale of the Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy with HeartMate 3 (MOMENTUM 3) Investigational Device Exemption Clinical Study Protocol. J. Heart Lung Transplant. 2016, 35, 528–536. [Google Scholar] [CrossRef] [Green Version]
- Borosch, S.; Dahmen, E.; Beckers, C.; Stoppe, C.; Buhl, M.; Denecke, B.; Goetzenich, A.; Kraemer, S. Characterization of Extracellular Vesicles Derived from Cardiac Cells in an in Vitro Model of Preconditioning. J. Extracell. Vesicles 2017, 6, 1390391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zayat, R.; Moza, A.; Grottke, O.; Grzanna, T.; Fechter, T.; Motomura, T.; Schmidt-Mewes, C.; Breuer, T.; Autschbach, R.; Rossaint, R.; et al. In Vitro Comparison of the Hemocompatibility of Two Centrifugal Left Ventricular Assist Devices. J. Thorac. Cardiovasc. Surg. 2019, 157, 591–599.e4. [Google Scholar] [CrossRef] [PubMed]
- Bourque, K.; Cotter, C.; Dague, C.; Harjes, D.; Dur, O.; Duhamel, J.; Spink, K.; Walsh, K.; Burke, E. Design Rationale and Preclinical Evaluation of the HeartMate 3 Left Ventricular Assist System for Hemocompatibility. ASAIO J. Am. Soc. Artif. Intern. Organs 1992 2016, 62, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Thoratec Corporation. HeartMate 3 Left Ventricular Assist System: Instructions for Use; Thoratec Corporation: Pleasanton, CA, USA, 2017. [Google Scholar]
- Yamazaki, K.; Litwak, P.; Tagusari, O.; Mori, T.; Kono, K.; Kameneva, M.; Watach, M.; Gordon, L.; Miyagishima, M.; Tomioka, J.; et al. An Implantable Centrifugal Blood Pump with a Recirculating Purge System (Cool-Seal System). Artif. Organs 1998, 22, 466–474. [Google Scholar] [CrossRef]
- Packham, M.A. The Behavior of Platelets at Foreign Surfaces. Proc. Soc. Exp. Biol. Med. 1988, 189, 261–274. [Google Scholar] [CrossRef]
- Gorbet, M.B.; Sefton, M.V. Biomaterial-Associated Thrombosis: Roles of Coagulation Factors, Complement, Platelets and Leukocytes. Biomaterials 2004, 25, 5681–5703. [Google Scholar] [CrossRef]
- Koh, L.B.; Rodriguez, I.; Venkatraman, S.S. The Effect of Topography of Polymer Surfaces on Platelet Adhesion. Biomaterials 2010, 31, 1533–1545. [Google Scholar] [CrossRef]
- Xu, L.-C.; Bauer, J.W.; Siedlecki, C.A. Proteins, Platelets, and Blood Coagulation at Biomaterial Interfaces. Colloids Surf. B Biointerfaces 2014, 124, 49–68. [Google Scholar] [CrossRef] [Green Version]
- Minelli, C.; Kikuta, A.; Tsud, N.; Ball, M.D.; Yamamoto, A. A Micro-Fluidic Study of Whole Blood Behaviour on PMMA Topographical Nanostructures. J. Nano Biotechnol. 2008, 6, 3. [Google Scholar] [CrossRef] [Green Version]
- Koc, Y.; de Mello, A.J.; McHale, G.; Newton, M.I.; Roach, P.; Shirtcliffe, N.J. Nano-Scale Superhydrophobicity: Suppression of Protein Adsorption and Promotion of Flow-Induced Detachment. Lab Chip 2008, 8, 582–586. [Google Scholar] [CrossRef] [Green Version]
- Zapanta, C.M.; Griffith, J.W.; Hess, G.D.; Doxtater, B.J.; Khalapyan, T.; Pae, W.E.; Rosenberg, G. Microtextured Materials for Circulatory Support Devices: Preliminary Studies. ASAIO J. Am. Soc. Artif. Intern. Organs 1992 2006, 52, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Consolo, F.; Sferrazza, G.; Motolone, G.; Contri, R.; Valerio, L.; Lembo, R.; Pozzi, L.; Della Valle, P.; De Bonis, M.; Zangrillo, A.; et al. Platelet Activation Is a Preoperative Risk Factor for the Development of Thromboembolic Complications in Patients with Continuous-Flow Left Ventricular Assist Device. Eur. J. Heart Fail. 2018, 20, 792–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahdi, A.; Cortese-Krott, M.M.; Kelm, M.; Li, N.; Pernow, J. Novel Perspectives on Redox Signaling in Red Blood Cells and Platelets in Cardiovascular Disease. Free Radic. Biol. Med. 2021, 168, 95–109. [Google Scholar] [CrossRef]
- Deguchi, K.; Noguchi, M.; Yuwasaki, E.; Endou, T.; Deguchi, A.; Wada, H.; Murashima, S.; Nishikawa, M.; Shirakawa, S.; Tanaka, K. Dynamic Fluctuations in Blood of Thrombin/Antithrombin III Complex (TAT). Am. J. Hematol. 1991, 38, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Sato, T.; Kondo, C.; Yada, I.; Yuasa, H.; Kusagawa, M.; Nasu, M.; Okada, Y.; Shomura, T. Hematological Problems during the Use of Cardiac Assist Devices: Clinical Experiences in Japan. Artif. Organs 1992, 16, 182–188. [Google Scholar] [CrossRef]
- Zayat, R.; Khattab, M.A.; Grottke, O.; Honickel, M.; Goetzenich, A.; Moza, A.; Stoppe, C.; Autschbach, R.; Tewarie, L. Survival of HeartMate II Patients Despite Cessation of Anticoagulation—Outcomes and Hemostatic Analysis. Circ. J. 2018, 82, 1309–1318. [Google Scholar] [CrossRef] [Green Version]
- Schibilsky, D.; Lenglinger, M.; Avci-Adali, M.; Haller, C.; Walker, T.; Wendel, H.P.; Schlensak, C. Hemocompatibility of Axial Versus Centrifugal Pump Technology in Mechanical Circulatory Support Devices. Artif. Organs 2015, 39, 723–728. [Google Scholar] [CrossRef]
- Schibilsky, D.; Takatani, S.; Schibilsky, B.; Graf, T.; da Silva, D.M.; Wendel, H.P.; Avci-Adali, M.; Schlensak, C. Hemocompatibility of New Magnetically-Levitated Centrifugal Pump Technology Compared to the CentriMag Adult Pump. Sci. Rep. 2020, 10, 22055. [Google Scholar] [CrossRef]
- Ragusa, R.; Di Molfetta, A.; D’Aurizio, R.; Del Turco, S.; Cabiati, M.; Del Ry, S.; Basta, G.; Pitto, L.; Amodeo, A.; Trivella, M.G.; et al. Variations of Circulating MiRNA in Paediatric Patients with Heart Failure Supported with Ventricular Assist Device: A Pilot Study. Sci. Rep. 2020, 10, 5905. [Google Scholar] [CrossRef]
- Ding, J.; Chen, Z.; Niu, S.; Zhang, J.; Mondal, N.; Griffith, B.; Wu, Z. Quantification of Shear-Induced Platelet Activation: High Shear Stresses for Short Exposure Time. Artif. Organs 2015, 39, 576–583. [Google Scholar] [CrossRef]
- MEyER, A.D.; GELFOND, J.A.L.; WILES, A.A.; FREISHTAT, R.J.; RAIS-BAHRAMI, K. Platelet-Derived Microparticles Generated by Neonatal Extracorporeal Membrane Oxygenation Systems. ASAIO J. Am. Soc. Artif. Intern. Organs 1992 2015, 61, 37–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, C.H.; Leverett, L.B.; Lewis, C.W.; Alfrey, C.P.; Hellums, J.D. Morphological, Biochemical, and Functional Changes in Human Platelets Subjected to Shear Stress. J. Lab. Clin. Med. 1975, 86, 462–471. [Google Scholar] [PubMed]
- Nomura, S.; Tandon, N.N.; Nakamura, T.; Cone, J.; Fukuhara, S.; Kambayashi, J. High-Shear-Stress-Induced Activation of Platelets and Microparticles Enhances Expression of Cell Adhesion Molecules in THP-1 and Endothelial Cells. Atherosclerosis 2001, 158, 277–287. [Google Scholar] [CrossRef]
- Veerman, R.E.; Teeuwen, L.; Czarnewski, P.; Güclüler Akpinar, G.; Sandberg, A.; Cao, X.; Pernemalm, M.; Orre, L.M.; Gabrielsson, S.; Eldh, M. Molecular Evaluation of Five Different Isolation Methods for Extracellular Vesicles Reveals Different Clinical Applicability and Subcellular Origin. J. Extracell. Vesicles 2021, 10, e12128. [Google Scholar] [CrossRef]
- Brennan, K.; Martin, K.; FitzGerald, S.P.; O’Sullivan, J.; Wu, Y.; Blanco, A.; Richardson, C.; Mc Gee, M.M. A Comparison of Methods for the Isolation and Separation of Extracellular Vesicles from Protein and Lipid Particles in Human Serum. Sci. Rep. 2020, 10, 1039. [Google Scholar] [CrossRef] [Green Version]
- Sódar, B.W.; Kittel, Á.; Pálóczi, K.; Vukman, K.V.; Osteikoetxea, X.; Szabó-Taylor, K.; Németh, A.; Sperlágh, B.; Baranyai, T.; Giricz, Z.; et al. Low-Density Lipoprotein Mimics Blood Plasma-Derived Exosomes and Microvesicles during Isolation and Detection. Sci. Rep. 2016, 6, 24316. [Google Scholar] [CrossRef] [Green Version]
- Comfort, N.; Cai, K.; Bloomquist, T.R.; Strait, M.D.; Ferrante, A.W.; Baccarelli, A.A. Nanoparticle Tracking Analysis for the Quantification and Size Determination of Extracellular Vesicles. J. Vis. Exp. JoVE 2021. [Google Scholar] [CrossRef]
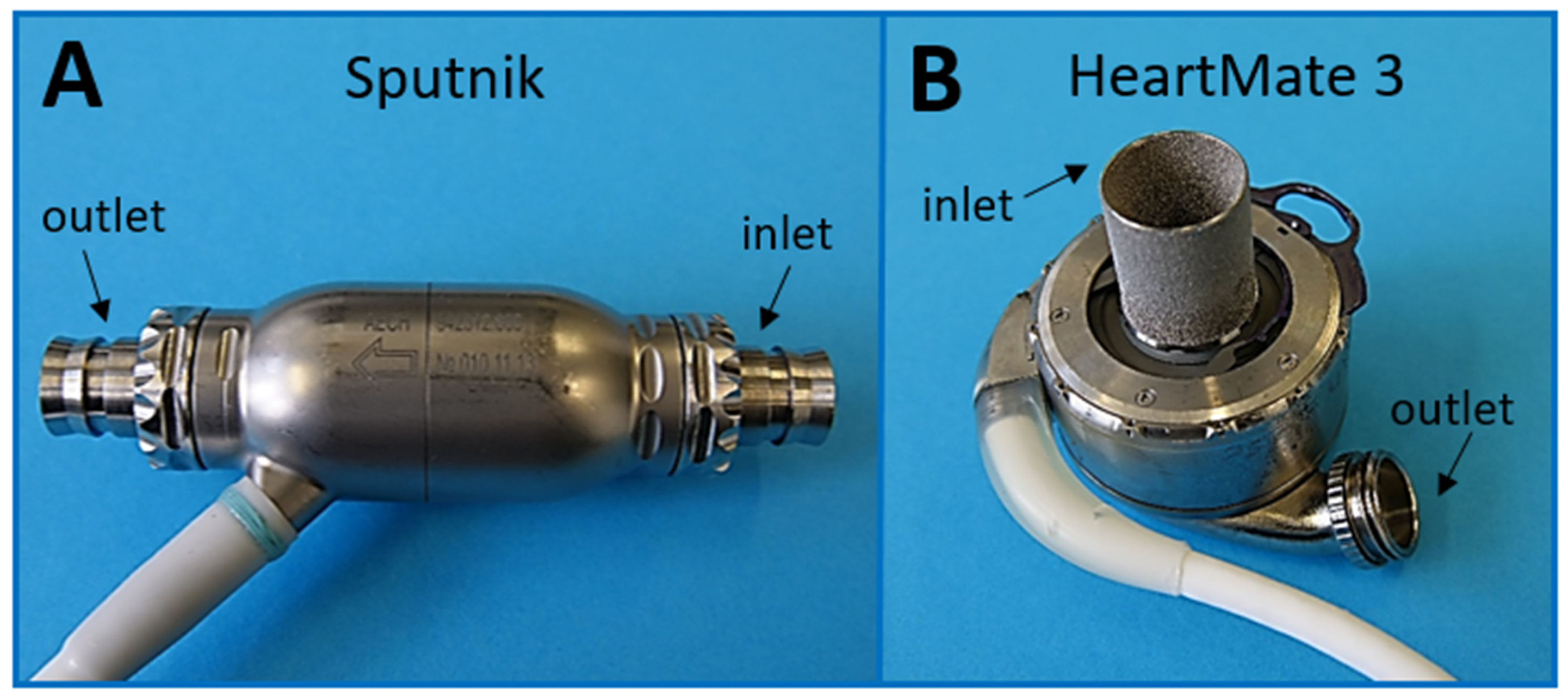


| Sputnik | HeartMate 3 | |
|---|---|---|
| Pump type | Axial-flow pump (2. generation) | Centrifugal-flow pump (3. generation) |
| Bearing | Mechanical (cup socket) | Magnetic |
| Implantation location | Extrathoracic/intrathoracic | Intrathoracic |
| Patients | >50 (2020) | >515 (2019) |
| Maximum speed range | 5000 rpm–10,000 rpm | 3000 rpm–9000 rpm |
| Clinical speed range | 7500 rpm–8600 rpm | 5000 rpm–6000 rpm |
| Artificial pulse | No | Yes |
| Maximum flow | Up to 10 L/min | Up to 10 L/min |
| Weight | 246 g | 200 g |
| Size | Diameter: 34 mm Length: 81 mm | Diameter: 69 mm Height: 30 mm |
| Flow gap | 0.2 mm | 0.5 mm–1 mm |
| Material | Titanium alloy | Titanium |
| Textured surfaces | No | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borchers, P.; Winnersbach, P.; Kraemer, S.; Beckers, C.; Buhl, E.M.; Leonhardt, S.; Rossaint, R.; Walter, M.; Breuer, T.; Bleilevens, C. Comparison of the Hemocompatibility of an Axial and a Centrifugal Left Ventricular Assist Device in an In Vitro Test Circuit. J. Clin. Med. 2022, 11, 3431. https://doi.org/10.3390/jcm11123431
Borchers P, Winnersbach P, Kraemer S, Beckers C, Buhl EM, Leonhardt S, Rossaint R, Walter M, Breuer T, Bleilevens C. Comparison of the Hemocompatibility of an Axial and a Centrifugal Left Ventricular Assist Device in an In Vitro Test Circuit. Journal of Clinical Medicine. 2022; 11(12):3431. https://doi.org/10.3390/jcm11123431
Chicago/Turabian StyleBorchers, Patrick, Patrick Winnersbach, Sandra Kraemer, Christian Beckers, Eva Miriam Buhl, Steffen Leonhardt, Rolf Rossaint, Marian Walter, Thomas Breuer, and Christian Bleilevens. 2022. "Comparison of the Hemocompatibility of an Axial and a Centrifugal Left Ventricular Assist Device in an In Vitro Test Circuit" Journal of Clinical Medicine 11, no. 12: 3431. https://doi.org/10.3390/jcm11123431







