Contact Lenses Loaded with Melatonin Analogs: A Promising Therapeutic Tool against Dry Eye Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Spectrophotometric Determination of Melatonin and Its Analogs
2.4. Preparation of Drug-Loaded CL and In Vitro Studies of CL Drug Release
2.5. In Vitro Studies of CL Drug Uptake
2.6. In Vivo Studies of the Secretagogue Activity of Melatonin Analogs Pre-Soaked CLs
2.7. In Vivo Studies of the Secretagogue Activity of Melatonin Analogs via Topical Administration
2.8. Statistical Analysis
3. Results
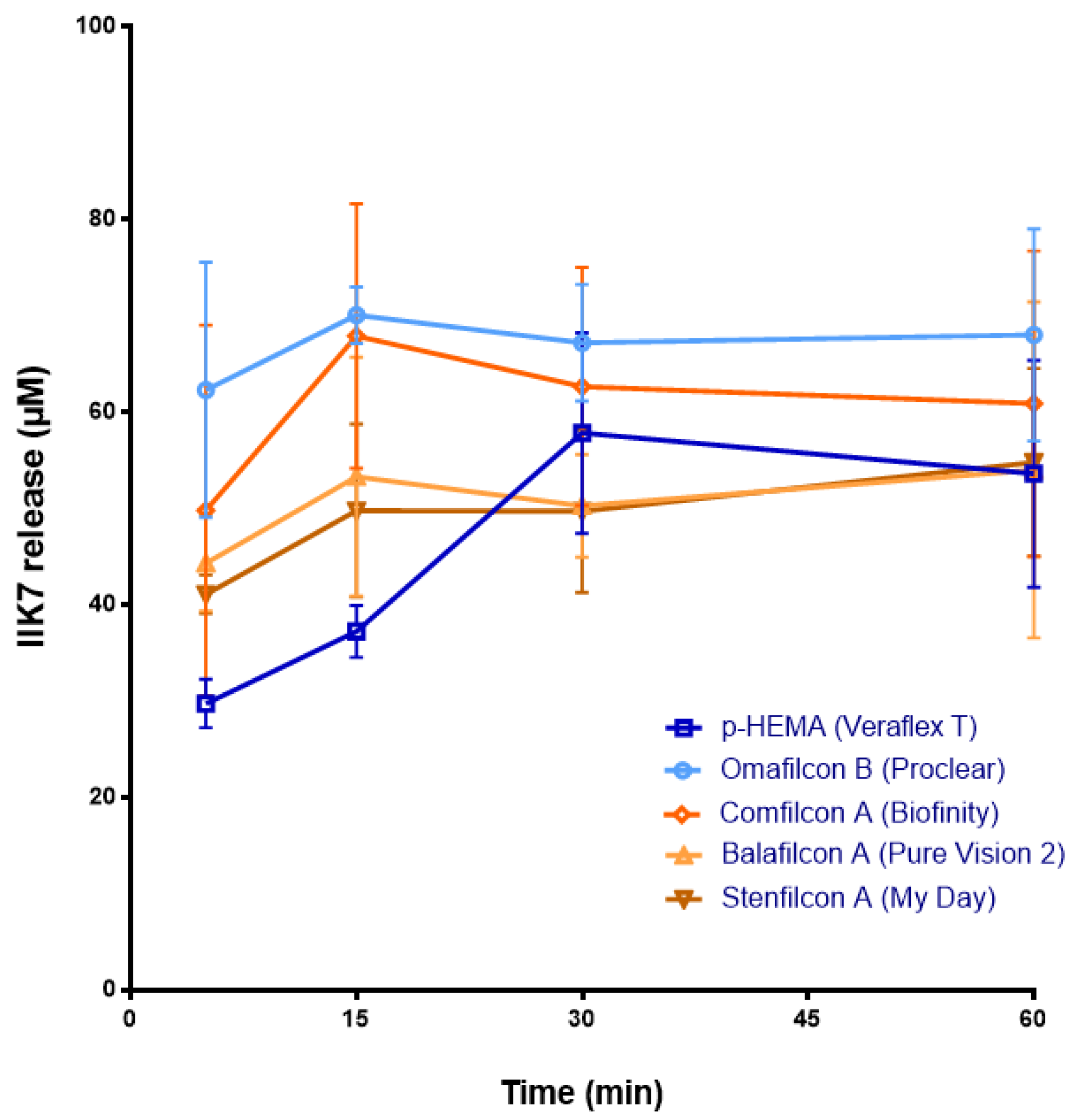
3.1. Release of Melatonin and Its Analogs from CLs
3.2. Uptake of Agomelatine and 5-MCA-NAT by CLs
3.3. Effect of Melatonin Analogs Released from CLs and Administered Topically in Rabbit Tear Secretion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef] [PubMed]
- Uchino, M.; Schaumberg, D.A. Dry Eye Disease: Impact on Quality of Life and Vision. Curr. Ophthalmol. Rep. 2013, 1, 51–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, A.; Hindman, H.B. Aging: A predisposition to dry eyes. J. Ophthalmol. 2014, 2014, 781683. [Google Scholar] [CrossRef]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Seen, S.; Tong, L. Dry eye disease and oxidative stress. Acta Ophthalmol. 2018, 96, e412–e420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aragona, P.; Giannaccare, G.; Mencucci, R.; Rubino, P.; Cantera, E.; Rolando, M. Modern approach to the treatment of dry eye, a complex multifactorial disease: A P.I.C.A.S.S.O. board review. Br. J. Ophthalmol. 2021, 105, 446–453. [Google Scholar] [CrossRef]
- Bron, A.J.; de Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II pathophysiology report. Ocul. Surf. 2017, 15, 438–510. [Google Scholar] [CrossRef]
- Ganesalingam, K.; Ismail, S.; Sherwin, T.; Craig, J.P. Molecular evidence for the role of inflammation in dry eye disease. Clin. Exp. Optom. 2019, 102, 446–454. [Google Scholar] [CrossRef] [Green Version]
- Jones, L.; Downie, L.E.; Korb, D.; Benitez-Del-Castillo, J.M.; Dana, R.; Deng, S.X.; Dong, P.N.; Geerling, G.; Hida, R.Y.; Liu, Y.; et al. TFOS DEWS II Management and Therapy Report. Ocul. Surf. 2017, 15, 575–628. [Google Scholar] [CrossRef]
- Kojima, T.; Dogru, M.; Kawashima, M.; Nakamura, S.; Tsubota, K. Advances in the diagnosis and treatment of dry eye. Prog. Retin. Eye Res. 2020, 78, 100842. [Google Scholar] [CrossRef]
- Wolffsohn, J.S.; Travé Huarte, S.; Jones, L.; Craig, J.P.; Wang, M.T.M. Clinical practice patterns in the management of dry eye disease: A TFOS international survey. Ocul. Surf. 2021, 21, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Colligris, B.; Crooke, A.; Huete-Toral, F.; Pintor, J. An update on dry eye disease molecular treatment: Advances in drug pipelines. Expert Opin. Pharmacother. 2014, 15, 1371–1390. [Google Scholar] [CrossRef] [PubMed]
- Navarro Gil, F.J.; Huete-Toral, F.; Crooke, A.; Dominguez Godinez, C.O.; Carracedo, G.; Pintor, J. Effect of Melatonin and Its Analogs on Tear Secretion. J. Pharmacol. Exp. Ther. 2019, 371, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Alarma-Estrany, P.; Pintor, J. Melatonin receptors in the eye: Location, second messengers and role in ocular physiology. Pharmacol. Ther. 2007, 113, 507–522. [Google Scholar] [CrossRef]
- Crooke, A.; Guzman-Aranguez, A.; Mediero, A.; Alarma-Estrany, P.; Carracedo, G.; Pelaez, T.; Peral, A.; Pintor, J. Effect of melatonin and analogues on corneal wound healing: Involvement of Mt2 melatonin receptor. Curr. Eye Res. 2015, 40, 56–65. [Google Scholar] [CrossRef]
- Gilad, E.; Shanas, U.; Terkel, J.; Zisapel, N. Putative melatonin receptors in the blind mole rat harderian gland. J. Exp. Zool. 1997, 277, 435–441. [Google Scholar] [CrossRef]
- Tomás-Zapico, C.; Antonio Boga, J.; Caballero, B.; Vega-Naredo, I.; Sierra, V.; Alvarez-García, O.; Tolivia, D.; Josefa Rodríguez-Colunga, M.; Coto-Montes, A. Coexpression of MT1 and RORalpha1 melatonin receptors in the Syrian hamster Harderian gland. J. Pineal. Res. 2005, 39, 21–26. [Google Scholar] [CrossRef]
- Claustrat, B.; Brun, J.; Chazot, G. The basic physiology and pathophysiology of melatonin. Sleep Med. Rev. 2005, 9, 11–24. [Google Scholar] [CrossRef]
- Crooke, A.; Colligris, B.; Pintor, J. Update in glaucoma medicinal chemistry: Emerging evidence for the importance of melatonin analogues. Curr. Med. Chem. 2012, 19, 3508–3522. [Google Scholar] [CrossRef]
- Crooke, A.; Huete-Toral, F.; Colligris, B.; Pintor, J. The role and therapeutic potential of melatonin in age-related ocular diseases. J. Pineal. Res. 2017, 63, e12430. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Zuo, X.; Peng, L.; Wang, X.; Zeng, H.; Zhong, J.; Li, S.; Xiao, Y.; Wang, L.; Ouyang, H.; et al. Melatonin ameliorates oxidative stress-mediated injuries through induction of HO-1 and restores autophagic flux in dry eye. Exp. Eye Res. 2021, 205, 108491. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, P.; Zhao, G.; Wei, S.; Li, Q.; Guo, C.; Cao, Q.; Wu, X.; Di, G. Copolymer Micelle-administered Melatonin Ameliorates Hyperosmolarity-induced Ocular Surface Damage through Regulating PINK1-mediated Mitophagy. Curr Eye Res. 2022, 47, 688–703. [Google Scholar] [CrossRef] [PubMed]
- Besharse, J.C.; Dunis, D.A. Methoxyindoles and photoreceptor metabolism: Activation of rod shedding. Science 1983, 219, 1341–1343. [Google Scholar] [CrossRef] [PubMed]
- Huete-Toral, F.; Crooke, A.; Martínez-Águila, A.; Pintor, J. Melatonin receptors trigger cAMP production and inhibit chloride movements in nonpigmented ciliary epithelial cells. J. Pharmacol. Exp. Ther. 2015, 352, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Pierce, M.E.; Besharse, J.C. Circadian regulation of retinomotor movements. I. Interaction of melatonin and dopamine in the control of cone length. J. Gen. Physiol. 1985, 86, 671–689. [Google Scholar] [CrossRef] [Green Version]
- Pintor, J.; Martin, L.; Pelaez, T.; Hoyle, C.H.; Peral, A. Involvement of melatonin MT(3) receptors in the regulation of intraocular pressure in rabbits. Eur. J. Pharmacol. 2001, 416, 251–254. [Google Scholar] [CrossRef]
- Dominguez-Godinez, C.O.; Martin-Gil, A.; Carracedo, G.; Guzman-Aranguez, A.; González-Méijome, J.M.; Pintor, J. In vitro and in vivo delivery of the secretagogue diadenosine tetraphosphate from conventional and silicone hydrogel soft contact lenses. J. Optom. 2013, 6, 205–211. [Google Scholar] [CrossRef] [Green Version]
- Hughes, P.M.; Olejnik, O.; Chang-Lin, J.E.; Wilson, C.G. Topical and systemic drug delivery to the posterior segments. Adv. Drug Deliv. Rev. 2005, 57, 2010–2032. [Google Scholar] [CrossRef]
- Liu, S.; Dozois, M.D.; Chang, C.N.; Ahmad, A.; Ng, D.L.; Hileeto, D.; Liang, H.; Reyad, M.M.; Boyd, S.; Jones, L.W.; et al. Prolonged Ocular Retention of Mucoadhesive Nanoparticle Eye Drop Formulation Enables Treatment of Eye Diseases Using Significantly Reduced Dosage. Mol. Pharm. 2016, 13, 2897–2905. [Google Scholar] [CrossRef]
- Franco, P.; De Marco, I. Contact Lenses as Ophthalmic Drug Delivery Systems: A Review. Polymers 2021, 13, 1102. [Google Scholar] [CrossRef]
- Xu, J.; Xue, Y.; Hu, G.; Lin, T.; Gou, J.; Yin, T.; He, H.; Zhang, Y.; Tang, X. A comprehensive review on contact lens for ophthalmic drug delivery. J. Control. Release 2018, 281, 97–118. [Google Scholar] [CrossRef] [PubMed]
- Pereira-da-Mota, A.F.; Phan, C.M.; Concheiro, A.; Jones, L.; Alvarez-Lorenzo, C. Testing drug release from medicated contact lenses: The missing link to predict in vivo performance. J. Control. Release 2022, 343, 672–702. [Google Scholar] [CrossRef] [PubMed]
- van Bijsterveld, O.P. Diagnostic tests in the Sicca syndrome. Arch. Ophthalmol. 1969, 82, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Boone, A.; Hui, A.; Jones, L. Uptake and release of dexamethasone phosphate from silicone hydrogel and group I, II, and IV hydrogel contact lenses. Eye Contact Lens. 2009, 35, 260–267. [Google Scholar] [CrossRef]
- Hui, A.; Boone, A.; Jones, L. Uptake and release of ciprofloxacin-HCl from conventional and silicone hydrogel contact lens materials. Eye Contact Lens. 2008, 34, 266–271. [Google Scholar] [CrossRef]
- Karlgard, C.C.; Wong, N.S.; Jones, L.W.; Moresoli, C. In vitro uptake and release studies of ocular pharmaceutical agents by silicon-containing and p-HEMA hydrogel contact lens materials. Int. J. Pharm. 2003, 257, 141–151. [Google Scholar] [CrossRef]
- Bengani, L.C.; Chauhan, A. Extended delivery of an anionic drug by contact lens loaded with a cationic surfactant. Biomaterials 2013, 34, 2814–2821. [Google Scholar] [CrossRef]
- Kim, J.; Chauhan, A. Dexamethasone transport and ocular delivery from poly(hydroxyethyl methacrylate) gels. Int. J. Pharm. 2008, 353, 205–222. [Google Scholar] [CrossRef]
- Mahomed, A.; Tighe, B.J. The design of contact lens based ocular drug delivery systems for single-day use: Part (I) Structural factors, surrogate ophthalmic dyes and passive diffusion studies. J. Biomater. Appl. 2014, 29, 341–353. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, D.C.T.; Dowling, J.; Ryan, R.; McLoughlin, P.; Fitzhenry, L. Pharmaceutical-loaded contact lenses as an ocular drug delivery system: A review of critical lens characterization methodologies with reference to ISO standards. Cont. Lens. Anterior. Eye 2021, 44, 101487. [Google Scholar] [CrossRef]
- Read, M.L.; Morgan, P.B.; Maldonado-Codina, C. Measurement errors related to contact angle analysis of hydrogel and silicone hydrogel contact lenses. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 91, 662–668. [Google Scholar] [CrossRef]
- Ergun, S.; Demir, P.; Uzbay, T.; Severcan, F. Agomelatine strongly interacts with zwitterionic DPPC and charged DPPG membranes. Biochim. Biophys. Acta 2014, 1838, 2798–2806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maulvi, F.A.; Soni, T.G.; Shah, D.O. Extended release of hyaluronic acid from hydrogel contact lenses for dry eye syndrome. J. Biomater. Sci. Polym. Ed. 2015, 26, 1035–1050. [Google Scholar] [CrossRef]
- Tieppo, A.; Boggs, A.C.; Pourjavad, P.; Byrne, M.E. Analysis of release kinetics of ocular therapeutics from drug releasing contact lenses: Best methods and practices to advance the field. Cont. Lens. Anterior. Eye 2014, 37, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Serramito, M.; Pereira-da-Mota, A.F.; Carpena-Torres, C.; Huete-Toral, F.; Alvarez-Lorenzo, C.; Carracedo, G. Melatonin-Eluting Contact Lenses Effect on Tear Volume: In Vitro and In Vivo Experiments. Pharmaceutics 2022, 14, 1019. [Google Scholar] [CrossRef] [PubMed]
- Nagahara, Y.; Koh, S.; Nishida, K.; Watanabe, H. Prolonged increase in tear meniscus height by 3% diquafosol ophthalmic solution in eyes with contact lenses. Clin. Ophthalmol. 2015, 9, 1029–1031. [Google Scholar] [CrossRef] [Green Version]
- Nagahara, Y.; Koh, S.; Oshita, Y.; Nagano, T.; Mano, H.; Nishida, K.; Watanabe, H. Diquafosol Ophthalmic Solution Increases Pre- and Postlens Tear Film During Contact Lens Wear in Rabbit Eyes. Eye Contact Lens. 2017, 43, 378–382. [Google Scholar] [CrossRef]
- Dominguez-Godinez, C.; Carracedo, G.; Pintor, J. Diquafosol Delivery from Silicone Hydrogel Contact Lenses: Improved Effect on Tear Secretion. J. Ocul. Pharmacol. Ther. 2018, 34, 170–176. [Google Scholar] [CrossRef]
- Fukuoka, S.; Arita, R. Tear film lipid layer increase after diquafosol instillation in dry eye patients with meibomian gland dysfunction: A randomized clinical study. Sci. Rep. 2019, 9, 9091. [Google Scholar] [CrossRef]
- Nakamura, M.; Imanaka, T.; Sakamoto, A. Diquafosol ophthalmic solution for dry eye treatment. Adv. Ther. 2012, 29, 579–589. [Google Scholar] [CrossRef]
- Hassanzadeh, S.; Varmaghani, M.; Zarei-Ghanavati, S.; Heravian Shandiz, J.; Azimi Khorasani, A. Global Prevalence of Meibomian Gland Dysfunction: A Systematic Review and Meta-Analysis. Ocul. Immunol. Inflamm. 2021, 29, 66–75. [Google Scholar] [CrossRef]
- Morgan, P.B.; Efron, N. Global contact lens prescribing 2000–2020. Clin. Exp. Optom. 2022, 105, 298–312. [Google Scholar] [CrossRef]
- Chae, J.J.; Shin, Y.J.; Lee, J.D.; Seo, K.; Elisseeff, J.H. Nictitating membrane fixation improves stability of the contact lens on the animal corneal surface. PLoS ONE 2018, 13, e0194795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Q.; Zhou, X.; Li, J.; Ma, Y.; Lu, L.; Xiong, J.; Xu, P.; Li, Y.; Chen, Y.; Gu, W.; et al. Sub-Acute Oral Toxicity of a Novel Derivative of Agomelatine in Rats in a Sex-Dependent Manner. Front. Pharmacol. 2019, 10, 242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atanasova, D.; Lazarov, N.; Stoyanov, D.S.; Spassov, R.H.; Tonchev, A.B.; Tchekalarova, J. Reduced neuroinflammation and enhanced neurogenesis following chronic agomelatine treatment in rats undergoing chronic constant light. Neuropharmacology 2021, 197, 108706. [Google Scholar] [CrossRef] [PubMed]
- Chumboatong, W.; Khamchai, S.; Tocharus, C.; Govitrapong, P.; Tocharus, J. Agomelatine Exerts an Anti-inflammatory Effect by Inhibiting Microglial Activation Through TLR4/NLRP3 Pathway in pMCAO Rats. Neurotox. Res. 2022, 40, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Abd El-Ghafar, O.A.M.; Alzoghaibi, M.A.; Hassanein, E.H.M. Agomelatine prevents gentamicin nephrotoxicity by attenuating oxidative stress and TLR-4 signaling, and upregulating PPARγ and SIRT1. Life Sci. 2021, 278, 119600. [Google Scholar] [CrossRef]







| Trade Name | PureVision | Biofinity | MyDay | Proclear | Veraflex |
|---|---|---|---|---|---|
| Unit states adopted name (USAN) | Balafilcon A | Comfilcon A | Stenfilcon A | Omafilcon B | p-HEMA |
| Manufacturer | Bausch & Lomb | Coopervision | Coopervision | Coopervision | Interlenco |
| Center thickness | 0.07 | 0.08 | 0.08 | 0.07 | 0.08 |
| Water Content (%) | 36 | 48 | 54 | 62 | 38 |
| Oxygen Permeability (×10−11) | 91 | 128 | 80 | 27 | NA |
| Oxygen Transmissibility (×10−11) | 130 | 160 | 100 | 42 | 30 |
| FDA Group | III | I | II | II | I |
| Surface Treatment | Plasma oxidation process | None | None | None | None |
| Principal monomers | NVA, NVP, PBVC, TPVC | FM0411M, HOB, IBM, M3U, NVP, TAIC, VMA | EGDMA, EGMA, NB, PDMS, PMMA, TEGDVE, VMA | EGDMA, HEMA, MPC | HEMA, NVP |
| Unit States Adopted Name (USAN) | Maximum Absorbed Concentration (μM) | Maximum Released Concentration (μM) | μM Ratio |
|---|---|---|---|
| p-HEMA | 613.97 ± 21.36 | 166.60 ± 3.97 | 3.7 |
| Omafilcon B | 691.67 ± 19.64 | 151.07 ± 14.43 | 4.6 |
| Comfilcon A | 884.7 ± 41.42 | 140.43 ± 17.45 | 6.3 |
| Stenfilcon A | 906.44 ± 19.56 | 138.25 ± 12.37 | 6.6 |
| Balafilcon A | 895.43 ± 11.45 | 101.04 ± 11.52 | 8.9 |
| Unit States Adopted Name (USAN) | Maximum Absorbed Concentration (μM) | Maximum Released Concentration (μM) | μM Ratio |
|---|---|---|---|
| p-HEMA | 507.98 ± 20.14 | 591.38 ± 15.46 | 0.9 |
| Omafilcon B | 407.55 ± 26.88 | 521.38 ± 69.58 | 0.8 |
| Comfilcon A | 648.00 ± 38.21 | 747.89 ± 32.24 | 0.9 |
| Stenfilcon A | 681.44 ± 23.68 | 781.88 ± 25.13 | 0.9 |
| Balafilcon A | 397.04 ± 15.86 | 728.95 ± 36.04 | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro-Gil, F.J.; Huete-Toral, F.; Domínguez-Godínez, C.O.; Carracedo, G.; Crooke, A. Contact Lenses Loaded with Melatonin Analogs: A Promising Therapeutic Tool against Dry Eye Disease. J. Clin. Med. 2022, 11, 3483. https://doi.org/10.3390/jcm11123483
Navarro-Gil FJ, Huete-Toral F, Domínguez-Godínez CO, Carracedo G, Crooke A. Contact Lenses Loaded with Melatonin Analogs: A Promising Therapeutic Tool against Dry Eye Disease. Journal of Clinical Medicine. 2022; 11(12):3483. https://doi.org/10.3390/jcm11123483
Chicago/Turabian StyleNavarro-Gil, Francisco Javier, Fernando Huete-Toral, Carmen Olalla Domínguez-Godínez, Gonzalo Carracedo, and Almudena Crooke. 2022. "Contact Lenses Loaded with Melatonin Analogs: A Promising Therapeutic Tool against Dry Eye Disease" Journal of Clinical Medicine 11, no. 12: 3483. https://doi.org/10.3390/jcm11123483
APA StyleNavarro-Gil, F. J., Huete-Toral, F., Domínguez-Godínez, C. O., Carracedo, G., & Crooke, A. (2022). Contact Lenses Loaded with Melatonin Analogs: A Promising Therapeutic Tool against Dry Eye Disease. Journal of Clinical Medicine, 11(12), 3483. https://doi.org/10.3390/jcm11123483







