The Role of ICG in Robot-Assisted Liver Resections
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Design and Study Population
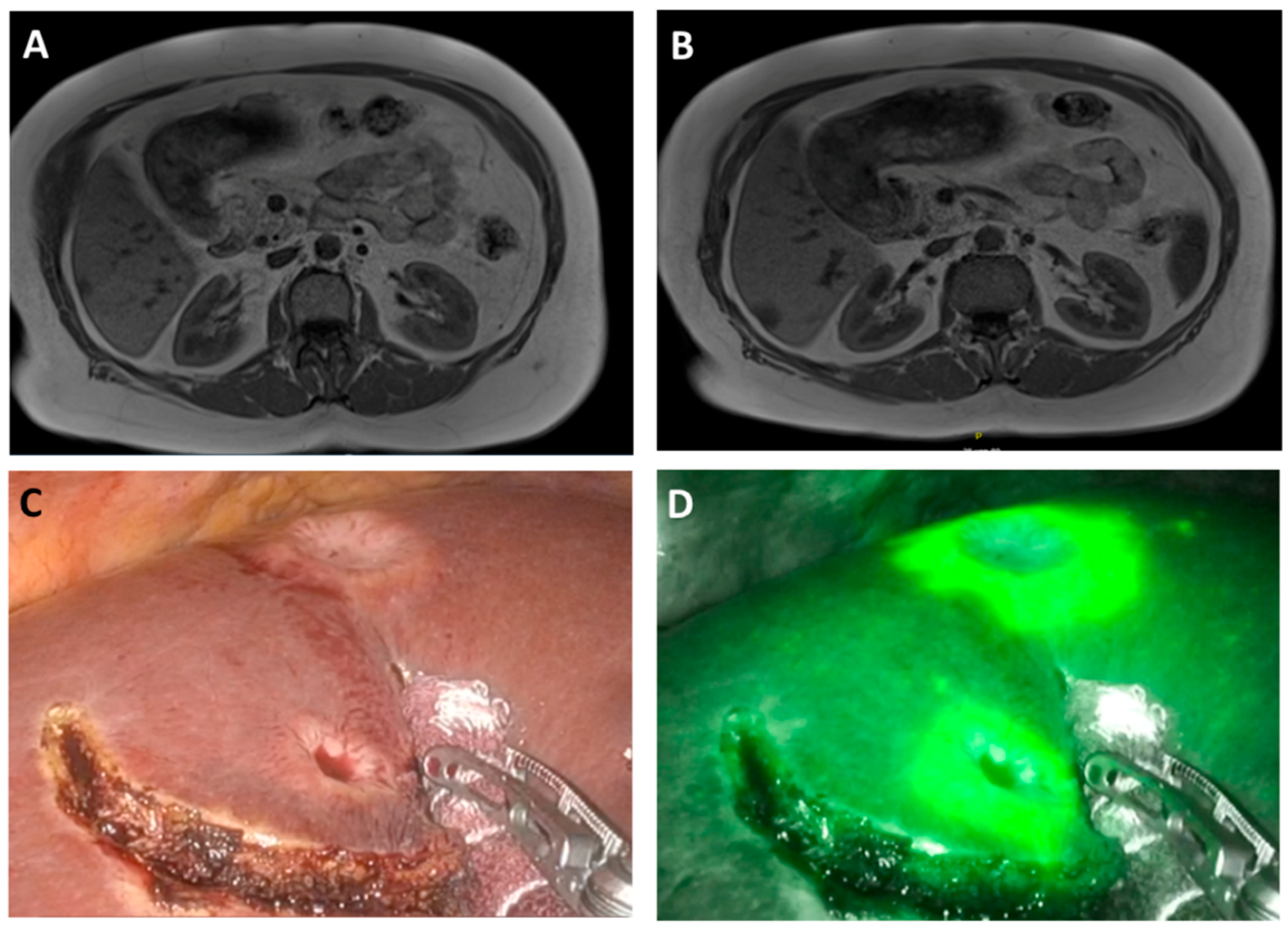
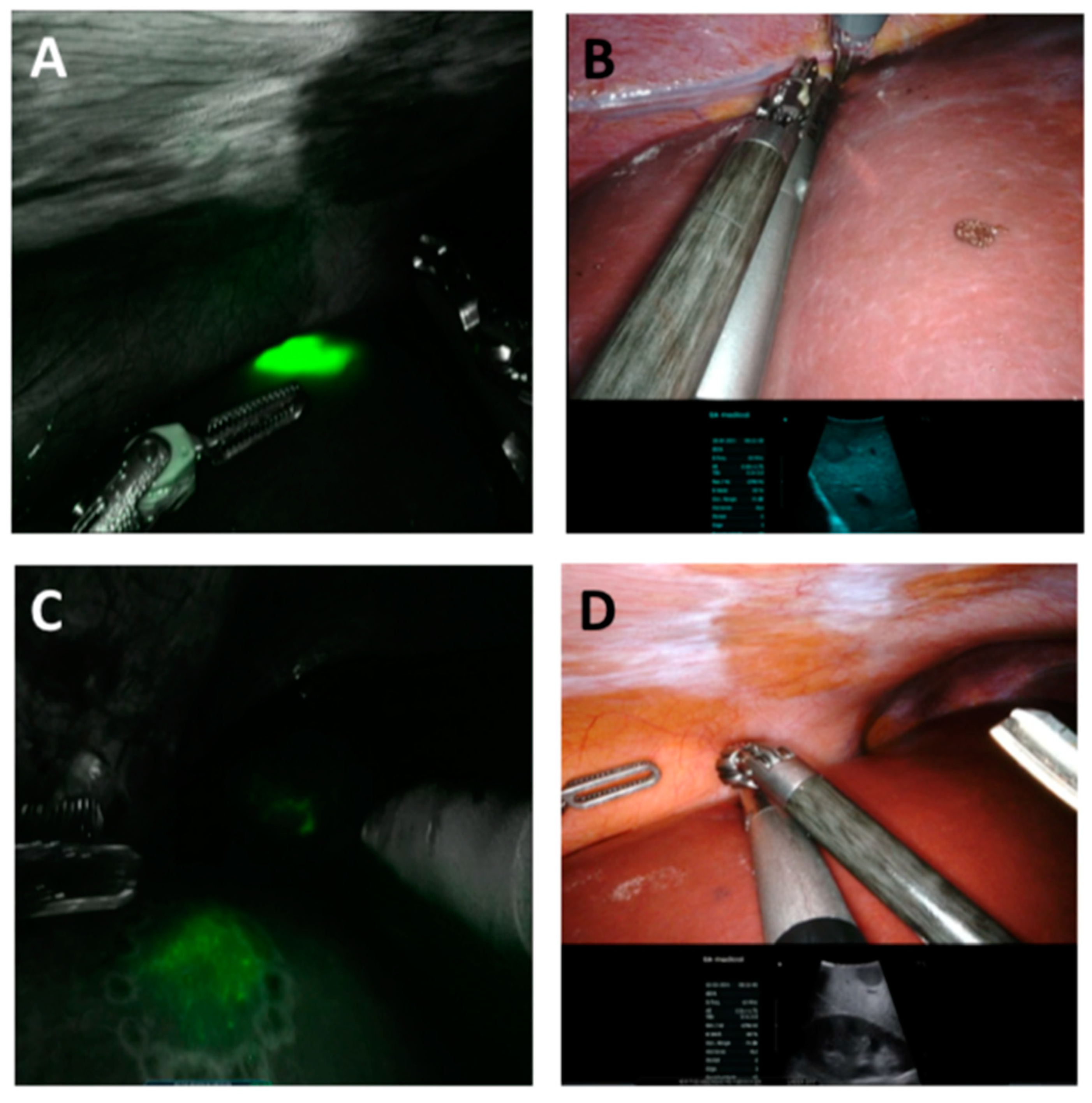
2.2. ICG Application and Evaluation
2.3. Surgery
2.4. Histological Analysis
2.5. Outcome Measures
2.6. Statistical Analysis
3. Results
| Total (n = 54) | ICG (n = 28) | HC (n = 26) | p-Value * | |
|---|---|---|---|---|
| Clinical Data | ||||
| Age (years) mean ± SD | 64.0 ± 14.3 | 65.4 ± 11.1 | 62.5 ± 17.1 | 0.465 a |
| Sex (males) % | 50.0 | 53.6 | 46.2 | 0.586 b |
| BMI (kg/m2) mean ± SD | 27.5 ± 5.3 | 28.7 ± 7.0 | 26.8 ± 4.3 | 0.389 a |
| Liver fibrosis (histopathologically proven) | 13.0 | 14.3 | 11.5 | 0.764 b |
| Liver cirrhosis (histopathologically proven) | 24.1 | 17.9 | 30.8 | 0.264 b |
| Previous abdominal surgery (yes) % | 44.4 | 50.0 | 38.5 | 0.394 b |
| Type of previous surgery (open vs. MI) % | 38.5/61.5 | 21.4/78.6 | 58.3/41.7 | 0.054 b |
| Open | 38.5 | 21.4 | 58.3 | 0.054 b |
| Minimally-invasive | 30.8 | 42.9 | 16.7 | 0.149 b |
| Robot-assisted | 30.8 | 35.7 | 25.0 | 0.555 b |
| Previous procedure | ||||
| Sigma/LAR | 22.2 | 21.4 | 23.1 | 0.918 b |
| Appendectomy | 18.5 | 7.1 | 30.8 | 0.114 b |
| Cholecystectomy | 11.1 | 21.4 | 0.0 | 0.077 b |
| Herniotomy | 7.4 | 7.1 | 7.7 | 0.957 b |
| Right nephrectomy | 7.4 | 7.1 | 7.7 | 0.957 b |
| Cystoprostatectomy | 7.4 | 14.3 | 0.0 | 0.157 b |
| Caesarean section | 7.4 | 0.0 | 7.7 | 0.290 b |
| Gynecological surgery | 7.4 | 0.0 | 15.4 | 0.127 b |
| Esophagectomy | 3.7 | 7.1 | 0.0 | 0.326 b |
| Right hemicolectomy | 3.7 | 7.1 | 0.0 | 0.326 b |
| Left hemicolectomy | 3.7 | 0.0 | 7.7 | 0.290 b |
| Adrenalectomy | 3.7 | 7.1 | 0.0 | 0.326 b |
| Total (n = 54) | ICG (n = 28) | HC (n = 26) | p-Value * | |
|---|---|---|---|---|
| Post-operative Data | ||||
| Post-operative complications (yes) % | 16.7 | 14.3 | 19.2 | 0.636 b |
| Clavien–Dindo I | 11.3 | 10.7 | 11.5 | 0.923 b |
| Clavien–Dindo II | 5.7 | 3.6 | 7.7 | 0.486 b |
| Clavien–Dindo III | 0.0 | 0.0 | 0.0 | na |
| Clavien–Dindo IV | 1.9 | 3.6 | 0.0 | 0.331 b |
| Clavien–Dindo V | 0.0 | 0.0 | 0.0 | na |
| Size of tumor (mm) mean ± SD | 36.9 ± 31.8 | 27.1 ± 25.0 | 47.6 ± 35.2 | 0.021 a |
| Histopathological results | ||||
| Hepatocellular carcinoma | 32.1 | 29.6 | 34.6 | 0.633 b |
| Hepatocellular carcinoma in cirrhosis | 18.5 | 14.3 | 23.1 | 0.406 b |
| Colorectal cancer | 17.0 | 22.2 | 11.5 | 0.330 b |
| Focal nodular hyperplasia | 15.1 | 11.1 | 19.2 | 0.379 b |
| Cholanciocellular carcinoma | 7.3 | 0.0 | 15.3 | 0.015 b |
| Haemangioma | 5.7 | 0.0 | 11.5 | 0.135 b |
| Breast cancer | 3.8 | 7.4 | 0.0 | 0.165 b |
| Neuroendocrine tumor | 3.8 | 7.4 | 0.0 | 0.165 b |
| Gastrointestinal stroma tumor | 1.9 | 0.0 | 3.8 | 0.295 b |
| Anal cancer | 1.9 | 0.0 | 3.8 | 0.295 b |
| Choroid coat melanoma | 1.9 | 3.7 | 0.0 | 0.331 b |
| Non-small cell lung cancer | 1.9 | 3.7 | 0.0 | 0.331 b |
| Leiomyosarcoma | 1.9 | 3.7 | 0.0 | 0.331 b |
| No malignancy proven | 5.7 | 11.1 | 0.0 | 0.086 b |
| Distance to resection margin (mm) mean ± SD | 7.8 ± 12.1 | 5.8 ± 10.9 | 10.2 ± 13.4 | 0.200 a |
| Resection margin positive-resections (yes) % | 11.3 | 3.7 | 19.2 | 0.075 b |
| Length of hospital stay (days) mean ± SD | 6.4 ± 4.0 | 5.9 ± 5.0 | 6.9 ± 2.7 | 0.383 a |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Becker, F.; Morgül, H.; Katou, S.; Juratli, M.; Hölzen, J.P.; Pascher, A.; Struecker, B. Robotic Liver Surgery-Current Standards and Future Perspectives. Z. Für Gastroenterol. 2020, 59, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, F.F.G.; Magistri, P.; Fontani, A.; Menonna, F.; Annecchiarico, M.; Lauterio, A.; De Carlis, L.; Coratti, A.; Boggi, U.; Ceccarelli, G.; et al. Pure laparoscopic versus robotic liver resections: Multicentric propensity score-based analysis with stratification according to difficulty scores. J. Hepato-Biliary-Pancreat. Sci. 2021, 1–16. [Google Scholar] [CrossRef]
- Hajibandeh, S.H.S.; Dosis, A.; Qayum, M.K.; Hassan, K.; Kausar, A.; Satyadas, T. Level 2a evidence comparing robotic versus laparoscopic left lateral hepatic sectionectomy: A meta-analysis. Langenbeck’s Arch. Surg. 2021, 407, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.G.C.; Salloum, C.; Tudisco, A.; Napoli, N.; Boggi, U.; Azoulay, D.; Scatton, O. Outcomes after 3D laparoscopic and robotic liver resection for hepatocellular carcinoma: A multicenter comparative study. Surg. Endosc. 2020, 37, 3258–3266. [Google Scholar] [CrossRef]
- Lin, C.M.; Huang, H.L.; Chu, F.Y.; Fan, H.C.; Chen, H.A.; Chu, D.M.; Wu, L.W.; Wang, C.C.; Chen, W.L.; Ho, S.Y.; et al. Association between Gastroenterological Malignancy and Diabetes Mellitus and Anti-Diabetic Therapy: A Nationwide, Population-Based Cohort Study. PLoS ONE 2015, 10, e0125421. [Google Scholar] [CrossRef]
- Duong, L.M.C.H.; Shrubsole, M.J.; Bailey, C.D.; Idrees, K.; Shu, X.O. Outcomes of Robotic-Assisted Liver Surgery Versus Laparoscopic Liver Surgery for Treatment of Stage I Hepatocellular Carcinoma. Cancer 2022, 128, 762–769. [Google Scholar] [CrossRef]
- Broering, D.S.M.; Zidan, A. Robotic donor hepatectomy: A major breakthrough in living donor liver transplantation. Am. J. Transplant. 2021, 22, 14–23. [Google Scholar] [CrossRef]
- Mehdorn, A.S.B.J.; Braun, F.; Becker, T.; Egberts, J.H. Usability of Indocyanine Green in Robot-Assisted Hepatic Surgery. J. Clin. Med. 2021, 10, 456. [Google Scholar] [CrossRef]
- Kokudo, N.T.N.; Ito, K.; Mihara, F. The history of liver surgery: Acheivements over the past 50 years. J. Am. Coll. Surg. 2019, 193, 210–222. [Google Scholar] [CrossRef]
- Marino, M.V.P.M.; Fernandez, C.C.; Ruiz, M.G.; Fleitas, M.G. The application of indocyanine green-fluorescence imaging during robotic-assisted liver resection for malignant tumors: A single-arm feasibility cohort study. HPB 2019, 22, 422–431. [Google Scholar] [CrossRef]
- Alfano, M.S.M.S.; Benedicenti, S.; Molteni, S.; Porsio, P.; Arici, E.; Gheza, F.; Botticini, M.; Portolani, N.; Baiocchi, G.L. Intraoperative ICG-based imaging of liver neoplasms: A simple yet powerful tool. Preliminary results. Surg. Endosc. 2019, 33, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.T.A.; Baiocchi, G.; de’Angelis, G.L.; Gaiani, F.; Di Mario, F.; Catena, F.; Dalla Valle, R. Fluorescence guided surgery in liver tumors: Applications and advantages. Acta Biomed. 2018, 89, 135–140. [Google Scholar]
- Wang, X.T.C.; Ishizawa, T.; Aoki, T.; Cavallucci, D.; Lee, S.Y.; Panganiban, K.M.; Perini, M.V.; Shah, S.R.; Wang, H.; Xu, Y.; et al. Consensus Guidelines for the Use of Fluoresecence Imaging in Hepatobiliary Surgery. Ann. Surg. 2021, 274, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, T.C.A.; Abe, Y.; Bona, E.D.; Nicolini, D.; Mocchegiani, F.; Kabeshima, Y.; Vivarelli, M.; Wakabayashi, G.; Kitagawa, Y. Indocyanine green fluorescence navigation in liver surgery: A systematic review on dose and timing of administration. Ann. Surg. 2022, 275, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Ishizawa, I.F.N.; Shibahara, J.; Masuda, K.; Tamura, S.; Aoki, T.; Hasegawa, K.; Beck, Y.; Fukayama, M.; Kokudo, N. Real-Time Identification of Liver Cancers by Using Indocyanine Green Fluorescent Imaging. Cancer 2009, 115, 2491–2505. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, J.K.G.; Inagaki, Y.; Hasegawa, K. Innovative treatment for hepatocellular carcinoma HCC. Transl. Gastroenterol. Hepatol. 2018, 3, 78. [Google Scholar] [CrossRef] [PubMed]
- Franz, M.A.J.; Wolff, S.; Perrakis, A.; Rahimli, M.; Negrini, V.N.; Stockheim, J.; Lorenz, E.; Croner, R. Tumor visualization and fluorescence angiography with indocyanine green ICG in laparoscopic and robotic hepatobiliary surgery-valuation of early adopters from Germany. Innov. Surg. Sci. 2021, 6, 59–66. [Google Scholar] [CrossRef]
- Achterberg, F.B.M.B.; Meijer, R.P.J.; Bonsing, B.A.; Hartgrink, H.H.; Mieog, J.S.D.; Zlitni, A.; Park, S.M.; Sarasqueta, A.F.; Vahrmeijer, A.L.; Swijnenburg, R.J. Real-time surgical margin assessment using ICG-fluorescence during laparoscopic and robot-assisted resections of colorectal liver metastases. Ann. Transl. Med. 2020, 8, 1448. [Google Scholar] [CrossRef]
- van der Vorst, J.R.S.B.; Hutteman, M.; Verbeek, F.P.R.; Liefers, G.J.; Hartgrink, H.H.; Smit, V.T.H.M.B.; Löwik, C.W.G.M.; van de Velde, C.J.H.; Frangioni, J.V.; Vahrmeijer, A.L. Near-infrared fluorescence-guided resection of colorectal liver metastases. Cancer 2013, 119, 3411–3418. [Google Scholar] [CrossRef]
- Ishizawa, T.S.A.; Kokudo, N. Clinical application of indocyanine green-fluorescence imaging during hepatectomy. Hepatobiliary Surg. Nutr. 2015, 5, 322–328. [Google Scholar] [CrossRef] [Green Version]
- Speich, R.S.B.; Hoffmann, U.; Neftel, K.A.; Reichen, J. Anaphylactoid reactions after indocyanine-green administration. Ann. Intern. Med. 1988, 109, 345–346. [Google Scholar] [CrossRef] [PubMed]
- Aselmann, H.; Möller, T.; Kersebaum, J.N.; Egberts, J.H.; Croner, R.; Brunner, M.; Grützmann, R.; Becker, T. Roboterassistierte Leberresektion. Der Chir. 2017, 88, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Nagtegaal, I.D.O.R.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A.; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marino, M.V.; Di Saverio, S.; Podda, M.; Gomez Ruiz, M.; Gomez Fleitas, M. The Application of Indocyanine Green Fluorescence Imaging during Robotic Liver Resection: A Case-Match-Study. World J. Surg. 2019, 43, 2595–2606. [Google Scholar] [CrossRef] [PubMed]
- Hilal, M.A.; Aldrighetti, L.; Dagher, I.; Edwin, B.; Troisi, R.I.; Alikhanov, R.; Cherqui, D. The Southampton Consensus Guidelines for Laparoscopic Liver Surgery: From Indication to Imple-mentation. Ann. Surg. 2018, 268, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Feldbrügge, L.; Ortiz Galindo, S.A.; Frisch, O.; Benzing, C.; Krenzien, F.; Riddermann Kästner, A.; Nevermann, N.F.; Malinka, T.; Schöning, W.; Pratschke, J.; et al. Safety and feasibility of robotic liver resection after previous abdominal surgeries. Surg. Endosc. 2021, 36, 2842–2849. [Google Scholar] [CrossRef]
- Zwart, M.J.; Görgec, B.; Arabiyat, A.; Nota, C.L.M.; van der Poel, M.J.; Fichtinger, R.S.; Berrevoet, F.; van Dam, R.M.; Aldrighetti, L.; Fuks, D.; et al. Pan-European survey on the implementation of robotic and laparoscopic minimally invasive liver surgery. HPB 2022, 24, 322–331. [Google Scholar] [CrossRef]
- Giulianotti, P.C.; Bianco, F.M.; Daskalaki, D.; Gonzalez-Ciccarelli, L.F.; Kim, J.; Benedetti, E. Robotic liver surgery: Technical aspects and review of the literature. Hepatobiliary Surg. Nutr. 2016, 5, 311–321. [Google Scholar] [CrossRef] [Green Version]
- Giulianotti, P.C.; Coratti, A.; Angelini, M.; Sbrana, F.; Cecconi, S.; Balestracci, T.; Caravaglios, G. Robotics in general surgery: Personal experience in a large community hospital. Arch. Surg. 2003, 138, 777–784. [Google Scholar] [CrossRef] [Green Version]
- Stoffels, B.G.T.; von Websky, M.M.; Kalff, J.C.; Vilz, T.O. Robertassistierte Operationen in der Viszeralchirurgie. Der Chir. 2020, 91, 190–194. [Google Scholar] [CrossRef]
- Sucandy, I.G.A.; Ross, S.; Rosemurgy, A. Institutional First 100 Case Experience and Outcomes of Robotic Hepatectomy for Liver Tumors. Am. Surg. 2020, 86, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Sucandy, I.S.E.; Crespo, K.; Syblis, C.; Przetocki, V.; Ross, S.; Rosemurgy, A. The effect of the robotic platform in hepatectomy after prior liver and non-liver abdominal operations: A comparative study of clinical outcomes. J. Robot. Surg. 2021, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kamarajah, S.K.; Bundred, J.; Manas, D.; Jiao, L.R.; Hilal, M.A.; White, S.A. Robotic versus conventional laparoscopic liver resections: A systematic review and meta-analysis. Scand. J. Surg. 2020, 110, 290–300. [Google Scholar] [CrossRef]
- Lai, A.; Lipka, S.; Kumar, A.; Sethi, S.; Bromberg, D.; Li, N.; Shen, H.; Stefaniwsky, L.; Brady, P. Role of Esophageal Metal Stents Placement and Combination Therapy in Inoperable Esophageal Carcinoma: A Systematic Review and Meta-analysis. Dig. Dis. Sci. 2018, 63, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Rocca, A.; Scacchi, A.; Cappuccio, M.; Avella, P.; Bugiantella, W.; De Rosa, M.; Costa, G.; Polistena, A.; Codacci-Pisanelli, M.; Amato, B.; et al. Robotic surgery for colorectal liver metastases resection: A systematic review. Int. J. Med. Robot. Comput. Assist. Surg. 2021, 17, e2330. [Google Scholar] [CrossRef]
- Efanov, M.; Alikhanov, R.; Tsvirkun, V.; Kazakov, I.; Melekhina, O.; Kim, P.; Vankovich, A.; Grendal, K.; Berelavichus, S.; Khatkov, I. Comparative analysis of learning curve in complex robot-assisted and laparoscopic liver resection. HPB 2017, 19, 818–824. [Google Scholar] [CrossRef] [Green Version]
- Zhu, P.; Liao, W.; Ding, Z.Y.; Chen, L.; Zhang, W.G.; Zhang, B.X.; Chen, X.P. Learning Curve in Robot-Assisted Laparoscopic Liver Resection. J. Gastrointest. Surg. 2019, 23, 1778–1787. [Google Scholar] [CrossRef]
- Lai, E.C.; Tang, C.N. Long-term survival analysis of robotic versus conventional laparoscopic hepatectomy for hepatocellular carcinoma: A comparative study. Surg. Laparosc. Endosc. Percutaneous Tech. 2016, 26, 162–166. [Google Scholar] [CrossRef]
- Hottenrott, S.; Schlesinger, T.; Helmer, P.; Meybohm, P.; Alkatout, I.; Kranke, P. Do Small Incisions Need Only Minimal Anesthesia?—Anesthetic Management in Laparoscopic and Robotic Surgery. J. Clin. Med. 2020, 9, 4058. [Google Scholar] [CrossRef]
- Mehdorn, A.S.; Möller, T.; Franke, F.; Richter, F.; Kersebaum, J.N.; Becker, T.; Egberts, J.H. Long-Term, Health-Related Quality of Life after Open and Robot-Assisted Ivor-Lewis Procedures-A Propensity Score-Matched Study. J. Clin. Med. 2020, 9, 3513. [Google Scholar] [CrossRef]
- Abo, T.; Nanashima, A.; Tobinaga, S.; Hidaka, S.; Taura, N.; Takagi, K.; Arai, J.; Miyaaki, H.; Shibata, H.; Nagayasu, T. Usefulness of intraoperative diagnosis of hepatic tumors located at the liver surface and hepatic segmental visualization using indocyanine green-photodynamic eye imaging. Eur. J. Surg. Oncol. 2015, 41, 257–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.G.; Zhou, Z.P.; Tan, X.L.; Wang, Z.Z.; Liu, Q.; Zhao, Z.M. Robotic resection of liver focal nodal hyperplasia guided by indocyanine green fluorescence imaging: A preliminary analysis of 23 cases. World J. Gastrointest. Oncol. 2020, 12, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Casciola, L.; Patriti, A.; Ceccarelli, G.; Bartoli, A.; Ceribelli, C.; Spaziani, A. Robot-assisted parenchymal-sparing liver surgery including lesions located in the posterosuperior segments. Surg. Endosc. 2011, 25, 3815–3824. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Zaidi, N.; Berber, E. An initial report on the intraoperative use of indocyanine green fluorescence imaging in the surgical management of liver tumors. J. Surg. Oncol. 2016, 114, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Giannone, F.; Felli, E.; Cherkaoui, Z.; Mascagni, P.; Pessaux, P. Augmented Reality and Image-Guided Robotic Liver Surgery. Cancers 2021, 13, 6268. [Google Scholar] [CrossRef]
- Available online: https://isfgs.org/ (accessed on 16 June 2022).
- Handgraaf, H.J.; Boogerd, L.S.; Höppener, D.J.; Peloso, A.; Sibinga Mulder, B.G.; Hoogstins, C.E.S.; Hartgrink, H.H.; van de Velde, C.J.H.; Mieog, C.S.D.; Swijnenburg, R.J.; et al. Long-term follow-up after near-infrared fluorescence-guided resection of colorectal liver metastases: A retrospective multicenter analysis. Eur. J. Surg. Oncol. 2017, 43, 1463–1471. [Google Scholar] [CrossRef]
- Kobayashi, K.; Kawaguchi, Y.; Kobayashi, Y.; Matsumura, M.; Ishizawa, T.; Akamatsu, N.; Kaneko, J.; Arita, J.; Sakamoto, Y.; Kokudo, N.; et al. Identification of liver lesions using fluorescence imaging: Comparison of methods for administering indocyanine green. HPB 2021, 23, 262–269. [Google Scholar] [CrossRef]
- Liu, D.; Liang, L.; Liu, L.; Zhu, Z. Does intraoperative indocyanine green fluorescence angiography decrease the incidence of anastomotic leakage in colorectal surgery? A systematic review and meta-analysis. Int. J. Colorectal Dis. 2020, 36, 57–66. [Google Scholar] [CrossRef]
- Andreyev, J.N.; Norman, A.R.; Cunningham, D.; Oates, J.; Dix, B.R.; Iacopetta, B.J.; Young, J.; Walsh, T.; Ward, R.; Hawkins, N.; et al. Kirsten ras mutations in patients with colorectal cancer: The ‘RASCAL II’ study. Br. J. Cancer 2001, 85, 692–696. [Google Scholar] [CrossRef]
- Tomassini, F.; Giglio, M.C.; De Simone, G.; Montalti, R.; Troisi, R.I. Hepatic function assessment to predict post-hepatectomy liver failure: What can we trust? A systematic review. Updates Surg. 2020, 72, 925–938. [Google Scholar] [CrossRef]
- Oldhafer, K.; Reese, T.; Fard-Aghaie, M.; Strohmaier, A.; Makridis, G.; Kantas, A.; Wagner, K.C. Intraoperative Fluoreszenzangio- und cholangiographie mit Indocyaningrün bei hepatobiliären Eingriffen. Der Chir. 2019, 90, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Naldini, A.; Vizzielli, G.; Perrone, E.; Gallotta, V.; Scambia, G. Robotic video endoscopic inguinal lymphadenectomy R-VEIL for vulvar cancer with sentinel node mapping using indocyanine green and near-infrared fluorescence imaging technology. Gynecol. Oncol. 2018, 150, 203–204. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.N.; Qiu, W.W.; Liu, C.H.; Chen, Q.Y.; Zheng, C.H.; Li, P.; Wang, J.B.; Lin, J.X.; Lu, J.; Cao, L.L.; et al. Assessment of indocyanine green tracer-guided lymphadenectomy in laparoscopic gastrectomy after neoadjuvant chemotherapy for locally advanced gastric cancer: Results from a multicenter analysis based on propensity matching. Gastric Cancer 2021, 24, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Arezzo, A.; Arolfo, S.; Mistrangelo, M.; Mussa, B.; Cassoni, P.; Morino, M. Transrectal sentinel lymph node biopsy for early rectal cancer during transanal endoscopic microsurgery. Minim. Invasive Ther. Allied Technol. 2014, 23, 17–20. [Google Scholar] [CrossRef]

|
Total (n = 54) |
ICG (n = 28) |
HC (n = 26) | p-Value * | |
|---|---|---|---|---|
| Operative Data | ||||
| Type of resection | ||||
| Wedge resection | 34.0 | 40.7 | 26.9 | 0.513 b |
| Segment resection | 35.8 | 44.4 | 26.9 | 0.221 b |
| Lobectomy | 5.7 | 0.0 | 11.5 | 0.135 b |
| Left hemihepatectomy | 18.9 | 14.8 | 23.1 | 0.626 b |
| Right hemihepatectomy | 5.7 | 0.0 | 11.5 | 0.031b |
| Segment resected | ||||
| I | 1.0 | 2.1 | 0.0 | 0.331 b |
| II | 19.4 | 17.0 | 21.6 | 0.178 b |
| III | 20.4 | 14.9 | 25.5 | 0.181 b |
| IV | 10.2 | 6.5 | 13.7 | 0.249 b |
| V | 16.3 | 17.0 | 15.7 | 0.675 b |
| VI | 14.4 | 17.0 | 11.8 | 0.637 b |
| VII | 5.1 | 8.5 | 2.0 | 0.186 b |
| VIII | 13.3 | 17.0 | 9.8 | 0.150 b |
| Length of surgery (min) mean ± SD | 192.3 ± 97.7 | 142.7 ± 61.8 | 246.4 ± 98.6 | <0.001 a |
| Intra-operatively realized complications (yes) % | 5.6 | 10.7 | 0.0 | 0.086 b |
| Simultaneous CHE (yes) % | 40.4 | 25.0 | 53.8 | 0.030b |
| Intra-operative placement of a drain (yes) % | 75.9 | 71.4 | 80.8 | 0.422 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehdorn, A.-S.; Richter, F.; Hess, K.; Beckmann, J.H.; Egberts, J.-H.; Linecker, M.; Becker, T.; Braun, F. The Role of ICG in Robot-Assisted Liver Resections. J. Clin. Med. 2022, 11, 3527. https://doi.org/10.3390/jcm11123527
Mehdorn A-S, Richter F, Hess K, Beckmann JH, Egberts J-H, Linecker M, Becker T, Braun F. The Role of ICG in Robot-Assisted Liver Resections. Journal of Clinical Medicine. 2022; 11(12):3527. https://doi.org/10.3390/jcm11123527
Chicago/Turabian StyleMehdorn, Anne-Sophie, Florian Richter, Katharina Hess, Jan Henrik Beckmann, Jan-Hendrik Egberts, Michael Linecker, Thomas Becker, and Felix Braun. 2022. "The Role of ICG in Robot-Assisted Liver Resections" Journal of Clinical Medicine 11, no. 12: 3527. https://doi.org/10.3390/jcm11123527
APA StyleMehdorn, A.-S., Richter, F., Hess, K., Beckmann, J. H., Egberts, J.-H., Linecker, M., Becker, T., & Braun, F. (2022). The Role of ICG in Robot-Assisted Liver Resections. Journal of Clinical Medicine, 11(12), 3527. https://doi.org/10.3390/jcm11123527






