Development of a Laboratory Risk-Score Model to Predict One-Year Mortality in Acute Myocardial Infarction Survivors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Participants
2.2. Definition of STEMI and NSTEMI
2.3. Data Acquisition
2.4. Statistics
3. Results
3.1. Baseline Demographics and Characteristics
3.2. Laboratory Parameters Associated with Post-Discharge Death
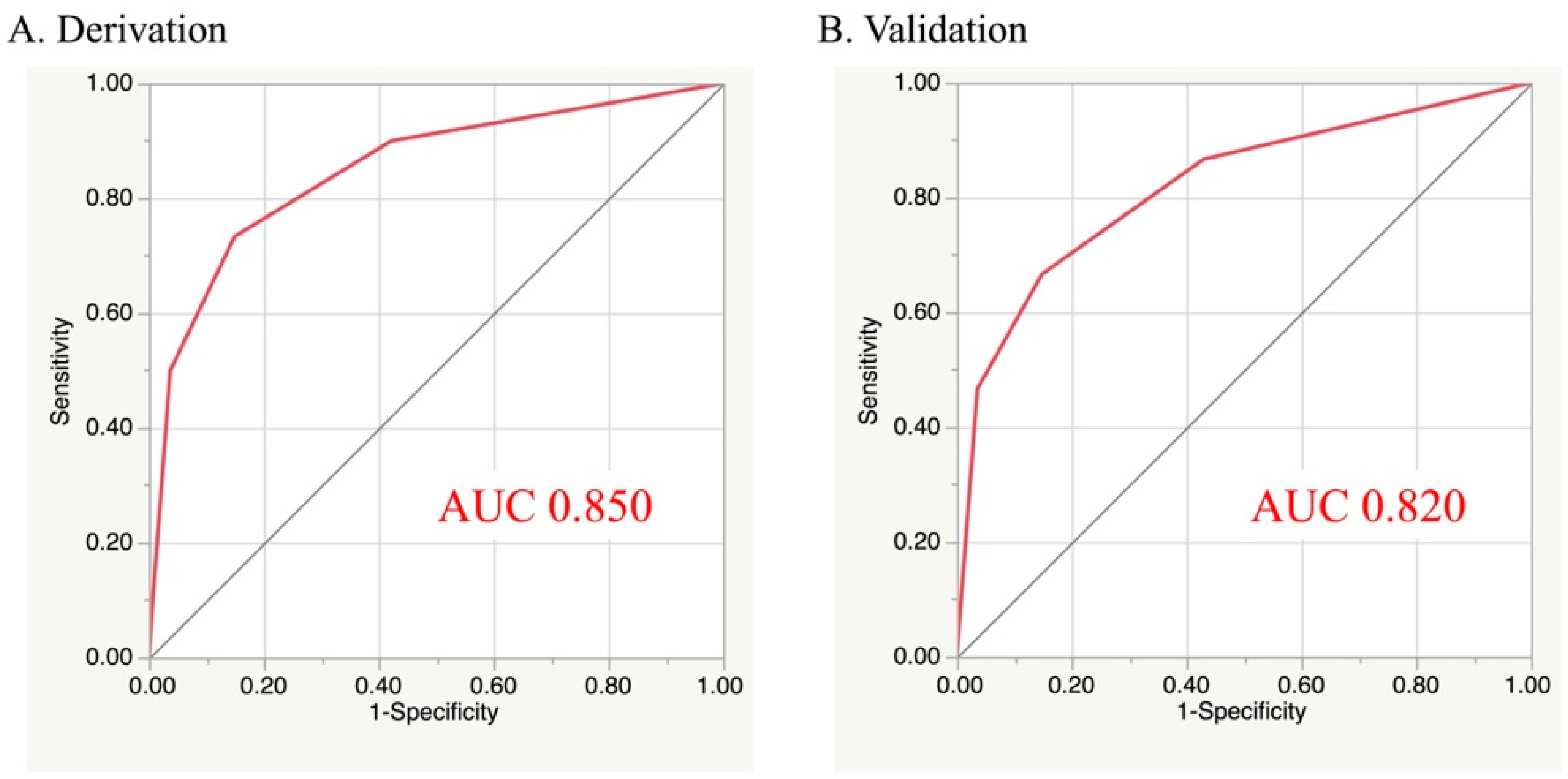
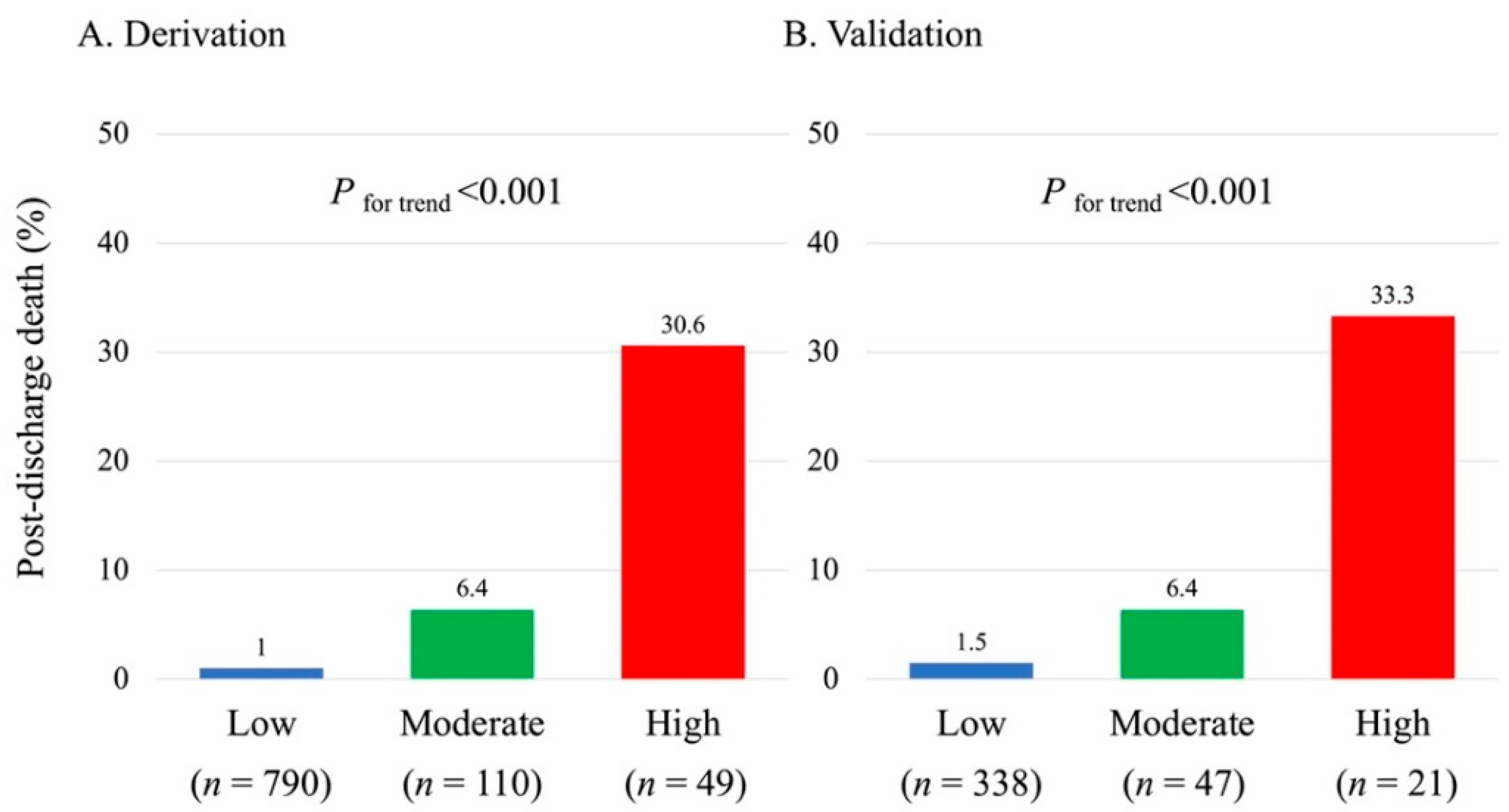
3.3. Predictive Model of Post-Discharge Death
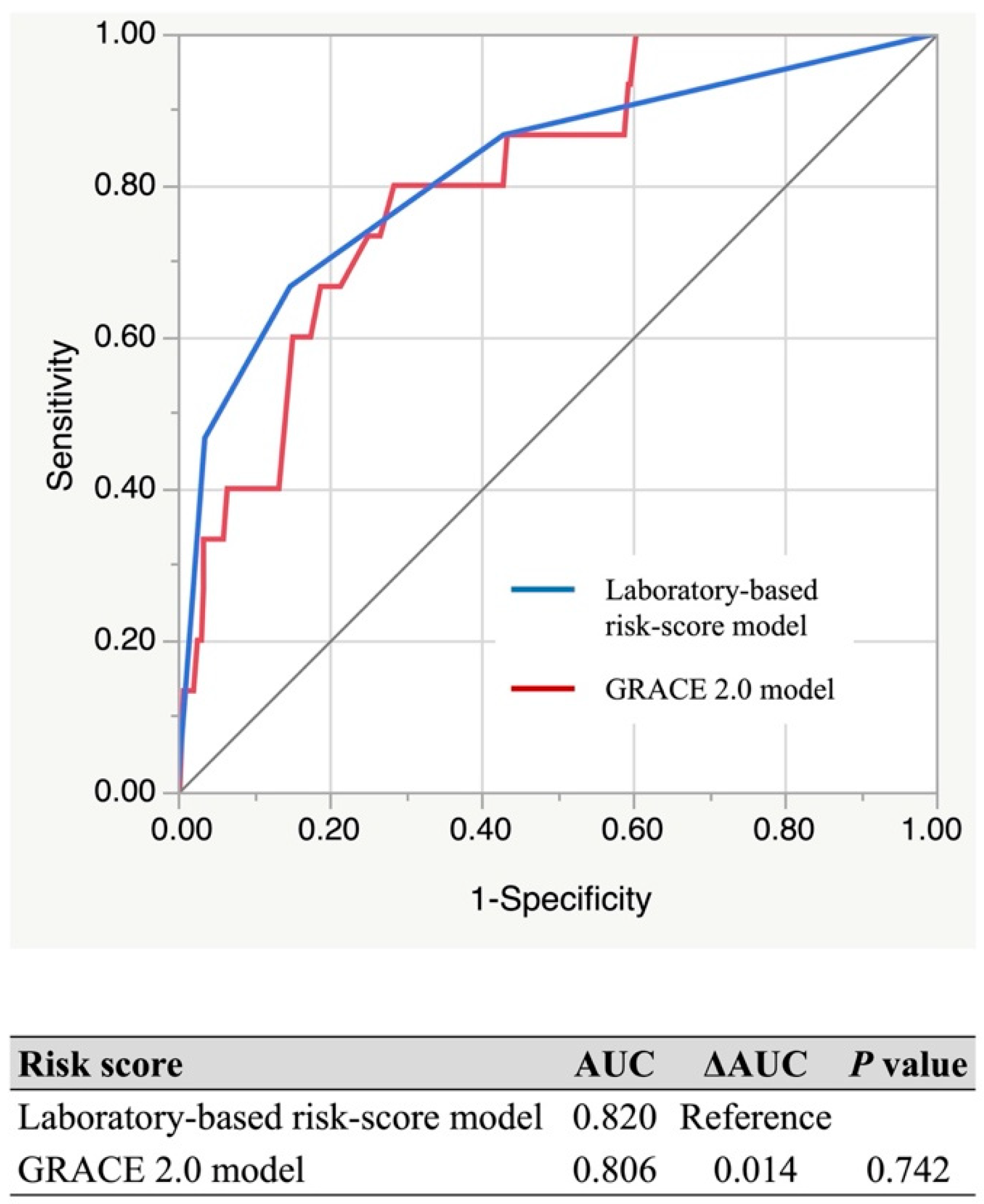
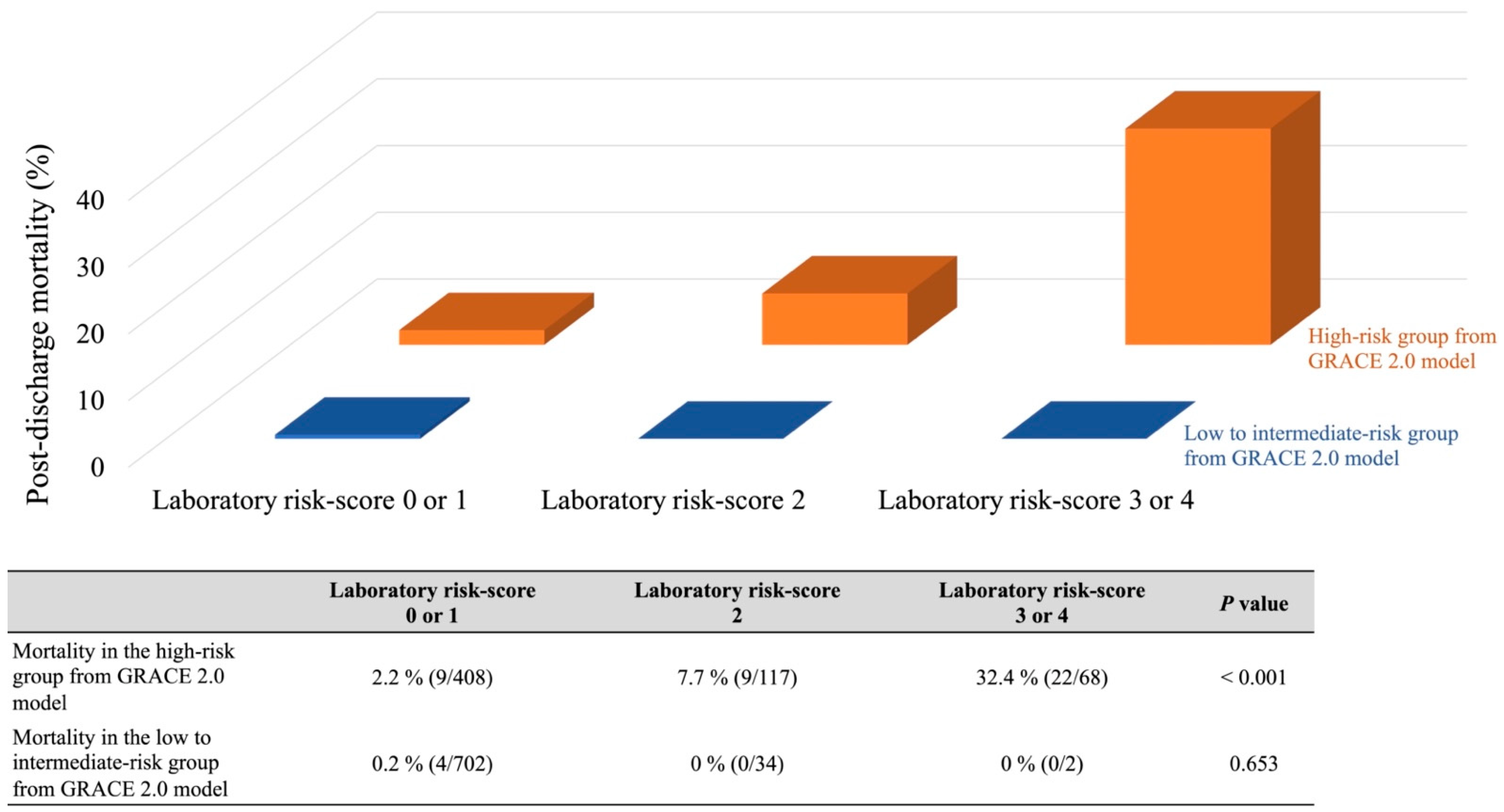
3.4. Comparison with GRACE 2.0 Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [PubMed] [Green Version]
- Puymirat, E.; Simon, T.; Cayla, G.; Cottin, Y.; Elbaz, M.; Coste, P.; Lemesle, G.; Motreff, P.; Popovic, B.; Khalife, K.; et al. Acute Myocardial Infarction: Changes in Patient Characteristics, Management, and 6-Month Outcomes over a Period of 20 Years in the FAST-MI Program (French Registry of Acute ST-Elevation or Non-ST-Elevation Myocardial Infarction) 1995 to 2015. Circulation 2017, 136, 1908–1919. [Google Scholar] [CrossRef] [PubMed]
- Fox, K.A.; Carruthers, K.F.; Dunbar, D.R.; Graham, C.; Manning, J.R.; De Raedt, H.; Buysschaert, I.; Lambrechts, D.; Van de Werf, F. Underestimated and under-recognized: The late consequences of acute coronary syndrome (GRACE UK-Belgian Study). Eur. Heart J. 2010, 31, 2755–2764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, K.A.; Fitzgerald, G.; Puymirat, E.; Huang, W.; Carruthers, K.; Simon, T.; Coste, P.; Monsegu, J.; Gabriel Steg, P.; Danchin, N.; et al. Should patients with acute coronary disease be stratified for management according to their risk? Derivation, external validation and outcomes using the updated GRACE risk score. BMJ Open 2014, 4, e004425. [Google Scholar] [CrossRef] [PubMed]
- Pocock, S.; Bueno, H.; Licour, M.; Medina, J.; Zhang, L.; Annemans, L.; Danchin, N.; Huo, Y.; Van de Werf, F. Predictors of one-year mortality at hospital discharge after acute coronary syndromes: A new risk score from the EPICOR (long-term follow up of antithrombotic management patterns in acute Coronary syndrome patients) study. Eur. Heart J. Acute Cardiovasc. Care 2015, 4, 509–517. [Google Scholar] [CrossRef]
- Simonsson, M.; Wallentin, L.; Alfredsson, J.; Erlinge, D.; Hellström Ängerud, K.; Hofmann, R.; Kellerth, T.; Lindhagen, L.; Ravn-Fischer, A.; Szummer, K.; et al. Temporal trends in bleeding events in acute myocardial infarction: Insights from the SWEDEHEART registry. Eur. Heart J. 2020, 41, 833–843. [Google Scholar] [CrossRef] [Green Version]
- O’Donoghue, M.L.; Morrow, D.A.; Cannon, C.P.; Jarolim, P.; Desai, N.R.; Sherwood, M.W.; Murphy, S.A.; Gerszten, R.E.; Sabatine, M.S. Multimarker Risk Stratification in Patients with Acute Myocardial Infarction. J. Am. Heart Assoc. 2016, 5, e002586. [Google Scholar] [CrossRef] [Green Version]
- Damman, P.; Beijk, M.A.; Kuijt, W.J.; Verouden, N.J.; van Geloven, N.; Henriques, J.P.; Baan, J.; Vis, M.M.; Meuwissen, M.; van Straalen, J.P.; et al. Multiple biomarkers at admission significantly improve the prediction of mortality in patients undergoing primary percutaneous coronary intervention for acute ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol. 2011, 57, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Goriki, Y.; Tanaka, A.; Nishihira, K.; Kawaguchi, A.; Natsuaki, M.; Watanabe, N.; Ashikaga, K.; Kuriyama, N.; Shibata, Y.; Node, K. A Novel Predictive Model for In-Hospital Mortality Based on a Combination of Multiple Blood Variables in Patients with ST-Segment-Elevation Myocardial Infarction. J. Clin. Med. 2020, 9, 852. [Google Scholar] [CrossRef] [Green Version]
- Tsai, T.T.; Patel, U.D.; Chang, T.I.; Kennedy, K.F.; Masoudi, F.A.; Matheny, M.E.; Kosiborod, M.; Amin, A.P.; Weintraub, W.S.; Curtis, J.P.; et al. Validated contemporary risk model of acute kidney injury in patients undergoing percutaneous coronary interventions: Insights from the National Cardiovascular Data Registry Cath-PCI Registry. J. Am. Heart Assoc. 2014, 3, e001380. [Google Scholar] [CrossRef] [Green Version]
- Goriki, Y.; Yoshioka, G.; Natsuaki, M.; Shinzato, K.; Nishihira, K.; Kuriyama, N.; Shimomura, M.; Inoue, Y.; Nishikido, T.; Kaneko, T.; et al. Simple risk-score model for in-hospital major bleeding based on multiple blood variables in patients with acute myocardial infarction. Int. J. Cardiol. 2022, 346, 1–7. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; White, H.D. Universal definition of myocardial infarction. J. Am. Coll. Cardiol. 2007, 50, 2173–2195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Ducrocq, G.; Puymirat, E.; Steg, P.G.; Henry, P.; Martelet, M.; Karam, C.; Schiele, F.; Simon, T.; Danchin, N. Blood transfusion, bleeding, anemia, and survival in patients with acute myocardial infarction: FAST-MI registry. Am. Heart J. 2015, 170, 726–734.e2. [Google Scholar] [CrossRef] [PubMed]
- Kunadian, V.; Mehran, R.; Lincoff, A.M.; Feit, F.; Manoukian, S.V.; Hamon, M.; Cox, D.A.; Dangas, G.D.; Stone, G.W. Effect of anemia on frequency of short- and long-term clinical events in acute coronary syndromes (from the Acute Catheterization and Urgent Intervention Triage Strategy Trial). Am. J. Cardiol. 2014, 114, 1823–1829. [Google Scholar] [CrossRef] [PubMed]
- Saltzman, A.J.; Stone, G.W.; Claessen, B.E.; Narula, A.; Leon-Reyes, S.; Weisz, G.; Brodie, B.; Witzenbichler, B.; Guagliumi, G.; Kornowski, R.; et al. Long-term impact of chronic kidney disease in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention: The HORIZONS-AMI (Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction) trial. JACC Cardiovasc. Interv. 2011, 4, 1011–1019. [Google Scholar] [PubMed] [Green Version]
- Kotwal, S.; Ranasinghe, I.; Brieger, D.; Clayton, P.A.; Cass, A.; Gallagher, M. The influence of chronic kidney disease and age on revascularization rates and outcomes in acute myocardial infarction—A cohort study. Eur. Heart J. Acute Cardiovasc. Care 2017, 6, 291–298. [Google Scholar] [CrossRef]
- Yadav, M.; Généreux, P.; Giustino, G.; Madhavan, M.V.; Brener, S.J.; Mintz, G.; Caixeta, A.; Xu, K.; Mehran, R.; Stone, G.W. Effect of Baseline Thrombocytopenia on Ischemic Outcomes in Patients with Acute Coronary Syndromes Who Undergo Percutaneous Coronary Intervention. Can. J. Cardiol. 2016, 32, 226–233. [Google Scholar] [CrossRef]
- Liu, R.; Hu, Y.; Yang, J.; Wang, Q.; Yang, H.; Wang, Z.; Su, S.; Yuan, J.; Yang, Y. Effect of Baseline Thrombocytopenia on Long-Term Outcomes in Patients with Acute ST-Segment Elevated Myocardial Infarction—A Large Propensity Score-Matching Analysis from the China Acute Myocardial Infarction (CAMI) Registry. Circ. J. 2021, 85, 150–158. [Google Scholar] [CrossRef]
- Yoshioka, G.; Tanaka, A.; Nishihira, K.; Natsuaki, M.; Kawaguchi, A.; Watanabe, N.; Shibata, Y.; Node, K. Prognostic impact of follow-up serum albumin after acute myocardial infarction. ESC Heart Fail. 2021, 8, 5456–5465. [Google Scholar] [CrossRef]
- Wanamaker, B.L.; Seth, M.M.; Sukul, D.; Dixon, S.R.; Bhatt, D.L.; Madder, R.D.; Rumsfeld, J.S.; Gurm, H.S. Relationship between Troponin on Presentation and in-Hospital Mortality in Patients with ST-Segment-Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. J. Am. Heart Assoc. 2019, 8, e013551. [Google Scholar] [CrossRef] [PubMed]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Hung, J.; Roos, A.; Kadesjö, E.; McAllister, D.A.; Kimenai, D.M.; Shah, A.S.V.; Anand, A.; Strachan, F.E.; Fox, K.A.A.; Mills, N.L.; et al. Performance of the GRACE 2.0 score in patients with type 1 and type 2 myocardial infarction. Eur. Heart J. 2021, 42, 2552–2561. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef] [PubMed]
- Fanali, G.; di Masi, A.; Trezza, V.; Marino, M.; Fasano, M.; Ascenzi, P. Human serum albumin: From bench to bedside. Mol. Asp. Med. 2012, 33, 209–290. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Goodman, S.G.; Yan, A.T.; Alexander, K.P.; Wong, C.L.; Cheema, A.N.; Udell, J.A.; Kaul, P.; D’Souza, M.; Hyun, K.; et al. Frailty and Outcomes after Myocardial Infarction: Insights from the CONCORDANCE Registry. J. Am. Heart Assoc. 2018, 7, e009859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.L.; Sung, S.H.; Cheng, H.M.; Hsu, P.F.; Guo, C.Y.; Yu, W.C.; Chen, C.H. Prognostic Nutritional Index and the Risk of Mortality in Patients with Acute Heart Failure. J. Am. Heart Assoc. 2017, 6, e004876. [Google Scholar] [CrossRef]
- Xia, M.; Zhang, C.; Gu, J.; Chen, J.; Wang, L.C.; Lu, Y.; Huang, C.Y.; He, Y.M.; Yang, X.J. Impact of serum albumin levels on long-term all-cause, cardiovascular, and cardiac mortality in patients with first-onset acute myocardial infarction. Clin. Chim. Acta 2018, 477, 89–93. [Google Scholar] [CrossRef]
- Yoshioka, G.; Tanaka, A.; Nishihira, K.; Shibata, Y.; Node, K. Prognostic impact of serum albumin for developing heart failure remotely after acute myocardial infarction. Nutrients 2020, 12, 2637. [Google Scholar] [CrossRef]
- Mahmoud, K.D.; Hillege, H.L.; Jaffe, A.S.; Lennon, R.J.; Holmes, D.R., Jr. Biochemical Validation of Patient-Reported Symptom Onset Time in Patients With ST-Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2015, 8, 778–787. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, A. What are the most useful predictors of cardiac mortality in patients post myocardial infarction? Circ. J. 2013, 77, 319–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goriki, Y.; Tanaka, A.; Nishihira, K.; Kuriyama, N.; Shibata, Y.; Node, K. A novel prediction model of acute kidney injury based on combined blood variables in STEMI. JACC Asia 2021, 1, 372–381. [Google Scholar] [CrossRef]
- Kimura, K.; Kimura, T.; Ishihara, M.; Nakagawa, Y.; Nakao, K.; Miyauchi, K.; Sakamoto, T.; Tsujita, K.; Hagiwara, N.; Miyazaki, S.; et al. JCS 2018 Guideline on Diagnosis and Treatment of Acute Coronary Syndrome. Circ. J. 2019, 83, 1085–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Derivation (n = 949) | Validation (n = 405) | p-Value | |
|---|---|---|---|
| Age, years | 69.2 ± 12.1 | 68.6 ± 12.6 | 0.435 |
| Female, n (%) | 250 (26.3) | 111 (27.4) | 0.635 |
| Body mass index, kg/m2 | 24.0 ± 3.7 | 24.0 ± 4.1 | 0.789 |
| Systolic blood pressure, mmHg | 142.1 ± 28.5 | 141.0 ± 28.0 | 0.464 |
| Pulse rate, bpm | 77.1 ± 19.7 | 77.3 ± 17.8 | 0.863 |
| Medical history | |||
| Hypertension, n (%) | 687 (72.4) | 277 (68.4) | 0.149 |
| Dyslipidemia, n (%) | 508 (53.5) | 211 (52.1) | 0.628 |
| Diabetes mellitus, n (%) | 264 (27.8) | 102 (25.2) | 0.317 |
| Smoking, n (%) | 467 (49.2) | 189 (46.7) | 0.391 |
| Previous myocardial infarction, n (%) | 70 (7.4) | 23 (5.7) | 0.274 |
| Peripheral artery disease, n (%) | 29 (3.1) | 13 (3.2) | 0.855 |
| Malignancy, n (%) | 45 (4.7) | 14 (3.5) | 0.288 |
| Laboratory parameters | |||
| White blood cell, ×102/μL | 96.4 ± 37.1 | 96.8 ± 33.3 | 0.857 |
| Hemoglobin, g/dL | 13.8 ± 2.1 | 13.8 ± 2.1 | 0.845 |
| Platelet, ×104/μL | 21.7 ± 6.2 | 22.1 ± 6.3 | 0.246 |
| Glycated hemoglobin A1c, % | 6.0 (5.7, 6.6) | 5.9 (5.6, 6.5) | 0.168 |
| Glucose, mg/dL | 148 (107, 189) | 141 (102, 181) | 0.196 |
| eGFR, mL/min/1.73 m2 | 66.2 ± 22.7 | 67.9 ± 24.1 | 0.899 |
| LDL-cholesterol, mg/dL | 122.8 ± 35.1 | 123.6 ± 37.7 | 0.698 |
| HDL-cholesterol, mg/dL | 46.8 ± 12.0 | 48.8 ± 13.2 | 0.097 |
| Albumin, g/dL | 4.0 ± 0.5 | 4.0 ± 0.5 | 0.648 |
| Creatine kinase, U/L | 156 (96, 356) | 169 (100, 395) | 0.286 |
| hs-TnI, ng/L | 300 (50, 3180) | 380 (60, 3010) | 0.653 |
| STEMI, n (%) | 640 (67.4) | 278 (68.5) | 0.709 |
| Killip classification ≥ 3, n (%) | 64 (6.7) | 19 (4.7) | 0.146 |
| LVEF (on admission), % | 52.4 ± 11.7 | 52.5 ± 10.6 | 0.550 |
| Peak creatine kinase, IU/L | 1231 (358, 2950) | 1185 (299, 2687) | 0.337 |
| IABP, n (%) | 93 (9.8) | 28 (6.9) | 0.086 |
| ECMO, n (%) | 9 (1.0) | 2 (0.5) | 0.166 |
| Medication at discharge | |||
| Antiplatelet therapy, n (%) | 940 (99.0) | 404 (99.5) | 0.214 |
| Aspirin (100 mg daily), n (%) | 899 (94.7) | 391 (96.5) | 0.131 |
| Prasugrel (3.75 mg daily), n (%) | 208 (21.9) | 73 (18.0) | 0.221 |
| Clopidogrel (75 mg daily), n (%) | 670 (70.6) | 308 (76.0) | 0.089 |
| Dual antiplatelet therapy, n (%) | 857 (90.3) | 377 (93.0) | 0.165 |
| Statin, n (%) | 860 (90.6) | 370 (91.1) | 0.844 |
| β-Blocker, n (%) | 424 (44.9) | 195 (48.0) | 0.326 |
| ACE inhibitor, n (%) | 390 (41.1) | 147 (36.2) | 0.091 |
| ARB, n (%) | 296 (31.2) | 147 (36.2) | 0.074 |
| Diuretic, n (%) | 177 (18.7) | 68 (16.8) | 0.401 |
| Post-discharge death during one-year follow-up, n (%) | 30 (3.2) | 14 (3.5) | 0.785 |
| Survivors | Non-Survivors (Post-Discharge Death) | p-Value | |
|---|---|---|---|
| White blood cell, ×103/μL | 9.6 ± 3.5 | 9.4 ± 4.2 | 0.656 |
| Hemoglobin, g/dL | 13.9 ± 2.0 | 11.7 ± 2.1 | <0.001 |
| Platelet, ×104/μL | 21.8 ± 6.1 | 18.1 ± 7.3 | 0.001 |
| Glycated hemoglobin A1c, % | 6.0 (5.4, 6.6) | 6.3 (5.4, 6.7) | 0.688 |
| Glucose, mg/dL | 148 (123, 187) | 154 (119, 212) | 0.531 |
| eGFR, mL/min/1.73 m2 | 66.9 ± 22.1 | 43.7 ± 28.1 | <0.001 |
| LDL-cholesterol, mg/dL | 123.7 ± 34.8 | 93.9 ± 32.9 | <0.001 |
| HDL-cholesterol, mg/dL | 47.0 ± 12.1 | 41.8 ± 10.7 | 0.020 |
| Albumin, mg/dL | 4.1 ± 0.5 | 3.4 ± 0.5 | <0.001 |
| Creatine kinase, U/L | 152 (96, 355) | 232 (77, 855) | 0.128 |
| hs-TnI, ng/L | 280 (50, 2030) | 5900 (560, 21300) | <0.001 |
| Odds Ratio | 95% Confidence Interval | p-Value | Risk-Score | |
|---|---|---|---|---|
| Hemoglobin < 11 g/dL | 4.01 | 1.65–9.72 | 0.002 | 1 |
| eGFR < 30 mL/min/1.73 m2 | 3.75 | 1.53–9.19 | 0.004 | 1 |
| Albumin < 3.8 mg/dL | 3.37 | 1.31–8.67 | 0.012 | 1 |
| hs-TnI > 2560 ng/L (normal upper limit × 80) | 3.78 | 1.64–8.72 | 0.002 | 1 |
| AUC of the Laboratory-Based Risk-Score Model | AUC of the GRACE 2.0 Model | ΔAUC | p-Value | ||
|---|---|---|---|---|---|
| Type of AMI | STEMI | 0.820 | 0.866 | −0.046 | 0.124 |
| NSTEMI | 0.871 | 0.855 | 0.016 | 0.738 | |
| Sex | Male | 0.831 | 0.861 | −0.036 | 0.397 |
| Female | 0.836 | 0.840 | −0.005 | 0.905 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goriki, Y.; Tanaka, A.; Yoshioka, G.; Nishihira, K.; Kuriyama, N.; Shibata, Y.; Node, K. Development of a Laboratory Risk-Score Model to Predict One-Year Mortality in Acute Myocardial Infarction Survivors. J. Clin. Med. 2022, 11, 3497. https://doi.org/10.3390/jcm11123497
Goriki Y, Tanaka A, Yoshioka G, Nishihira K, Kuriyama N, Shibata Y, Node K. Development of a Laboratory Risk-Score Model to Predict One-Year Mortality in Acute Myocardial Infarction Survivors. Journal of Clinical Medicine. 2022; 11(12):3497. https://doi.org/10.3390/jcm11123497
Chicago/Turabian StyleGoriki, Yuhei, Atsushi Tanaka, Goro Yoshioka, Kensaku Nishihira, Nehiro Kuriyama, Yoshisato Shibata, and Koichi Node. 2022. "Development of a Laboratory Risk-Score Model to Predict One-Year Mortality in Acute Myocardial Infarction Survivors" Journal of Clinical Medicine 11, no. 12: 3497. https://doi.org/10.3390/jcm11123497






