Real-World Analysis of the Therapeutic Management and Disease Burden in Chronic Myeloid Leukemia Patients with Later Lines in Italy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Source
2.2. Study Periods
2.3. Study Population
2.4. Study Variables
3. Results
3.1. Baseline Characteristics
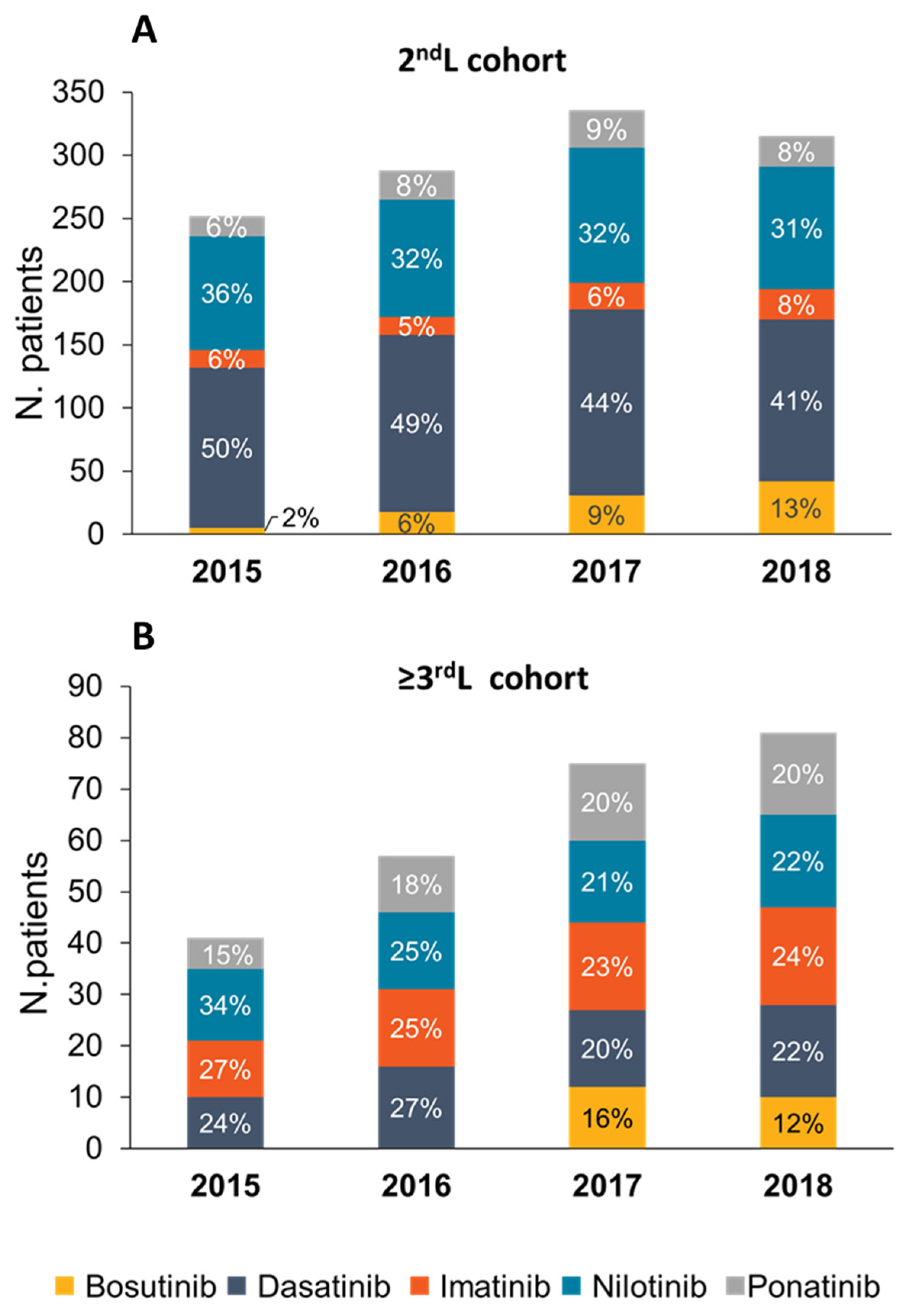
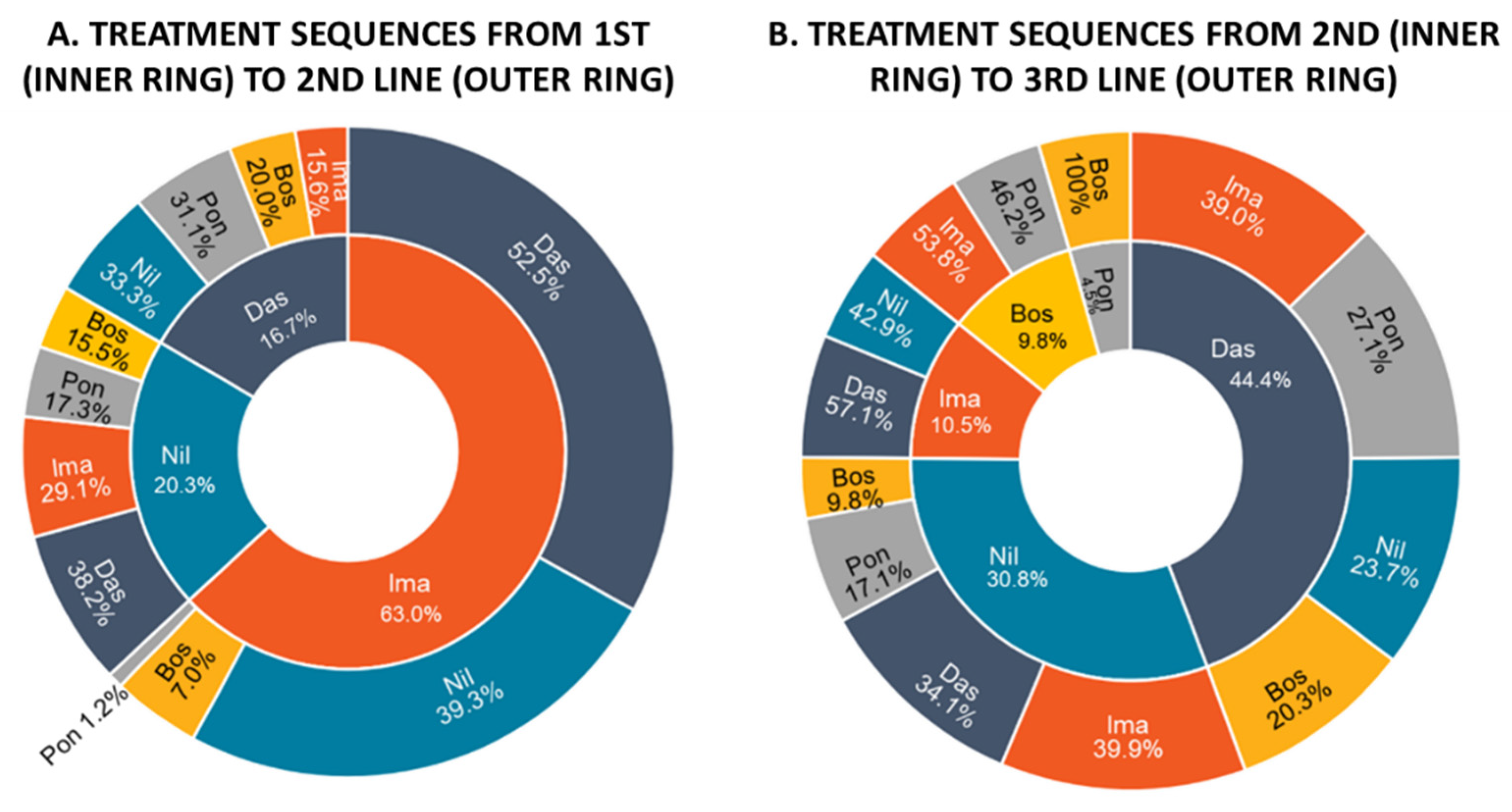
3.2. Treatment Patterns and Drug Utilization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hochhaus, A.; Saussele, S.; Rosti, G.; Mahon, F.-X.; Janssen, J.J.W.M.; Hjorth-Hansen, H.; Richter, J.; Buske, C. Chronic Myeloid Leukaemia: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2017, 28, iv41–iv51. [Google Scholar] [CrossRef]
- Eden, R.E.; Coviello, J.M. Chronic Myelogenous Leukemia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Hochhaus, A.; Baccarani, M.; Silver, R.T.; Schiffer, C.; Apperley, J.F.; Cervantes, F.; Clark, R.E.; Cortes, J.E.; Deininger, M.W.; Guilhot, F.; et al. European LeukemiaNet 2020 Recommendations for Treating Chronic Myeloid Leukemia. Leukemia 2020, 34, 966–984. [Google Scholar] [CrossRef] [Green Version]
- Di Maio, M.; Perrone, F.; Conte, P. Real-World Evidence in Oncology: Opportunities and Limitations. Oncologist 2020, 25, e746–e752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, P.; Kantarjian, H.; Alattar, M.L.; Jabbour, E.; Sasaki, K.; Gonzalez, G.N.; Dellasala, S.; Pierce, S.; Verstovsek, S.; Wierda, W.; et al. Analysis of Long Term Responses and Their Impact on Outcomes in Patients with Chronic Phase CML Treated with Four Different TKI Modalities—Analysis of 5 Prospective Clinical Trials. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4854752/ (accessed on 21 June 2022).
- Jain, P.; Kantarjian, H.M.; Ghorab, A.; Sasaki, K.; Jabbour, E.J.; Nogueras Gonzalez, G.; Kanagal-Shamanna, R.; Issa, G.C.; Garcia-Manero, G.; Kc, D.; et al. Prognostic Factors and Survival Outcomes in Patients with Chronic Myeloid Leukemia in Blast Phase in the Tyrosine Kinase Inhibitor Era: Cohort Study of 477 Patients. Cancer 2017, 123, 4391–4402. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Kantarjian, H.; Alattar, M.L.; Jabbour, E.; Sasaki, K.; Nogueras Gonzalez, G.; Dellasala, S.; Pierce, S.; Verstovsek, S.; Wierda, W.; et al. Long-Term Molecular and Cytogenetic Response and Survival Outcomes with Imatinib 400 Mg, Imatinib 800 Mg, Dasatinib, and Nilotinib in Patients with Chronic-Phase Chronic Myeloid Leukaemia: Retrospective Analysis of Patient Data from Five Clinical Trials. Available online: https://www.thelancet.com/journals/lanhae/article/PIIS2352-3026(15)00021-6/fulltext (accessed on 21 June 2022).
- Annunziata, M.; Bonifacio, M.; Breccia, M.; Castagnetti, F.; Gozzini, A.; Iurlo, A.; Pregno, P.; Stagno, F.; Specchia, G. Current Strategies and Future Directions to Achieve Deep Molecular Response and Treatment-Free Remission in Chronic Myeloid Leukemia. Front. Oncol. 2020, 10, 883. [Google Scholar] [CrossRef]
- Baccarani, M.; Abruzzese, E.; Accurso, V.; Albano, F.; Annunziata, M.; Barulli, S.; Beltrami, G.; Bergamaschi, M.; Binotto, G.; Bocchia, M.; et al. Managing Chronic Myeloid Leukemia for Treatment-Free Remission: A Proposal from the GIMEMA CML WP. Blood Adv. 2019, 3, 4280–4290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Booth, C.M.; Karim, S.; Mackillop, W.J. Real-World Data: Towards Achieving the Achievable in Cancer Care. Nat. Rev. Clin. Oncol. 2019, 16, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.; Lang, F. Third-Line Therapy for Chronic Myeloid Leukemia: Current Status and Future Directions. J. Hematol. Oncol. 2021, 14, 44. [Google Scholar] [CrossRef] [PubMed]
- Gambacorti-Passerini, C.; Coutre, P.L.; Piazza, R. The Role of Bosutinib in the Treatment of Chronic Myeloid Leukemia. Future Oncol. 2020, 16, 4395–4408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruppo Italiano Malattie EMatologiche dell’Adulto. Bosutinib Efficacy, Safety, Tolerability (BEST) Study in Elderly Chronic Myeloid Leukemia Patients Failing Front.-Line Treatment With Other Tyrosine Kinase Inhibitors. Available online: https://clinicaltrials.gov/ct2/show/NCT02810990 (accessed on 21 June 2022).
- Latagliata, R.; Attolico, I.; Trawinska, M.M.; Capodanno, I.; Annunziata, M.; Elena, C.; Luciano, L.; Crugnola, M.; Bergamaschi, M.; Bonifacio, M.; et al. Bosutinib in the Real-Life Treatment of Chronic Phase Chronic Myeloid Leukemia (CML) Patients Aged > 65 Years Resistant/Intolerant to Frontline Tyrosine-Kynase Inhibitors. Blood 2019, 134, 1649. [Google Scholar] [CrossRef]
- Banegas, M.P.; Rivera, D.R.; O’Keeffe-Rosetti, M.C.; Carroll, N.M.; Pawloski, P.A.; Tabano, D.C.; Epstein, M.M.; Yeung, K.; Hornbrook, M.C.; Lu, C.; et al. Long-Term Patterns of Oral Anticancer Agent Adoption, Duration, and Switching in Patients With CML. J. Natl. Compr. Cancer Netw. 2019, 17, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Milojkovic, D.; Cross, N.C.P.; Ali, S.; Byrne, J.; Campbell, G.; Dignan, F.L.; Drummond, M.; Huntly, B.; Marshall, S.; McMullin, M.F.; et al. Real-world tyrosine kinase inhibitor treatment pathways, monitoring patterns and responses in patients with chronic myeloid leukaemia in the United Kingdom: The UK TARGET CML study. Br. J. Haematol. 2021, 192, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Dahlen, T.; Kockerols, C.; Ferreira, G.; Westerweel, P.E.; Mayer, J.; Sahmoud, T.; Wormser, D.; Yau, L.; Žácková, D. Treatment Patterns in Patients with Chronic Myeloid Leukemia in Chronic Phase in the Third Line of TKI Therapy and Beyond Based on Real-World Evidence. Blood 2021, 138, 1485. [Google Scholar] [CrossRef]
- Campiotti, L.; Suter, M.B.; Guasti, L.; Piazza, R.; Gambacorti-Passerini, C.; Grandi, A.M.; Squizzato, A. Imatinib Discontinuation in Chronic Myeloid Leukaemia Patients with Undetectable BCR-ABL Transcript Level: A Systematic Review and a Meta-Analysis. Eur. J. Cancer 2017, 77, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Hochhaus, A.; Gambacorti-Passerini, C.; Abboud, C.; Gjertsen, B.T.; Brümmendorf, T.H.; Smith, B.D.; Ernst, T.; Giraldo-Castellano, P.; Olsson-Strömberg, U.; Saussele, S.; et al. Bosutinib for pretreated patients with chronic phase chronic myeloid leukemia: Primary results of the phase 4 BYOND study. Leukemia 2020, 34, 2125–2137. [Google Scholar] [CrossRef] [PubMed]
- Lomaia, E.; Zaritskey, A.; Shuvaev, V.; Martynkevich, I.; Fominykh, M.; Ovsyannikova, E.; Lazorko, N.; Matyuhina, N.; Butylin, P.; Machyulaitene, E.; et al. Efficacy of Tyrosine Kinase Inhibitors in Third Line Therapy in Chronic Phase Chronic Myeloid Leukemia. Blood 2015, 126, 4051. [Google Scholar] [CrossRef]
- Atallah, E.; Schiffer, C.A. Discontinuation of tyrosine kinase inhibitors in chronic myeloid leukemia: When and for whom? Haematologica 2020, 105, 2738–2745. [Google Scholar] [CrossRef] [PubMed]
- Bower, H.; Björkholm, M.; Dickman, P.W.; Höglund, M.; Lambert, P.C.; Andersson, T.M.-L. Life Expectancy of Patients With Chronic Myeloid Leukemia Approaches the Life Expectancy of the General Population. J. Clin. Oncol. 2016, 34, 2851–2857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negi, H.; Agrawal, R.; Vieira, J.; Ryan, J.; Thakur, D.; Viana, R. PCN231 Humanistic and Economic Burden in Patients with Chronic Myeloid Leukemia –A Review of the Literature. Value Health 2021, 24, S63. [Google Scholar] [CrossRef]

| (a) 2nd L Cohort | Bosutinib | Dasatinib | Imatinib | Nilotinib | Ponatinib | Overall |
| N. of patients | 60 | 201 | 38 | 142 | 50 | 491 |
| Age (mean, SD) | 68.2 (10.4) | 60.3 (14.3) | 63.0 (15.6) | 60.4 (15.4) | 56.1 (16.3) | 61.1 (14.8) |
| Male (n, %) | 32 (53.3) | 119 (59.2) | 25 (65.8) | 74 (52.1) | 32 (64.0) | 282 (57.4) |
| Comorbidities 1 | ||||||
| Hypertension (n, %) | 55 (91.7) | 131 (65.2) | 33 (86.8) | 91 (64.1) | 34 (68.0) | 344 (70.1) |
| Cardiovascular (n, %) | 28 (46.7) | 33 (16.4) | 14 (36.8) | 28 (19.7) | 9 (18.0) | 112 (22.8) |
| Ischemic heart disease | 10 (16.7) | 20 (10.0) | 8 (21.1) | 11 (7.7) | 5 (10.0) | 54 (11.0) |
| Diseases of pulmonary circulation | 0 (0.0) | 0 (0.0) | NR | NR | 0 (0.0) | NR |
| Other forms of heart disease | 17 (28.3) | 14 (7.0) | 8 (21.1) | 18 (12.7) | 5 (10.0) | 62 (12.6) |
| Cerebrovascular disease | 13 (21.7) | 11 (5.5) | 7 (18.4) | 8 (5.6) | NR | 42 (8.6) |
| Diseases of veins and lymphatics | NR | NR | 0 (0.0) | NR | NR | 7 (1.4) |
| Pneumonia and pleurisy (n, %) | 13 (21.7) | 12 (6.0) | NR | 23 (16.2) | 15 (30.0) | 66 (13.4) |
| Pneumonia and influenza | NR | NR | NR | 7 (4.9) | 6 (12.0) | 18 (3.7) |
| COPD and allied condition | NR | 5 (2.5) | 0 (0.0) | 7 (4.9) | 5 (10.0) | 20 (4.1) |
| Other diseases respiratory system | 10 (16.7) | 8 (4.0) | NR | 17 (12.0) | 7 (14.0) | 45 (9.2) |
| Gastrointestinal (n, %) | 5 (8.3) | 15 (7.5) | NR | 11 (7.7) | 6 (12.0) | 40 (8.1) |
| Liver (n, %) | NR | 12 (6.0) | 0 (0.0) | 5 (3.5) | 4 (8.0) | 24 (4.9) |
| Renal (n, %) | 4 (6.7) | 5 (2.5) | 4 (10.5) | 4 (2.8) | 6 (12.0) | 23 (4.7) |
| Edema (n, %) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Blood count alterations (n, %) | 29 (48.3) | 57 (28.4) | 13 (34.2) | 47 (33.1) | 29 (58.0) | 175 (35.6) |
| Metabolic alterations (n, %) | 33 (55.0) | 56 (27.9) | 24 (63.2) | 48 (33.8) | 16 (32.0) | 177 (36.0) |
| Pre-index period, years (mean, SD) | 6.3 (1.9) | 5.6 (1.6) | 5.6 (2.0) | 5.5 (1.8) | 5.5 (1.9) | 5.7 (1.8) |
| (b) ≥3rd L Cohort | Bosutinib | Dasatinib | Imatinib | Nilotinib | Ponatinib | Overall |
| N. of patients | 24 | 23 | 38 | 27 | 32 | 144 |
| Age (mean, SD) | 69.5 (9.8) | 63.4 (16.6) | 64.6 (12.5) | 57.0 (14.7) | 64.8 (12.9) | 63.8 (13.7) |
| Male (n, %) | 15 (62.5) | 14 (60.9) | 16 (42.1) | 12 (44.4) | 13 (40.6) | 70 (48.6) |
| Comorbidities 1 | ||||||
| Hypertension (n, %) | 24 (100.0) | 15 (65.2) | 30 (78.9) | 19 (70.4) | 26 (81.3) | 114 (79.2) |
| Cardiovascular (n, %) | 13 (54.2) | 6 (26.1) | 14 (36.8) | 6 (22.2) | 12 (37.5) | 51 (35.4) |
| Ischemic heart disease | 7 (29.2) | NR | 6 (15.8) | NR | 4 (12.5) | 22 (15.3) |
| Diseases of pulmonary circulation | 0 (0.0) | NR | 0 (0.0) | NR | NR | NR |
| Other forms of heart disease | 8 (33.3) | NR | 10 (26.3) | NR | 9 (28.1) | 31 (21.5) |
| Cerebrovascular disease | 5 (20.8) | NR | 5 (13.2) | NR | 4 (12.5) | 17 (11.8) |
| Diseases of veins and lymphatics | NR | 0 (0.0) | 0 (0.0) | NR | NR | NR |
| Pneumonia and pleurisy (n, %) | 11 (45.8) | 4 (17.4) | 10 (26.3) | 6 (22.2) | 5 (15.6) | 36 (25.0) |
| Pneumonia and influenza | NR | NR | 0 (0.0) | NR | NR | 10 (6.9) |
| COPD and allied conditions | NR | 0 (0.0) | 4 (10.5) | NR | NR | 10 (6.9) |
| Other diseases respiratory system | 8 (33.3) | NR | 9 (23.7) | 4 (14.8) | NR | 26 (18.1) |
| Gastrointestinal (n, %) | NR | NR | 4 (10.5) | NR | NR | 15 (10.4) |
| Liver (n, %) | 4 (16.7) | 0 (0.0) | 4 (10.5) | NR | NR | 11 (7.6) |
| Renal (n, %) | 4 (16.7) | 0 (0.0) | NR | NR | NR | 11 (7.6) |
| Edema (n, %) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | NR | NR |
| Blood count alterations (n, %) | 14 (58.3) | 8 (34.8) | 15 (39.5) | 13 (48.1) | 19 (59.4) | 69 (47.9) |
| Metabolic alterations (n, %) | 12 (50.0) | 11 (47.8) | 18 (47.4) | 10 (37.0) | 12 (37.5) | 63 (43.8) |
| Pre-index period, years (mean, SD) | 6.6 (2.3) | 5.0 (1.9) | 5.8 (1.5) | 5.0 (2.0) | 5.8 (1.7) | 5.7 (1.9) |
| 2nd L Cohort | 2015 | 2016 | 2017 | 2018 |
| N. of patients | 252 | 288 | 336 | 315 |
| Incident to line (n, %) | 57 (22.6) | 83 (28.7) | 84 (24.9) | 74 (23.5) |
| Switch to subsequent line, same year (n, %) | 17 (6.7) | 19 (6.6) | 21 (6.2) | 22 (7.0) |
| Death, same year (n, %) | 6 (2.4) | 9 (3.1) | 10 (3.0) | 4 (1.3) |
| Interruptions (n, %) | 24 (9.1) | 8 (2.8) | 64 (19.0) | - |
| Follow-up, years (mean, SD) | 3.9 (1.2) | 3.1 (1.0) | 2.2 (0.9) | 1.6 (0.5) |
| ≥3rd L Cohort | 2015 | 2016 | 2017 | 2018 |
| N. of patients | 41 | 60 | 75 | 81 |
| Incident to line (n, %) | 18 (43.9) | 28 (46.7) | 29 (38.7) | 30 (37.0) |
| Switch to subsequent line, same year (n, %) | NR | 4 (6.7) | NR | NR |
| Death, same year (n, %) | NR | NR | 4 (5.3) | 6 (7.4) |
| Interruptions (n, %) | 7 (17.1) | 8 (13.3) | 13 (17.3) | - |
| Follow-up, years (mean, SD) | 3.9 (1.4) | 3.4 (1.0) | 2.3 (0.9) | 1.7 (0.6) |
| (a) 2nd L cohort | Bosutinib | Dasatinib | Imatinib | Nilotinib | Ponatinib | Overall |
| N. of patients | 60 | 201 | 38 | 142 | 50 | 491 |
| Incident to 2nd L (n, %) | 52 (86.7) | 91 (45.3) | 23 (60.5) | 79 (55.6) | 39 (78.0) | 284 (57.8) |
| Follow-up, years (mean, SD) | 2.1 (1.1) | 3.2 (1.4) | 2.7 (1.6) | 3.3 (1.5) | 2.1 (1.4) | 3.0 (1.5) |
| No switch to 3rd L (n, %) | 42 (70.0) | 152 (75.6) | 30 (78.9) | 119 (83.8) | 39 (78.0) | 382 (77.8) |
| Patients still in treatment (n, %) | 32 (53.3%) | 112 (55.7%) | 18 (47.4%) | 89 (62.7%) | 25 (50%) | - |
| Switch to 3rd L (n, %) | 15 (25.0) | 43 (21.4) | 6 (15.8) | 20 (14.1) | 8 (16.0) | 92 (18.7) |
| Switch to 4th L or more (n, %) | NR | 6 (3.0) | NR | NR | NR | 17 (3.5) |
| Death (n, %) | 4 (6.7) | 27 (13.4) | 4 (10.5) | 17 (12.0) | 13 (26.0) | 65 (13.2) |
| Mean treatment length (years) | 1.42 | 3.21 | 1.84 | 3.24 | 1.50 | - |
| (b) ≥3rd L cohort | Bosutinib | Dasatinib | Imatinib | Nilotinib | Ponatinib | Overall |
| N. of patients | 24 | 23 | 38 | 27 | 32 | 144 |
| Incident to 3rd L (n, %) | 21 (87.5) | 10 (43.5) | 16 (42.1) | 12 (44.4) | 23 (71.9) | 82 (56.9) |
| Follow-up, years (mean, SD) | 1.6 (1.0) | 3.7 (1.5) | 2.8 (1.4) | 3.3 (1.6) | 2.2 (1.4) | 2.7 (1.6) |
| No switch to 4th L (n, %) | 19 (79.2) | 14 (60.9) | 30 (78.9) | 17 (63.0) | 26 (81.3) | 106 (73.6) |
| Patients still in treatment (n, %) | 12 (50) | 8 (34.8) | 21 (55.3) | 14 (51.9) | 13 (40.6) | - |
| Switch to 4th L or more (n, %) | 5 (20.8) | 9 (39.1) | 8 (21.1) | 10 (37.0) | 6 (18.8) | 38 (26.4) |
| Death (n, %) | 8 (33.3) | NR | 4 (10.5) | 4 (14.8) | 10 (31.3) | 28 (19.4) |
| Mean treatment length (years) | 1.14 | 2.78 | 2.42 | 3.16 | 1.50 | - |
| (a) 2nd L Cohort | Bosutinib | Dasatinib | Imatinib | Nilotinib | Ponatinib | Overall |
| N. of patients | 60 | 201 | 38 | 142 | 50 | 491 |
| All-cause visits (mean, SD) | 8.6 (7.9) | 6.6 (5.4) | 5.4 (5.4) | 6.6 (6.6) | 8.3 (9.5) | 6.9 (6.6) |
| All-cause hospitalizations (mean, SD) | 0.7 (1.3) | 0.5 (1.1) | 0.5 (1.2) | 0.4 (1.0) | 1.1 (1.5) | 0.6 (1.1) |
| (b) ≥3rd L Cohort | Bosutinib | Dasatinib | Imatinib | Nilotinib | Ponatinib | Overall |
| N. of patients | 24 | 23 | 38 | 27 | 32 | 144 |
| All-cause visits (mean, SD) | 7.4 (6.7) | 8.0 (7.7) | 5.1 (4.9) | 4.5 (3.5) | 8.4 (6.5) | 6.6 (6.0) |
| All-cause hospitalizations (mean, SD) | 0.5 (0.9) | 0.5 (1.0) | 0.4 (1.2) | 0.4 (0.7) | 0.7 (1.2) | 0.5 (1.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breccia, M.; Chiodi, F.; Nardozza, A.P.; Valsecchi, D.; Perrone, V.; Sangiorgi, D.; Giacomini, E.; Rendace, M.C.; Coco, P.; Premoli, E.; et al. Real-World Analysis of the Therapeutic Management and Disease Burden in Chronic Myeloid Leukemia Patients with Later Lines in Italy. J. Clin. Med. 2022, 11, 3597. https://doi.org/10.3390/jcm11133597
Breccia M, Chiodi F, Nardozza AP, Valsecchi D, Perrone V, Sangiorgi D, Giacomini E, Rendace MC, Coco P, Premoli E, et al. Real-World Analysis of the Therapeutic Management and Disease Burden in Chronic Myeloid Leukemia Patients with Later Lines in Italy. Journal of Clinical Medicine. 2022; 11(13):3597. https://doi.org/10.3390/jcm11133597
Chicago/Turabian StyleBreccia, Massimo, Francesca Chiodi, Aurelio Pio Nardozza, Diletta Valsecchi, Valentina Perrone, Diego Sangiorgi, Elisa Giacomini, Maria Chiara Rendace, Paola Coco, Eleonora Premoli, and et al. 2022. "Real-World Analysis of the Therapeutic Management and Disease Burden in Chronic Myeloid Leukemia Patients with Later Lines in Italy" Journal of Clinical Medicine 11, no. 13: 3597. https://doi.org/10.3390/jcm11133597
APA StyleBreccia, M., Chiodi, F., Nardozza, A. P., Valsecchi, D., Perrone, V., Sangiorgi, D., Giacomini, E., Rendace, M. C., Coco, P., Premoli, E., & Degli Esposti, L. (2022). Real-World Analysis of the Therapeutic Management and Disease Burden in Chronic Myeloid Leukemia Patients with Later Lines in Italy. Journal of Clinical Medicine, 11(13), 3597. https://doi.org/10.3390/jcm11133597







