Ocular Tolerability of Bimatoprost 0.1 mg/mL Preservative-Free versus Bimatoprost 0.1 mg/mL with Benzalkonium Chloride or Bimatoprost 0.3 mg/mL Preservative-Free in Patients with Primary Open-Angle Glaucoma
Abstract
:1. Introduction
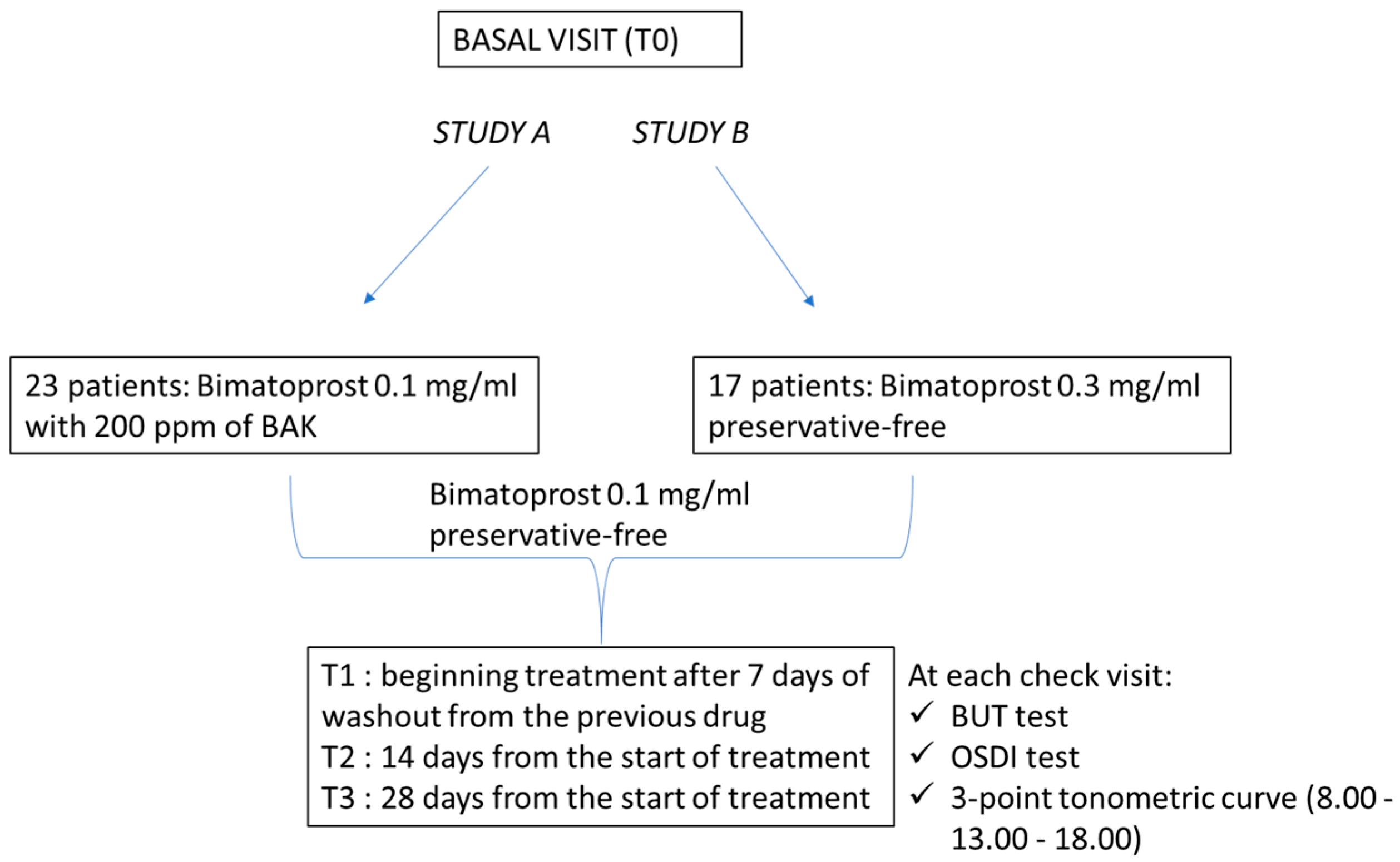
2. Materials and Methods
- Inclusion criteria:
- Exclusion criteria:
2.1. Sample Size Study A
2.2. Sample Size Study B
2.3. Statistical Analysis
3. Results
3.1. Study A
3.2. Study B
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, D.; Chen, P.P. Glaucoma. Am. Fam. Physician 2016, 93, 668–674. [Google Scholar] [PubMed]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Allison, K.; Patel, D.; Alabi, O. Epidemiology of Glaucoma: The Past, Present, and Predictions for the Future. Cureus 2020, 12, e11686. [Google Scholar] [CrossRef] [PubMed]
- Meleppat, R.K.; Zhang, P.; Ju, M.J.; Manna, S.K.; Jian, Y.; Pugh, E.N.; Zawadzki, R.J. Directional optical coherence tomography reveals melanin concentration-dependent scattering properties of retinal pigment epithelium. J. Biomed. Opt. 2019, 24, 066011. [Google Scholar] [CrossRef]
- Meleppat, R.K.; Matham, M.V.; Seah, L.K. Optical frequency domain imaging with a rapidly swept laser in the 1300 nm bio-imaging window. In Proceedings of the SPIE 9524, International Conference on Optical and Photonic Engineering (icOPEN 2015), Singapore, 14–16 April 2015; p. 95242R. [Google Scholar] [CrossRef]
- Imrie, C.; Tatham, A.J. Glaucoma: The patient’s perspective. Br. J. Gen. Pract. 2016, 66, e371–e373. [Google Scholar] [CrossRef] [Green Version]
- Hashemi, H.; Mohammadi, M.; Zandvakil, N.; Khabazkhoob, M.; Emamian, M.H.; Shariati, M.; Fotouhi, A. Prevalence and risk factors of glaucoma in an adult population from Shahroud, Iran. J. Curr. Ophthalmol. 2018, 31, 366–372. [Google Scholar] [CrossRef]
- McMonnies, C.W. Glaucoma history and risk factors. J. Optom. 2017, 10, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Sit, A.J.; Liu, J.H. Pathophysiology of glaucoma and continuous measurements of intraocular pressure. Mol. Cell Biomech. 2009, 6, 57–69. [Google Scholar] [PubMed]
- Tang, W.; Zhang, F.; Liu, K.; Duan, X. Efficacy and safety of prostaglandin analogues in primary open-angle glaucoma or ocular hypertension patients: A meta-analysis. Medicine 2019, 98, e16597. [Google Scholar] [CrossRef]
- European Glaucoma Society Terminology and Guidelines for Glaucoma, 5th Edition. Br. J. Ophthalmol. 2021, 105 (Suppl. S1), 1–169. [CrossRef]
- Li, F.; Huang, W.; Zhang, X. Efficacy and safety of different regimens for primary open-angle glaucoma or ocular hypertension: A systematic review and network meta-analysis. Acta Ophthalmol. 2018, 96, e277–e284. [Google Scholar] [CrossRef]
- Costagliola, C.; dell’Omo, R.; Romano, M.R.; Rinaldi, M.; Zeppa, L.; Parmeggiani, F. Pharmacotherapy of intraocular pressure: Part I. Parasympathomimetic, sympathomimetic and sympatholytics. Expert Opin. Pharmacother. 2009, 10, 2663–2677. [Google Scholar] [CrossRef] [PubMed]
- Assessment Report For Lumigan European Medicines Agency 7 Westferry Circus, Canary Wharf, London, E14 4HB, UK, London, 7 January 2010. Ref: EMA/105752/2010. Available online: https://www.ema.europa.eu/en/documents/variation-report/lumigan-h-c-391-x-0026-epar-assessment-report-extension_en.pdf (accessed on 7 February 2022).
- Figus, M.; Nardi, M.; Piaggi, P.; Sartini, M.; Guidi, G.; Martini, L.; Lazzeri, S. Bimatoprost 0.01% vs. bimatoprost 0.03%: A 12-month prospective trial of clinical and in vivo confocal microscopy in glaucoma patients. Eye 2014, 28, 422–429, Erratum in Eye 2014, 28, 506. [Google Scholar] [CrossRef] [PubMed]
- Costagliola, C.; Prete, A.D.; Incorvaia, C.; Fusco, R.; Parmeggiani, F.; Di Giovanni, A. Ocular surface changes induced by topical application of latanoprost and timolol: A short-term study in glaucomatous patients with and without allergic conjunctivitis. Graefe’s Arch. Clin. Exp. Ophthalmol. 2001, 239, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Pisella, P.J.; Fillacier, K.; Elena, P.P.; Debbasch, C.; Baudouin, C. Comparison of the effects of preserved and unpreserved formulations of timolol on the ocular surface of albino rabbits. Ophthalmic. Res. 2000, 32, 3–8. [Google Scholar] [CrossRef]
- Rosin, L.M.; Bell, N.P. Preservative toxicity in glaucoma medication: Clinical evaluation of benzalkonium chloride-free 0.5% timolol eye drops. Clin. Ophthalmol. 2013, 7, 2131–2135. [Google Scholar] [CrossRef] [Green Version]
- Methodologies to diagnose and monitor dry eye disease: Report of the Diagnostic Methodology Subcommittee of the International Dry Eye WorkShop (2007). Ocul. Surf. 2007, 5, 108–152. [CrossRef]
- El Hajj Moussa, W.G.; Farhat, R.G.; Nehme, J.C.; Sahyoun, M.A.; Schakal, A.R.; Jalkh, A.E.; Abi Karam, M.P.; Azar, G.G. Comparison of Efficacy and Ocular Surface Disease Index Score between Bimatoprost, Latanoprost, Travoprost, and Tafluprost in Glaucoma Patients. J. Ophthalmol. 2018, 2018, 1319628. [Google Scholar] [CrossRef] [Green Version]
- Aguayo Bonniard, A.; Yeung, J.Y.; Chan, C.C.; Birt, C.M. Ocular surface toxicity from glaucoma topical medications and associated preservatives such as benzalkonium chloride (BAK). Expert Opin. Drug Metab. Toxicol. 2016, 12, 1279–1289. [Google Scholar] [CrossRef]
- Debbasch, C.; Brignole, F.; Pisella, P.J.; Warnet, J.M.; Rat, P.; Baudouin, C. Quaternary ammoniums and other preservatives’ contribution in oxidative stress and apoptosis on Chang conjunctival cells. Investig. Ophthalmol. Vis. Sci. 2001, 42, 642–652. [Google Scholar]
- Baudouin, C.; Labbé, A.; Liang, H.; Pauly, A.; Brignole-Baudouin, F. Preservatives in eyedrops: The good, the bad and the ugly. Prog. Retin. Eye Res. 2010, 29, 312–334. [Google Scholar] [CrossRef] [PubMed]
- Baudouin, C. Un nouveau schéma pour mieux comprendre les maladies de la surface oculaire [A new approach for better comprehension of diseases of the ocular surface]. J. Fr. Ophtalmol. 2007, 30, 239–246. (In French) [Google Scholar] [CrossRef]
- Sarkar, J.; Chaudhary, S.; Namavari, A.; Ozturk, O.; Chang, J.H.; Yco, L.; Sonawane, S.; Khanolkar, V.; Hallak, J.; Jain, S. Corneal neurotoxicity due to topical benzalkonium chloride. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1792–1802. [Google Scholar] [CrossRef] [PubMed]
- Desbenoit, N.; Schmitz-Afonso, I.; Baudouin, C.; Laprévote, O.; Touboul, D.; Brignole-Baudouin, F.; Brunelle, A. Localisation and quantification of benzalkonium chloride in eye tissue by TOF-SIMS imaging and liquid chromatography mass spectrometry. Anal. Bioanal. Chem. 2013, 405, 4039–4049. [Google Scholar] [CrossRef] [PubMed]
- Kallab, M.; Szegedi, S.; Hommer, N.; Stegmann, H.; Kaya, S.; Werkmeister, R.M.; Schmidl, D.; Schmetterer, L.; Garhöfer, G. Topical Low Dose Preservative-Free Hydrocortisone Reduces Signs and Symptoms in Patients with Chronic Dry Eye: A Randomized Clinical Trial. Adv. Ther. 2020, 37, 329–341, Erratum in Adv. Ther. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baudouin, C.; Denoyer, A.; Desbenoit, N.; Hamm, G.; Grise, A. In vitro and in vivo experimental studies on trabecular meshwork degeneration induced by benzalkonium chloride (an American Ophthalmological Society thesis). Trans. Am. Ophthalmol. Soc. 2012, 110, 40–63. [Google Scholar] [PubMed]
- Boimer, C.; Birt, C.M. Preservative exposure and surgical outcomes in glaucoma patients: The PESO study. J. Glaucoma 2013, 22, 730–735. [Google Scholar] [CrossRef]
- Alm, A. Latanoprost in the treatment of glaucoma. Clin. Ophthalmol. 2014, 8, 1967–1985. [Google Scholar] [CrossRef] [Green Version]
- Myers, J.S.; Vold, S.; Zaman, F.; Williams, J.M.; Hollander, D.A. Bimatoprost 0.01% or 0.03% in patients with glaucoma or ocular hypertension previously treated with latanoprost: Two randomized 12-week trials. Clin. Ophthalmol. 2014, 8, 643–652. [Google Scholar] [CrossRef] [Green Version]






| Parameter | Bimatoprost 0.1 mg/mL with BAK at Baseline Mean ± SD (95% CI) | Bimatoprost 0.1 mg/mL Preservative-Free at 14 Days Mean ± SD (95% CI) | Bimatoprost 0.1 mg/mL Preservative-Free at 28 Days Mean ± SD (95% CI) | p |
|---|---|---|---|---|
| OSDI (score) | 33.74 ± 12.02 (28.54 to 38.93) | 25.61 ± 10.88 (20.9 to 30.31) | 23.00 ± 10.70 (18.37 to 27.63) | <0.0001 |
| BUT (sec) | 6.87 ± 2.16 (5.94 to 7.80) | 8.04 ± 2.16 (7.11 to 8.98) | 8.17 ± 2.29 (7.18 to 9.16) | 0.0003 |
| IOP (mmHg) | 15.83 ± 0.86 (15.45 to 16.20) | 15.73 ± 1.29 (15.17 to 16.29) | 15.75 ± 1.61 (15.05 to 16.45) | 0.92 |
| Parameter | Bimatoprost 0.1 mg/mL with BAK at Baseline Mean ± SD (95% CI) | Bimatoprost 0.1 mg/mL Preservative-Free at 14 Days Mean ± SD (95% CI) | Bimatoprost 0.1 mg/mL Preservative-Free at 28 Days Mean ± SD (95% CI) |
|---|---|---|---|
| IOP, mmHg | |||
| At 8 h | 16.00 ± 1.04 (15.55 to 16.45) | 15.83 ± 1.40 (15.22 to 16.43) | 15.65 ± 1.75 (14.90 to 16.41) |
| At 13 h | 15.74 ± 0.86 (15.38 to 16.11) | 15.70 ± 1.26 (15.15 to 16.24) | 15.70 ± 1.58 (15.01 to 16.38) |
| At 18 h | 15.75 ± 1.25 (15.20 to 16.28) | 15.65 ± 1.50 (15.01 to 16.30) | 15.91 ± 1.70 (15.18 to 16.65) |
| Adverse Event | Bimatoprost 0.1 mg/mL with BAK at Baseline n (%) | Bimatoprost 0.1 mg/mL Preservative-Free at 14 Days n (%) | Bimatoprost 0.1 mg/mL Preservative-Free at 28 Days n (%) |
|---|---|---|---|
| Hyperemia | |||
| absent | 5 (21.74) | 7 (30.43) | 11 (47.83) |
| very mild | 7 (30.43) | 10 (43.48) | 9 (39.13) |
| mild | 9 (39.13) | 5 (21.74) | 3 (13.04) |
| severe/serious | 2 (8.70) | 1 (4.35) | 0 (0.00) |
| Photophobia | |||
| absent | 7 (30.43) | 14 (60.87) | 16 (69.57) |
| very mild | 11 (47.83) | 8 (34.78) | 6 (26.09) |
| mild | 4 (17.39) | 1 (4.35) | 1 (4.35) |
| severe/serious | 1 (4.35) | 0 (0.00) | 0 (0.00) |
| Tearing | |||
| absent | 4 (17.39) | 7 (30.43) | 11 (47.83) |
| very mild | 13 (56.52) | 16 (69.57) | 12 (52.17) |
| mild | 6 (26.09) | 0 (0.00) | 0 (0.00) |
| severe/serious | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Pain | |||
| 0 | 17 (73.91) | 21 (91.30) | 22 95.65) |
| 1 | 3 (13.04) | 0 (0.00) | 1 (4.35) |
| 2 | 2 (8.70) | 2 (8.70) | 0 (0.00) |
| 3 | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| 4 | 1 (4.35) | 0 (0.00) | 0 (0.00) |
| Parameter | Bimatoprost 0.3 mg/mL Preservative-Free at Baseline Mean ± SD (95% CI) | Bimatoprost 0.1 mg/mL Preservative-Free at 14 Days Mean ± SD (95% CI) | Bimatoprost 0.1 mg/mL Preservative-Free at 28 Days Mean ± SD (95% CI) | p |
|---|---|---|---|---|
| OSDI (score) | 38.88 ± 4.95 (36.34 to 41.43) | 31.65 ± 4.03 (29.57 to 33.72) | 33.06 ± 4.63 (30.68 to 35.44) | <0.0001 |
| BUT (sec) | 5.71 ± 1.16 (5.11 to 6.30) | 7.53 ± 1.37 (6.82 to 8.24) | 6.59 ± 1.12 (6.01 to 7.16) | <0.0001 |
| IOP (mmHg) | 16.78 ± 2.02 (15.75 to 17.83) | 16.84 ± 1.86 (15.89 to 17.80) | 16.87 ± 1.95 (15.87 to 17.87) | 0.97 |
| Parameter | Bimatoprost 0.3 mg/mL Preservative-Free at Baseline Mean ± SD (95% CI) | Bimatoprost 0.1 mg/mL Preservative-Free at 14 Days Mean ± SD (95% CI) | Bimatoprost 0.1 mg/mL Preservative-Free at 28 Days Mean ± SD (95% CI) |
|---|---|---|---|
| IOP, mmHg | |||
| At 8 h | 17.53 ± 2.40 (16.29 to 18.76) | 17.11 ± 1.93 (16.12 to 18.11) | 17.41 ± 2.43 (16.16 to 18.66) |
| At 13 h | 16.12 ± 2.06 (15.06 to 17.18) | 16.65 ± 1.80 (15.72 to 17.57) | 16.47 ± 1.62 (15.63 to 17.30) |
| At 18 h | 16.71 ± 2.23 (15.56 to 17.85) | 16.76 ± 2.28 (15.59 to 17.94) | 16.76 ± 2.19 (15.64 to 17.89) |
| Adverse Event | Bimatoprost 0.3 mg/mL Preservative-Free at Baseline n (%) | Bimatoprost 0.1 mg/mL Preservative-Free at 14 Days n (%) | Bimatoprost 0.1 mg/mL Preservative-Free at 28 Days n (%) |
|---|---|---|---|
| Hyperemia | |||
| absent | 0 (0.00) | 0 (0.00) | 1 (5.88) |
| very mild | 4 (23.53) | 12 (70.59) | 9 (52.94) |
| mild | 9 (52.94) | 5 (19.41) | 6 (35.29) |
| severe/serious | 4 (23.53) | 0 (0.00) | 1 (5.88) |
| Photophobia | |||
| absent | 6 (35.29) | 8 (47.06) | 5 (29.41) |
| very mild | 8 (47.06) | 8 (47.06) | 11 (64.71) |
| mild | 3 (17.65) | 1 (5.88) | 1 (5.88) |
| severe/serious | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Tearing | |||
| absent | 2 (11.76) | 2 (11.76) | 3 (17.65) |
| very mild | 3 (17.65) | 12 (70.59) | 10 (58.62) |
| mild | 12 (70.59) | 3 (17.65) | 3 (17.65) |
| severe/serious | 0 (0.00) | 0 (0.00) | 1 (5.88) |
| Pain | |||
| 0 | 10 (58.62) | 17 (100.00) | 16 (94.12) |
| 1 | 6 (35.29) | 0 (0.00) | 1 (5.88) |
| 2 | 1 (5.88) | 0 (0.00) | 0 (0.00) |
| 3 | 0 (0.00) | 0 (0.00) | 0 (0.00) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filippelli, M.; Campagna, G.; Ciampa, N.; Fioretto, G.; Giannini, R.; Marino, P.F.; dell’Omo, R.; Costagliola, C. Ocular Tolerability of Bimatoprost 0.1 mg/mL Preservative-Free versus Bimatoprost 0.1 mg/mL with Benzalkonium Chloride or Bimatoprost 0.3 mg/mL Preservative-Free in Patients with Primary Open-Angle Glaucoma. J. Clin. Med. 2022, 11, 3518. https://doi.org/10.3390/jcm11123518
Filippelli M, Campagna G, Ciampa N, Fioretto G, Giannini R, Marino PF, dell’Omo R, Costagliola C. Ocular Tolerability of Bimatoprost 0.1 mg/mL Preservative-Free versus Bimatoprost 0.1 mg/mL with Benzalkonium Chloride or Bimatoprost 0.3 mg/mL Preservative-Free in Patients with Primary Open-Angle Glaucoma. Journal of Clinical Medicine. 2022; 11(12):3518. https://doi.org/10.3390/jcm11123518
Chicago/Turabian StyleFilippelli, Mariaelena, Giuseppe Campagna, Nicola Ciampa, Gaetano Fioretto, Roberta Giannini, Pier Franco Marino, Roberto dell’Omo, and Ciro Costagliola. 2022. "Ocular Tolerability of Bimatoprost 0.1 mg/mL Preservative-Free versus Bimatoprost 0.1 mg/mL with Benzalkonium Chloride or Bimatoprost 0.3 mg/mL Preservative-Free in Patients with Primary Open-Angle Glaucoma" Journal of Clinical Medicine 11, no. 12: 3518. https://doi.org/10.3390/jcm11123518







