Forced Oscillation Measurements in Patients with Idiopathic Interstitial Pneumonia Subjected to Pulmonary Rehabilitation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Patients
2.2. Physiological Measurements
2.3. Rehabilitation Program
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Travis, W.D.; Costabel, U.; Hansell, D.M.; King, T.E., Jr.; Lynch, D.A.; Nicholson, A.G.; Ryerson, C.J.; Ryu, J.H.; Selman, M.; Wells, A.U.; et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2013, 188, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Wuyts, W.A.; Wijsenbeek, M.; Bondue, B.; Bouros, D.; Bresser, P.; Cordeiro, C.R.; Hilberg, O.; Magnusson, J.; Manali, E.D.; Morais, A.; et al. Idiopathic Pulmonary Fibrosis: Best Practice in Monitoring and Managing a Relentless Fibrotic Disease. Respiration 2019, 99, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Tan, B.; Yin, Y.; Wang, S.; Jia, L.; Warner, G.; Jia, G.; Jiang, W. Short- and long-term effects of pulmonary rehabilitation for idiopathic pulmonary fibrosis: A systematic review and meta-analysis. Clin. Rehabil. 2018, 32, 1299–1307. [Google Scholar] [CrossRef]
- Spruit, M.A.; Singh, S.J.; Garvey, C.; ZuWallack, R.; Nici, L.; Rochester, C.; Hill, K.; Holland, A.E.; Lareau, S.C.; Man, W.D.; et al. ATS/ERS Task Force on Pulmonary Rehabilitation. An official American Thoracic Socie-ty/European Respiratory Society statement: Key concepts and advances in pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 2013, 188, e13–e64. [Google Scholar] [CrossRef] [PubMed]
- Vainshelboim, B.; Oliveira, J.; Yehoshua, L.; Weiss, I.; Fox, B.D.; Fruchter, O.; Kramer, M.R. Exercise training-based pulmonary rehabili-tation program is clinically beneficial for idiopathic pulmonary fibrosis. Respiration 2014, 88, 378–388. [Google Scholar] [CrossRef]
- Wallaert, B.; Duthoit, L.; Drumez, E.; Behal, H.; Wemeau, L.; Chenivesse, C.; Grosbois, J.M. Long-term evaluation of home-based pul-monary rehabilitation in patients with fibrotic idiopathic interstitial pneumonias. ERJ Open Res. 2019, 5, 00045-2019. [Google Scholar] [CrossRef] [PubMed]
- Huppmann, P.; Sczepanski, B.; Boensch, M.; Winterkamp, S.; Schönheit-Kenn, U.; Neurohr, C.; Behr, J.; Kenn, K. Effects of inpatient pulmonary rehabilitation in patients with interstitial lung disease. Eur. Respir. J. 2012, 42, 444–453. [Google Scholar] [CrossRef] [Green Version]
- Ryerson, C.J.; Cayou, C.; Topp, F.; Hilling, L.; Camp, P.G.; Wilcox, P.G.; Khalil, N.; Collard, H.R.; Garvey, C. Pulmonary rehabilitation improves long-term outcomes in interstitial lung disease: A prospective cohort study. Respir. Med. 2014, 108, 203–210. [Google Scholar] [CrossRef] [Green Version]
- Raghu, G.; Collard, H.R.; Egan, J.J.; Martinez, F.J.; Behr, J.; Brown, K.K.; Colby, T.V.; Cordier, J.-F.; Flaherty, K.R.; Lasky, J.A.; et al. An Official ATS/ERS/JRS/ALAT Statement: Idiopathic Pulmonary Fibrosis: Evidence-based Guidelines for Diagnosis and Management. Am. J. Respir. Crit. Care Med. 2011, 183, 788–824. [Google Scholar] [CrossRef] [Green Version]
- Nakazawa, A.; Cox, N.; Holland, A.E. Current best practice in rehabilitation in interstitial lung disease. Ther. Adv. Respir. Dis. 2017, 11, 115–128. [Google Scholar] [CrossRef]
- Gaunaurd, I.A.; Gómez-Marín, O.W.; Ramos, C.F.; Sol, C.M.; Cohen, M.I.; Cahalin, L.P.; Cardenas, D.D.; Jackson, R.M. Physical Activity and Quality of Life Improvements of Patients with Idiopathic Pulmonary Fibrosis Completing a Pulmonary Rehabilitation Program. Respir. Care 2014, 59, 1872–1879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishiyama, O.; Kondoh, Y.; Kimura, T.; Kato, K.; Kataoka, K.; Ogawa, T.; Watanabe, F.; Arizono, S.; Nishimura, K.; Taniguchi, H. Effects of pulmonary rehabilitation in patients with idiopathic pulmonary fibrosis. Respirology 2008, 13, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Ozalevli, S.; Karaali, H.K.; Ilgin, D.; Ucan, E.S. Effect of home-based pulmonary rehabilitation in patients with idiopathic pulmonary fibrosis. Multidiscip. Respir. Med. 2010, 5, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Holland, A.E.; Dowman, L.M.; Hill, C.J. Principles of Rehabilitation and Reactivation: Interstitial Lung Disease, Sarcoidosis and Rheumatoid Disease with Respiratory Involvement. Respiration 2015, 89, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.E.; Hill, C.J.; Conron, M.; Munro, P.; McDonald, C.F. Short term improvement in exercise capacity and symptoms following exercise training in interstitial lung disease. Thorax 2008, 63, 549–554. [Google Scholar] [CrossRef] [Green Version]
- National Institute for Health and Care Excellence (NICE). Diagnosis and Management of Suspected Idiopathic Pulmonary Fibrosis: Idiopathic Pulmonary Fibrosis; Nice Clinical Guideline 163; Royal College of Physicians: London, UK, 2013. [Google Scholar]
- du Bois, R.M.; Albera, C.; Bradford, W.Z.; Costabel, U.; Leff, J.A.; Noble, P.W.; Sahn, S.A.; Valeyre, D.; Weycker, D.; King, T.E., Jr. 6-Minute walk distance is an independent predictor of mortality in patients with idiopathic pulmonary fibrosis. Eur. Respir. J. 2014, 43, 1421–1429. [Google Scholar] [CrossRef] [Green Version]
- Flaherty, K.R.; Andrei, A.C.; Murray, S.; Fraley, C.; Colby, T.V.; Travis, W.D.; Lama, V.; Kazerooni, E.A.; Gross, B.H.; Toews, G.B.; et al. Idiopathic pulmonary fibrosis: Prognostic value of changes in physiology and six-minute-walk test. Am. J. Respir. Crit. Care Med. 2006, 174, 803–809. [Google Scholar] [CrossRef] [Green Version]
- Dowman, L.; McDonald, C.F.; Hill, C.J.; Lee, A.L.; Barker, K.; Boote, C.; Glaspole, I.; Goh, N.S.L.; Southcott, A.M.; Burge, A.T.; et al. The evidence of benefits of exercise training in interstitial lung disease: A randomised controlled trial. Thorax 2017, 72, 610–619. [Google Scholar] [CrossRef] [Green Version]
- Tomalak, W.; Radliński, J.; Latawiec, W. Jakość badania spirometrycznego u dzieci 10-letnich i młodszych w świetle zaleceń standaryzowanych. Pneumonol. Alergol. Pol. 2008, 6, 421–425. [Google Scholar] [CrossRef]
- Czajkowska-Malinowska, M.; Tomalak, W.; Radliński, J. Quality of spirometry in elderly. Pneumonol. Alergol. Pol. 2013, 81, 511–517. [Google Scholar] [CrossRef]
- Kaminsky, D.A.; Simpson, S.J.; Berger, K.I.; Calverley, P.; de Melo, P.L.; Dandurand, R.; Dellacà, R.L.; Farah, C.S.; Farré, R.; Hall, G.L.; et al. Clinical significance and applications of oscillometry. Eur. Respir. Rev. 2022, 31, 210208. [Google Scholar] [CrossRef] [PubMed]
- Brashier, B.; Salvi, S. Measuring lung function using sound waves: Role of the forced oscillation technique and impulse oscillometry system. Breathe 2015, 11, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Oostveen, E.; MacLeod, D.; Lorino, H.; Farre, R.; Hantos, Z.; Desager, K.; Marchal, F. The forced oscillation technique in clinical practice: Methodology, recommendations and future developments. Eur. Respir. J. 2003, 22, 1026–1041. [Google Scholar] [CrossRef] [PubMed]
- Urbankowski, T.; Przybyłowski, T. Methods of airway resistance assessment. Pneumonol. Alergol. Polska 2016, 84, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Goldman, M.D. Clinical Application of Forced Oscillation. Pulm. Pharmacol. Ther. 2001, 14, 341–350. [Google Scholar] [CrossRef]
- King, G.G.; Bates, J.; Berger, K.I.; Calverley, P.; De Melo, P.L.; Dellacà, R.L.; Farre, R.; Hall, G.; Ioan, I.; Irvin, C.G.; et al. Technical standards for respiratory oscillometry. Eur. Respir. J. 2020, 55, 1900753. [Google Scholar] [CrossRef]
- Smith, H.; Reinhold, P.; Goldman, M. Forced oscillation technique and impulse oscillometry. Eur. Respir. Monogr. 2005, 31, 72–105. [Google Scholar] [CrossRef] [Green Version]
- Kaczka, D.W.; Dellacá, R.L. Oscillation mechanics of the respiratory system: Applications to lung disease. Crit. Rev. Biomed. Eng. 2011, 39, 337–359. [Google Scholar] [CrossRef] [Green Version]
- Sugiyama, A.; Hattori, N.; Haruta, Y.; Nakamura, I.; Nakagawa, M.; Miyamoto, S.; Onari, Y.; Iwamoto, H.; Ishikawa, N.; Fujitaka, K.; et al. Characteristics of inspiratory and expiratory reactance in interstitial lung disease. Respir. Med. 2013, 107, 875–882. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.; Long, G.; Cançado, J.E.D.; Higham, A. Small airway disease in chronic obstructive pulmonary disease: Insights and im-plications for the clinician. Curr. Opin. Pulm. Med. 2020, 26, 162–168. [Google Scholar] [CrossRef]
- Takeichi, N.; Yamazaki, H.; Fujimoto, K. Comparison of impedance measured by the forced oscillation technique and pulmonary functions, including static lung compliance, in obstructive and interstitial lung disease. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 1109–1118. [Google Scholar] [CrossRef] [Green Version]
- Dandurand, R.; Dandurand, M.; Estepar, R.; Bourbeau, J.; Eidelman, D. Oscillometry in community practice ild is characterized by abnormal reactance but normal resistance. Quart. J. Med. 2016, 109, S50. [Google Scholar]
- Lundblad, L.K.A.; Robichaud, A. Oscillometry of the respiratory system: A translational opportunity not to be missed. Am. J. Physiol. Cell. Mol. Physiol. 2021, 320, L1038–L1056. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, S.C.; Thamrin, C.; Chan, A.S.; Bertolin, A.; Chapman, D.G.; King, G.G. Relationships Between Forced Oscillatory Im-pedance and 6-minute Walk Distance After Pulmonary Rehabilitation in COPD. Int. J. Chron. Obs. Pulmon. Dis. 2020, 15, 157–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, K.; Shirai, T.; Mikamo, M.; Shishido, Y.; Akita, T.; Morita, S.; Asada, K.; Fujii, M.; Hozumi, H.; Suda, T.; et al. Respiratory mechanics measured by forced oscillation technique in combined pulmonary fibrosis and emphysema. Respir. Physiol. Neurobiol. 2013, 185, 235–240. [Google Scholar] [CrossRef]
- Mikamo, M.; Fujisawa, T.; Oyama, Y.; Kono, M.; Enomoto, N.; Nakamura, Y.; Inui, N.; Sumikawa, H.; Johkoh, T.; Suda, T. Clinical Significance of Forced Oscillation Technique for Evaluation of Small Airway Disease in Interstitial Lung Diseases. Lung 2016, 194, 975–983. [Google Scholar] [CrossRef]
- Hu, P.-W.; Ko, H.-K.; Su, K.-C.; Feng, J.-Y.; Su, W.-J.; Hsiao, Y.-H.; Perng, D.-W. Functional parameters of small airways can guide bronchodilator use in idiopathic pulmonary fibrosis. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- American Thoracic Society (ATS). European Respiratory Society (ERS): Idiopathic pulmonary fibrosis: Diagnosis and treatment—international consensus statement. Am. J. Respir. Crit. Care. Med. 2000, 161, 646–664. [Google Scholar] [CrossRef] [Green Version]
- Piotrowski, W.J.; Bestry, I.; Białas, A.J.; Boros, P.W.; Grzanka, P.; Jassem, E.; Jastrzębski, D.; Klimczak, D.; Langfort, R.; Lewandowska, K.; et al. Guidelines of the Polish Respiratory Society for diagnosis and treatment of idiopathic pulmonary fibrosis. Adv. Respir. Med. 2020, 88, 41–93. [Google Scholar] [CrossRef] [Green Version]
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.M.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wanger, J.; Clausen, J.L.; Coates, A.; Pedersen, O.F.; Brusasco, V.; Burgos, F.; Casaburi, R.; Crapo, R.; Enright, P.; Van Der Grinten, C.P.M.; et al. Standardisation of the measurement of lung volumes. Eur. Respir. J. 2005, 26, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Sallinen, J.; Stenholm, S.; Rantanen, T.; Heliövaara, M.; Sainio, P.; Koskinen, S. Hand-Grip Strength Cut Points to Screen Older Persons at Risk for Mobility Limitation. J. Am. Geriatr. Soc. 2010, 58, 1721–1726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, B.L.; Brusasco, V.; Burgos, F.; Cooper, B.G.; Jensen, R.; Kendrick, A.; MacIntyre, N.R.; Thomspon, B.R.; Wagner, J. ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur. Respir. J. 2017, 49, 1600016. [Google Scholar] [CrossRef] [Green Version]
- Oostveen, E.; Boda, K.; Van Der Grinten, C.P.; James, A.L.; Young, S.; Nieland, H.; Hantos, Z. Respiratory impedance in healthy subjects: Baseline values and bronchodilator response. Eur. Respir. J. 2013, 42, 1513–1523. [Google Scholar] [CrossRef]
- Jastrzebski, D.; Gumola, A.; Gawlik, R.; Kozielski, J. Dyspnea and quality of life in patients with pulmonary fibrosis after six weeks of respiratory rehabilitation. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2006, 57, 139–148. [Google Scholar]
- Jastrzebski, D.; Kozielski, J.; Zebrowska, A. Pulmonary rehabilitation in patients with idiopathic pulmonary fibrosis with inspiratory muscle training. Pneumonol. Alergol. Polska 2008, 76, 131–141. [Google Scholar] [CrossRef]
- Kimihiro, N.; Gel, Y.R.; Brunner, E.; Konietschke, F. NparLD: An R Software Package for the Nonparametric Analysis of Longitudinal Data in Factorial Experiments. J. Stat. Softw. 2012, 50, 1–23. [Google Scholar]
- Collard, H.R. The age of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2010, 181, 771–772. [Google Scholar] [CrossRef]
- King, T.E., Jr.; Bradford, W.Z.; Castro-Bernardini, S.; Fagan, E.A.; Glaspole, I.; Glassberg, M.K.; Gorina, E.; Hopkins, P.M.; Kardatzke, D.; Lancaster, L.; et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014, 370, 2083–2092. [Google Scholar] [CrossRef] [Green Version]
- Richeldi, L.; Du Bois, R.M.; Raghu, G.; Azuma, A.; Brown, K.K.; Costabel, U.; Cottin, V.; Flaherty, K.R.; Hansell, D.M.; Inoue, Y.; et al. Efficacy and Safety of Nintedanib in Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2014, 370, 2071–2082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.; Dai, H.P.; Kang, J.; Chen, B.Y.; Sun, T.Y.; Xu, Z.J. Double-Blind Randomized Trial of Pirfenidone in Chinese Idiopathic Pulmonary Fibrosis Patients. Medicine 2015, 94, e1600. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.-Y.; Kim, D.S.; Song, J.W. Efficacy and Safety of Pirfenidone in Advanced Idiopathic Pulmonary Fibrosis. Respiration 2019, 97, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Rammaert, B.; Leroy, S.; Cavestri, B.; Wallaert, B.; Grosbois, J.-M. Home-based pulmonary rehabilitation in idiopathic pulmonary fibrosis. Rev. Mal. Respir. 2011, 28, e52–e57. [Google Scholar] [CrossRef]
- Danoff, S.K.; Schonhoft, E.H. Role of support measures and palliative care. Curr. Opin. Pulm. Med. 2013, 19, 480–484. [Google Scholar] [CrossRef]
- Kostorz-Nosal, S.; Jastrzębski, D.; Ziora, D. Forced oscillation measurements in patients after lobectomy—A comparative analysis with IPF and COPD patients. Clin. Respir. J. 2020, 15, 310–319. [Google Scholar] [CrossRef]
- Pornsuriyasak, P.; Khiawwan, S.; Rattanasiri, S.; Unwanatham, N.; Petnak, T. Prevalence of small airways dysfunction in asthma with- and without-fixed airflow obstruction and chronic obstructive pulmonary disease. Asian Pac. J. Allergy Immunol. 2021, 39, 296–303. [Google Scholar] [CrossRef]
- Lopes, A.J.; Mafort, T.T.; da Cal, M.S.; Monnerat, L.B.; Litrento, P.F.; Ramos, I.; de Oliveira, R.F.J.; da Costa, C.H.; Rufino, R. Impulse Oscil-lometry Findings and Their Associations with Lung Ultrasound Signs in COVID-19 Survivors. Respir Care. 2021, 66, 1691–1698. [Google Scholar] [CrossRef]
- Yoshimi, K.; Ueki, J.; Seyama, K.; Takizawa, M.; Yamaguchi, S.; Kitahara, E.; Fukazawa, S.; Takahama, Y.; Ichikawa, M.; Takahashi, K.; et al. Pulmonary rehabilitation program including respiratory conditioning for chronic obstructive pulmonary disease (COPD): Improved hyperinflation and expiratory flow during tidal breathing. J. Thorac. Dis. 2012, 4, 259–264. [Google Scholar] [CrossRef]
- Gibson, G. Pulmonary hyperinflation a clinical overview. Eur. Respir. J. 1996, 9, 2640–2649. [Google Scholar] [CrossRef] [Green Version]
- Garfinkel, F.; Fitzgerald, R.S. The effect of hyperoxia, hypoxia and hypercapnia on FRC and occlusion pressure in human subjects. Respir. Physiol. 1978, 33, 241–250. [Google Scholar] [CrossRef]
- A Krumholz, R.; Albright, C.D. The compliance of the chest wall and thorax in emphysema. Am. Rev. Respir. Dis. 1968, 97, 827–831. [Google Scholar] [CrossRef] [PubMed]
- Jack, K.; McLean, S.M.; Moffett, J.K.; Gardiner, E. Barriers to treatment adherence in physiotherapy outpatient clinics: A systematic review. Man. Ther. 2010, 15, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Morera-Balaguer, J.; Botella-Rico, J.M.; Martínez-González, M.C.; Medina-Mirapeix, F.; Rodríguez-Nogueira, Ó. Physical therapists’ perceptions and experiences about barriers and facilitators of therapeutic patient-centred relationships during outpatient reha-bilitation: A qualitative study. Braz. J. Phys. Ther. 2018, 22, 484–492. [Google Scholar] [CrossRef]
- Salhi, B.; Troosters, T.; Behaegel, M.; Joos, G.; Derom, E. Effects of Pulmonary Rehabilitation in Patients with Restrictive Lung Diseases. Chest 2010, 137, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.E.; Hill, C.J.; Conron, M.; Munro, P.; McDonald, C.F. Small changes in six-minute walk distance are important in diffuse parenchymal lung disease. Respir. Med. 2009, 103, 1430–1435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swigris, J.J.; Wamboldt, F.S.; Behr, J.; du Bois, R.M.; King, T.E.; Raghu, G.; Brown, K.K. Brown. The six-minute walk in idiopathic pulmonary fibrosis: Longitudinal changes and minimum important difference. Thorax 2010, 65, 173–177. [Google Scholar] [CrossRef] [Green Version]
- du Bois, R.M.; Weycker, D.; Albera, C.; Bradford, W.Z.; Costabel, U.; Kartashov, A.; Lancaster, L.; Noble, P.W.; Sahn, S.A.; Szwarcberg, J.; et al. Faculty Opinions recommendation of Six-minute-walk test in idiopathic pulmonary fibrosis: Test validation and minimal clinically important difference. Am. J. Respir. Crit. Care Med. 2011, 183, 1231–1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dowman, L.; Hill, C.J.; Holland, A.E. Pulmonary rehabilitation for interstitial lung disease. Cochrane Database Syst. Rev. 2014, 6, CD006322. [Google Scholar] [CrossRef]
- Mura, M.; Porretta, M.A.; Bargagli, E.; Sergiacomi, G.; Zompatori, M.; Sverzellati, N.; Taglieri, A.; Mezzasalma, F.; Rottoli, P.; Saltini, C.; et al. Predicting survival in newly diagnosed idiopathic pulmonary fibrosis: A 3-year prospective study. Eur. Respir. J. 2012, 40, 101–109. [Google Scholar] [CrossRef]
- Markovitz, G.H.; Cooper, C.B. Exercise and interstitial lung disease. Curr. Opin. Pulm. Med. 1998, 4, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, O.; Taniguchi, H.; Kondoh, Y.; Kimura, T.; Ogawa, T.; Watanabe, F.; Arizono, S. Quadriceps Weakness Is Related to Exercise Capacity in Idiopathic Pulmonary Fibrosis. Chest 2005, 127, 2028–2033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eaton, T.; Withy, S.; Garrett, J.E.; Whitlock, R.M.L.; Rea, H.H.; Mercer, J. Spirometry in Primary Care Practice. Chest 1999, 116, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Ram, J.; Pineda-Cely, J.; Calhoun, W.J. Forced Oscillometry: A New Tool for Assessing Airway Function-Is It Ready for Prime Time? J. Allergy Clin. Immunol. Pract. 2019, 7, 2861–2862. [Google Scholar] [CrossRef]
- Hellinckx, J.; Cauberghs, M.; de Boeck, K.; Demedts, M. Evaluation of Impulse Oscillation System: Comparison with Forced Oscil-lation Technique and Body Plethysmography. Eur. Respir. J. 2001, 18, 564–570. [Google Scholar] [CrossRef] [Green Version]
- Meier-Sydow, J.; Rust, M.G.; Kappos, A.; Kronenberger, H.; Nerger, K.; Schultze-Werninghaus, G. The Long-term Course of Airflow Obstruction in Obstructive Variants of the Fibrotic Stage of Sarcoidosis and of Idiopathic Pulmonary Fibrosis. Ann. N. Y. Acad. Sci. 1986, 465, 515–522. [Google Scholar] [CrossRef]
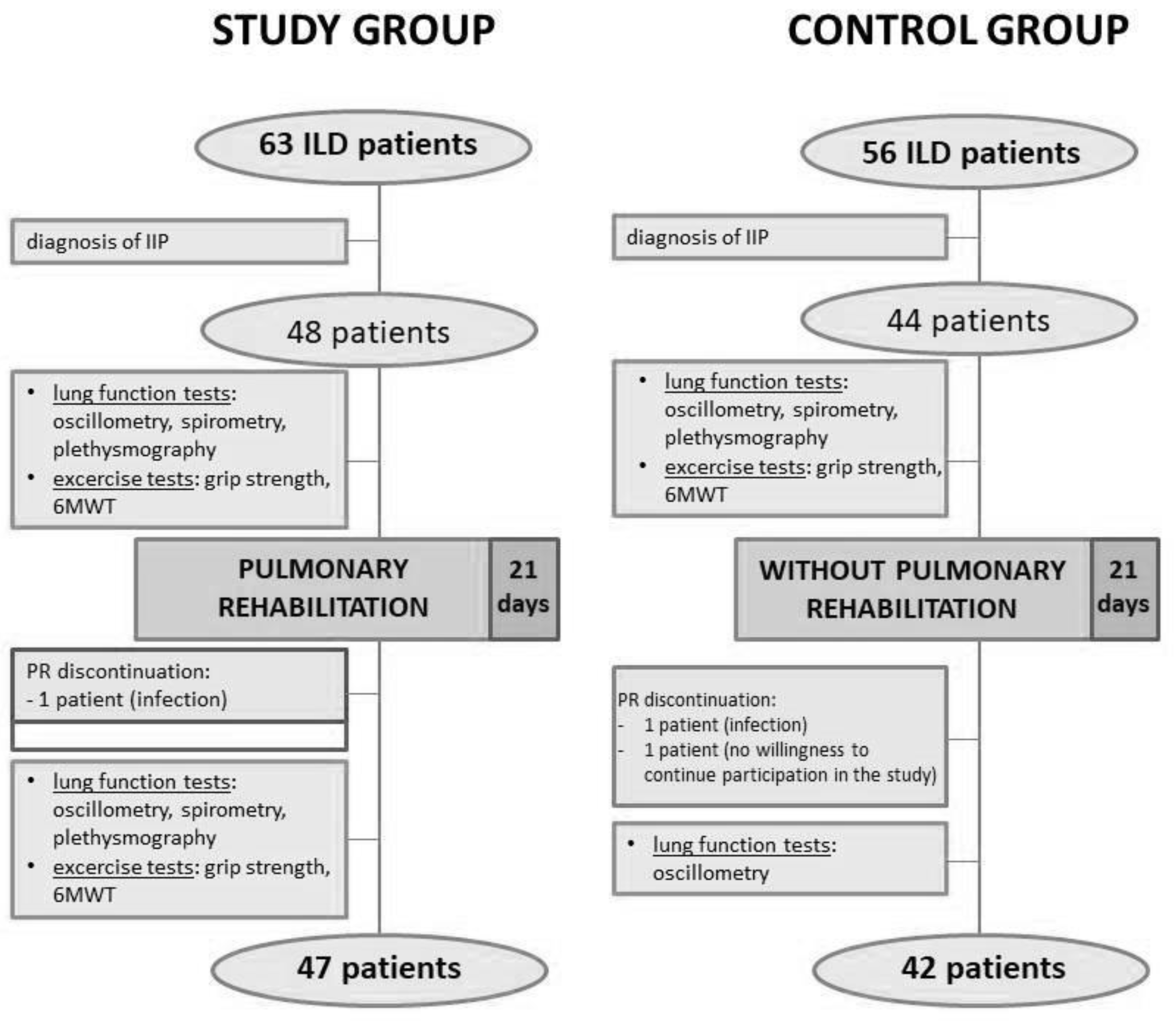


| Parameter | Study Group (48) | Control Group (44) | p |
|---|---|---|---|
| Sex | Female: 18 (37.5%) | Female: 6 (13.6%) | 0.01 * |
| Male: 30 (62.5%) | Male: 38 (86.4%) | ||
| Age (years) | 66.0 ± 7.4 | 66.7 ± 8.5 | 0.711 |
| BMI (kg/m2) | 28.3 ± 4.4 | 28.4 ± 4.3 | 0.913 |
| Idiopathic interstitial pneumonia type | IPF: 30 NSIP: 9 Unclassifiable IIP: 9 | IPF: 28 Unclassifiable IIPs: 16 | - |
| Comorbidities | - | ||
| Obstructive lung diseases | 3 (6%) | 2 (5%) | |
| Emphysema | 3 (6%) | 4 (9%) | |
| Cardiovascular diseases | 38 (79%) | 31 (70%) | |
| Diabetes mellitus | 6 (13%) | 11 (25%) | |
| Musculoskeletal disorders | 26 (54%) | 18 (41%) | |
| Gastrointestinal disorders | 14 (29%) | 10 (23%) | |
| Urogenital disorders | 15 (31%) | 19 (43%) | |
| Psychiatric disorders | 4 (8%) | - | |
| Smoking history | - | ||
| current | 1 (2%) | 3 (7%) | |
| previous | 24 (50%) | 26 (59%) | |
| never | 23 (48%) | 15 (34%) | |
| Antifibrotic treatment | total: 26 (54%) | total: 21 (48%) | - |
| pirfenidone: 22 (46%) | pirfenidone: 15 (34%) | ||
| nintedanib: 4 (8%) | nintedanib: 6 (14%) | ||
| Long-term oxygen therapy | 7 (15%) | 4 (9%) | - |
| Oral steroid consumption | 8 (17%) | 8 (18%) | - |
| Use of bronchodilator | 12 (25%) | 6 (14%) | - |
| Study Group | Control Group | p | |
|---|---|---|---|
| TLCO (%pred.) | 56.7 ± 20.8 | 58.7 ± 22.4 | 0.673 * |
| FEV1 (%pred.) | 82.9 ± 20.3 | 86.6 ± 22.7 | 0.415 * |
| FVC (%pred.) | 81.5 ± 19.8 | 84.7 ± 20.5 | 0.468 * |
| FEV1/FVC (%) | 80.9 ± 8.4 | 80.0 ± 8.3 | 0.587 # |
| 6MWD (m) | 439.6 ± 86.7 | 463.4 ± 62.0 | 0.192 * |
| SpO2 (%) | 95.1 ± 1.9 | 94.4 ± 2.9 | 0.536 # |
| RV/TLC (%pred.) | 106.0 ± 31.6 | 100.3 ± 22.7 | 0.366 * |
| RV (%pred.) | 78.5 ± 29.3 | 75.8 ± 25.8 | 0.662 * |
| TLC (%pred.) | 73.2 ± 15.7 | 73.4 ± 13.4 | 0.955 * |
| Raw (%pred.) | 115.2 ± 69.7 | 112.9 ± 36.4 | 0.422 # |
| RH strength (kg) | 32.3 ± 11.9 | 37.6 ± 10.2 | 0.052 # |
| LH strength (kg) | 30.1 ± 11.1 | 34.8 ± 10.0 | 0.078 # |
| Parameter | Study Group | Control Group | nparLD Test * | ||||
|---|---|---|---|---|---|---|---|
| Baseline | After 3-Week PR | Baseline | After 3-Week Interval | Study vs. Control Group (p) | Changes in 3-Week in Both Groups (p) | Differences in 3-Week Changes between Study and Control Group (p) | |
| Rinsp5 (%pred.) | 86.29 ± 30.6 | 84.93 ± 24.7 | 84.13 ± 26.0 | 83.92 ± 20.2 | 0.876 | 0.329 | 0.741 |
| Rexp5 (%pred.) | 105.26 ± 36.8 | 105.07 ± 31.5 | 96.23 ± 36.5 | 101.8 ± 41.0 | 0.28 | 0.283 | 0.588 |
| Rtot5 (%pred.) | 97.03 ± 32.0 | 96.18 ± 26.2 | 90.8 ± 30.0 | 94.13 ± 30.5 | 0.499 | 0.247 | 0.518 |
| Rinsp11 (%pred.) | 93.78 ± 26.0 | 91.58 ± 22.0 | 88.69 ± 24.8 | 88.44 ± 20.2 | 0.508 | 0.916 | 0.787 |
| Rexp11 (%pred.) | 113.15 ± 30.5 | 117.26 ± 30.2 | 105.68 ± 32.5 | 110.73 ± 37.2 | 0.249 | 0.298 | 0.984 |
| Rtot11 (%pred.) | 104.63 ± 27.2 | 105.63 ± 25.5 | 97.98 ± 28.4 | 101.04 ± 29.2 | 0.299 | 0.423 | 0.868 |
| Rinsp19 (%pred.) | 83.03 ± 20.5 | 82.08 ± 20.4 | 79.4 ± 22.4 | 79.35 ± 18.5 | 0.506 | 0.905 | 0.588 |
| Rexp19 (%pred.) | 94.7 ± 21.9 | 98.4 ± 25.2 | 90.92 ± 27.2 | 93.97 ± 29.3 | 0.383 | 0.414 | 0.888 |
| Rtot19 (%pred.) | 89.52 ± 20.2 | 90.96 ± 22.2 | 85.71 ± 24.4 | 87.54 ± 23.9 | 0.381 | 0.565 | 0.869 |
| Rinsp5-19 (cmH2O/L/s) | 0.29 ± 0.6 | 0.25 ± 0.6 | 0.32 ± 0.5 | 0.31 ± 0.5 | 0.491 | 0.843 | 0.995 |
| Rexp5-19 (cmH2O/L/s) | 0.58 ± 0.9 | 0.42 ± 0.7 | 0.36 ± 0.6 | 0.43 ± 0.7 | 0.853 | 0.885 | 0.199 |
| Rtot5-19 (cmH2O/L/s) | 0.46 ± 0.6 | 0.35 ± 0.6 | 0.35 ± 0.5 | 0.38 ± 0.5 | 0.708 | 0.786 | 0.427 |
| Parameter | Study Group | Control Group | nparLD Test * | ||||
|---|---|---|---|---|---|---|---|
| Baseline | After 3-Week PR | Baseline | After 3-Week Interval | Study vs. Control Group (p) | Changes in 3-Week in Both Groups (p) | Differences in 3-Week Changes between Study and Control Group (p) | |
| Xinsp5 (%pred.) | 94.95 ± 42.2 | 103.09 ± 44.0 | 102.89 ± 45.8 | 116.83 ± 66.9 | 0.378 | 0.203 | 0.735 |
| Xexp5 (%pred.) | 115.43 ± 64.8 | 115.13 ± 59.2 | 110.83 ± 95.9 | 117.15 ± 76.3 | 0.499 | 0.678 | 0.475 |
| Xtot5 (%pred.) | 106.78 ± 48.6 | 110.38 ± 44.8 | 106.56 ± 61.1 | 117.21 ± 64.3 | 0.906 | 0.349 | 0.577 |
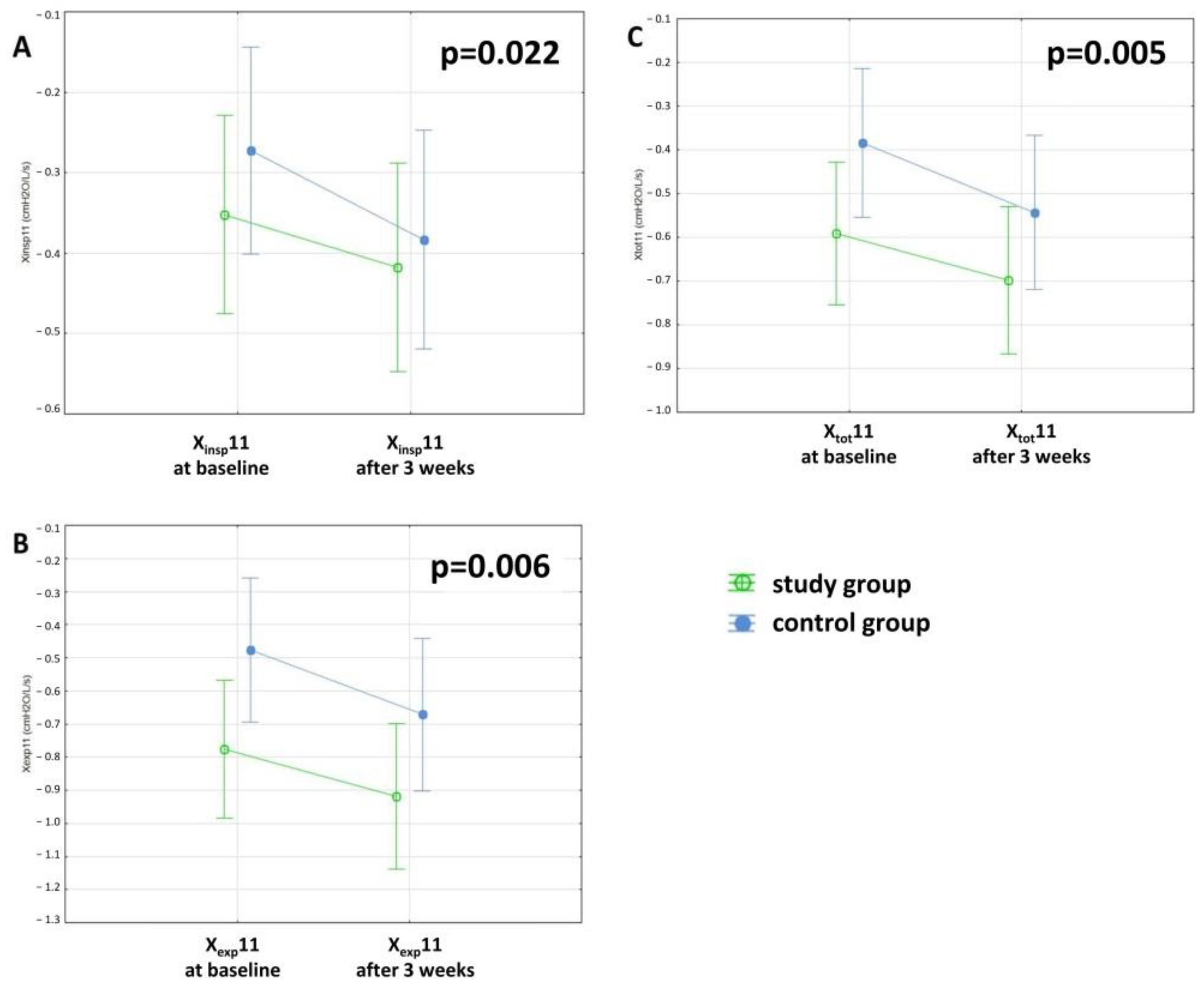
| Xinsp11 (cmH2O/L/s) | −0.36 ± 0.4 | −0.42 ± 0.5 | −0.28 ± 0.4 | −0.38 ± 0.4 | 0.323 | 0.022 | 0.402 |
| Xexp11 (cmH2O/L/s) | −0.78 ± 0.9 | −0.92 ± 0.9 | −0.6 ± 0.9 | −0.67 ± 0.6 | 0.134 | 0.006 | 0.458 |
| Xtot11 (cmH2O/L/s) | −0.6 ± 0.6 | −0.7 ± 0.7 | −0.45 ± 0.6 | −0.54 ± 0.5 | 0.172 | 0.005 | 0.386 |
| Xinsp19 (cmH2O/L/s) | 0.38 ± 0.4 | 0.39 ± 0.4 | 0.37 ± 0.4 | 0.32 ± 0.4 | 0.78 | 0.568 | 0.517 |
| Xexp19 (cmH2O/L/s) | −0.08 ± 0.6 | −0.12 ± 0.6 | −0.01 ± 0.6 | −0.06 ± 0.4 | 0.548 | 0.085 | 0.578 |
| Xtot19 (cmH2O/L/s) | 0.12 ± 0.5 | 0.11 ± 0.5 | 0.16 ± 0.5 | 0.11 ± 0.4 | 0.808 | 0.133 | 0.464 |
| ∆X5 (cmH2O/L/s) | 0.31 ± 0.8 | 0.19 ± 0.8 | 0.08 ± 1.1 | 0.02 ± 0.8 | 0.092 | 0.503 | 0.425 |
| Fres (Hz) | 14.96 ± 3.4 | 15.04 ± 3.4 | 14.28 ± 4.1 | 15.26 ± 4.2 | 0.584 | 0.095 | 0.342 |
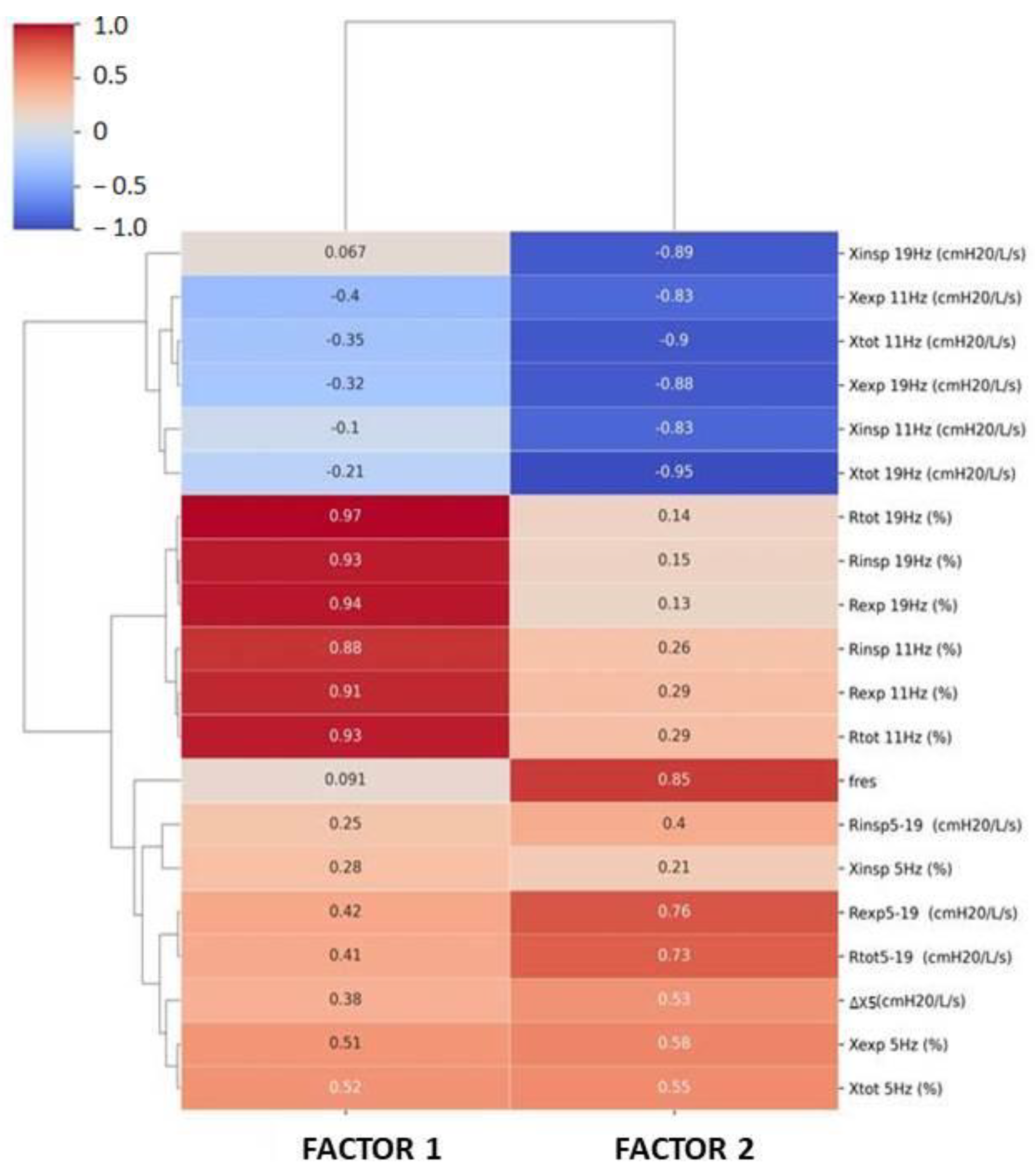
| % of Variance | Cumulative % of Variance | |
|---|---|---|
| Factor 1 | 58.63 | 58.63 |
| Factor 2 | 16.67 | 75.3 |
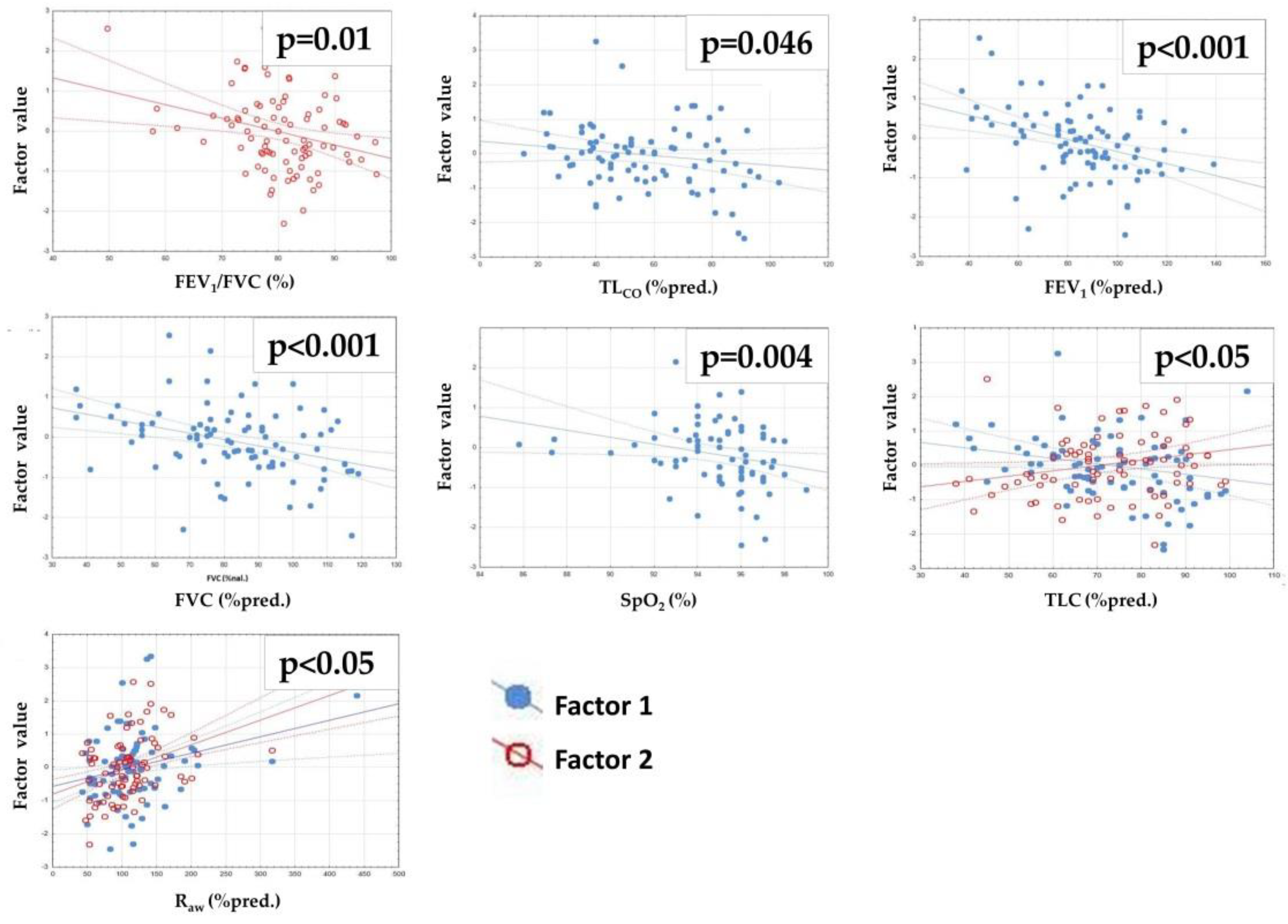
| TLCO | FEV1 | FVC | FEV1/FVC | Sp02 | RV | TLC | Raw | 6MWD | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Factor 1 | R p | 0.144 0.186 | −0.058 0.589 | 0.012 0.914 | −0.273 0.01 | 0.092 0.438 | 0.19 0.088 | 0.226 0.042 | 0.398 <0.001 | −0.158 0.18 |
| Factor 2 | R p | −0.215 0.046 | −0.384 <0.001 | −0.389 <0.001 | −0.078 0.468 | −0.333 0.004 | −0.194 0.081 | −0.318 0.004 | 0.246 0.026 | −0.031 0.793 |
| Results at Baseline | Results after 3-Week Rehabilitation | Mean Changes (∆) | 95% Cl | p | |
|---|---|---|---|---|---|
| FEV1 (%pred.) | 82.9 ± 20.3 | 84.5 ± 20.3 | 1.4 ± 7.93 | −1.05 to 3.84 | 0.255 * |
| FVC (%pred.) | 82.9 ± 19.8 | 84.4 ± 21.0 | 2.67 ± 7.1 | 0.5 to 4.85 | 0.017 * |
| FEV1/FVC (%) | 80.9 ± 8.4 | 80.6 ± 7.8 | −0.24 ± 4.4 | −1.58 to 1.1 | 0.646 # |
| 6MWD (m) | 439.6 ± 86.7 | 478.7 ± 87.0 | 35.61 ± 45.5 | 21.24 to 49.98 | <0.001 * |
| SpO2 before 6MWT (%) | 95.1 ± 1.9 | 95.5 ± 2.3 | 0.32 ± 2.4 | −0.45 to 1.09 | 0.360 # |
| SpO2 after 6MWT (%) | 84.5 ± 10.9 | 86.8 ± 8.7 | 2.24 ± 7.2 | −0.04 to 4.53 | 0.097 # |
| RV/TLC (%pred.) | 106.0 ± 31.6 | 103.8 ± 32.3 | −2.19 ± 26.7 | −10.51 to 6.13 | 0.644 # |
| RV (%pred.) | 78.5 ± 29.3 | 78.0 ± 27.2 | −0.12 ± 20.2 | −6.42 to 6.18 | 0.970 * |
| TLC (%pred.) | 73.2 ± 15.7 | 74.5 ± 14.9 | 1.02 ± 6.6 | −1.04 to 3.09 | 0.323 * |
| Raw (%pred.) | 115.2 ± 69.7 | 116.5 ± 60.7 | 12.14 ± 51.4 | −3.86 to 28.15 | 0.255 # |
| RH strength (kg) | 32.3 ± 11.9 | 32.7± 11.7 | 0.07 ± 3.5 | −0.96 to 1.1 | 0.397 # |
| LH strength (kg) | 30.1 ± 11.1 | 31.0 ± 10.2 | 0.58 ± 3.7 | −0.51 to 1.67 | 0.228 # |
| ∆FVC | ∆6MWD | ||
|---|---|---|---|
| Factor 1 | R p | 0.033 0.833 | −0.064 0.693 |
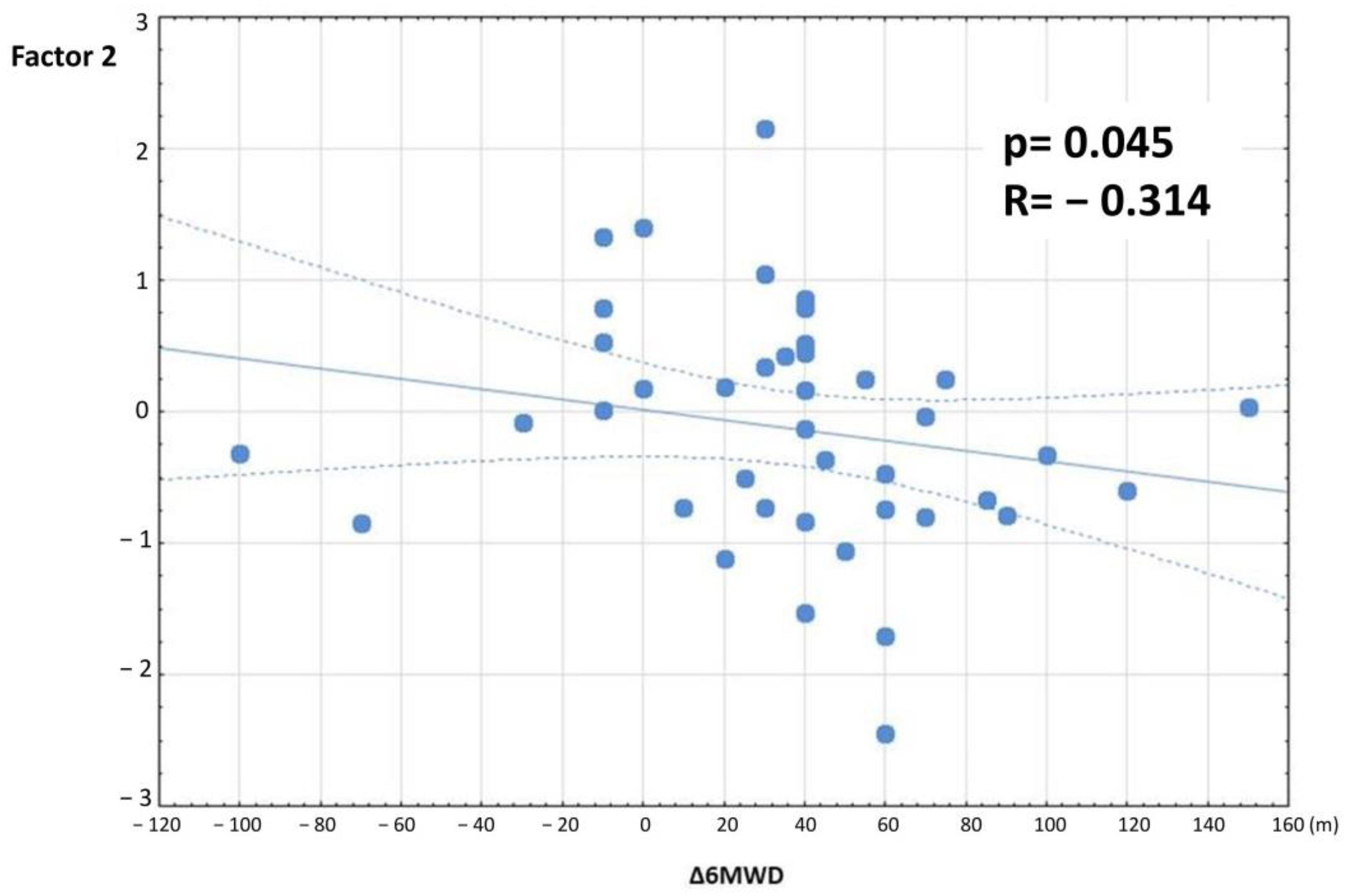
| Factor 2 | R p | 0.118 0.452 | −0.314 0.045 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostorz-Nosal, S.; Jastrzębski, D.; Kubicki, P.; Galle, D.; Gałeczka-Turkiewicz, A.; Toczylowska, B.; Ziora, D. Forced Oscillation Measurements in Patients with Idiopathic Interstitial Pneumonia Subjected to Pulmonary Rehabilitation. J. Clin. Med. 2022, 11, 3657. https://doi.org/10.3390/jcm11133657
Kostorz-Nosal S, Jastrzębski D, Kubicki P, Galle D, Gałeczka-Turkiewicz A, Toczylowska B, Ziora D. Forced Oscillation Measurements in Patients with Idiopathic Interstitial Pneumonia Subjected to Pulmonary Rehabilitation. Journal of Clinical Medicine. 2022; 11(13):3657. https://doi.org/10.3390/jcm11133657
Chicago/Turabian StyleKostorz-Nosal, Sabina, Dariusz Jastrzębski, Piotr Kubicki, Dagmara Galle, Alicja Gałeczka-Turkiewicz, Beata Toczylowska, and Dariusz Ziora. 2022. "Forced Oscillation Measurements in Patients with Idiopathic Interstitial Pneumonia Subjected to Pulmonary Rehabilitation" Journal of Clinical Medicine 11, no. 13: 3657. https://doi.org/10.3390/jcm11133657
APA StyleKostorz-Nosal, S., Jastrzębski, D., Kubicki, P., Galle, D., Gałeczka-Turkiewicz, A., Toczylowska, B., & Ziora, D. (2022). Forced Oscillation Measurements in Patients with Idiopathic Interstitial Pneumonia Subjected to Pulmonary Rehabilitation. Journal of Clinical Medicine, 11(13), 3657. https://doi.org/10.3390/jcm11133657








