Prognosis of Advanced Heart Failure Patients according to Their Hemodynamic Profile Based on the Modified Forrester Classification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Pulmonary Artery Catheterization
2.3. Data Collection
2.4. Definition of Events
2.5. Modified Forrester Classification
2.6. Primary and Secondary Endpoints
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Primary Endpoint
3.2.1. Analysis According to Pulmonary Congestion
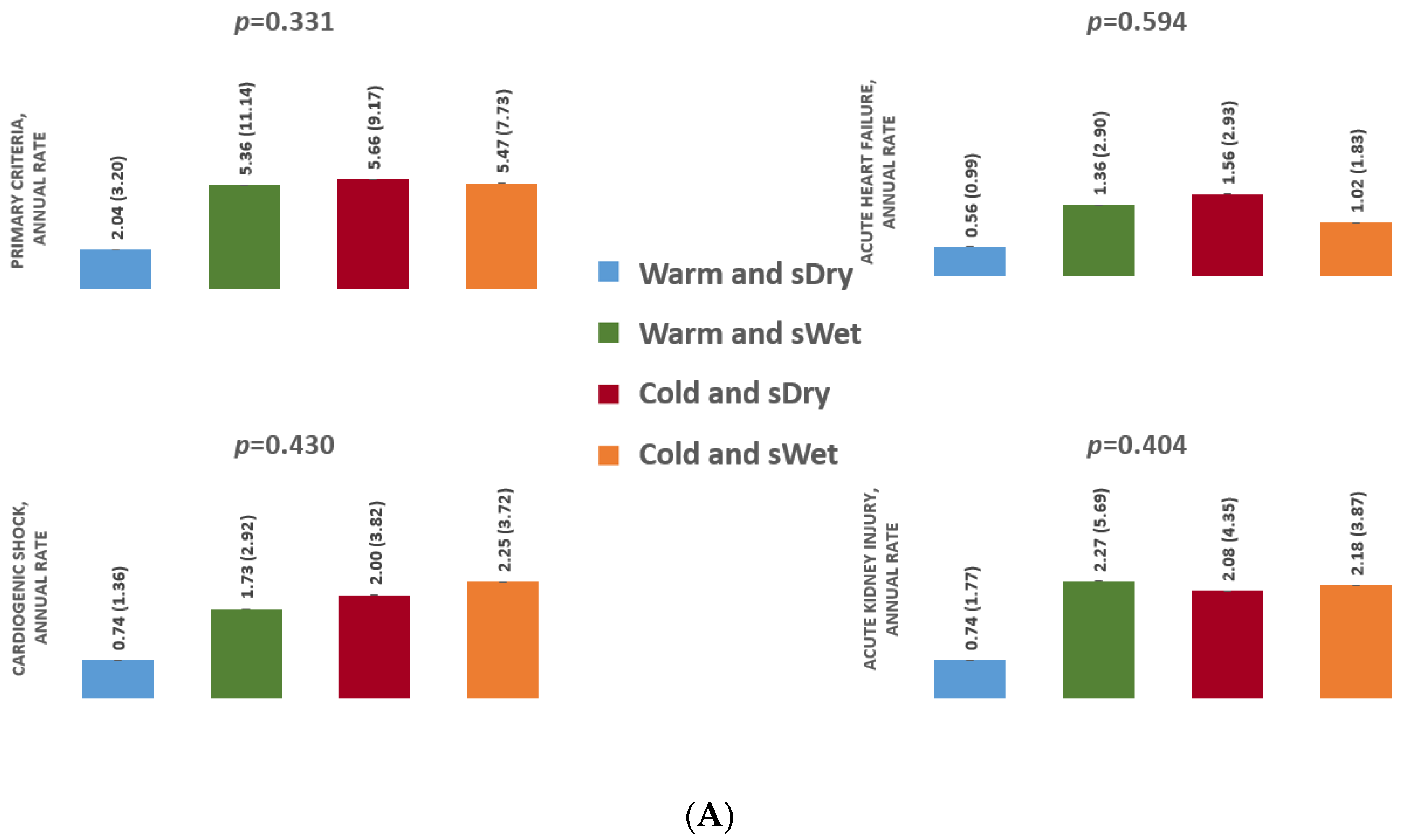
3.2.2. Analysis According to Systemic Congestion
3.3. Secondary Endpoint
3.3.1. Analysis According to Pulmonary Congestion
3.3.2. Analysis According to Systemic Congestion
3.3.3. Mortality on Waitlist
3.4. Correlation between PCWP and CVP
4. Discussion
4.1. Interest of Forrester Classification for Predicting Cardiorenal Outcomes
4.2. Congestion Is the Main Driver of Cardiorenal Events on Heart Transplant Waitlist
4.3. Systemic Congestion Has Higher Prognostic Value to Predict Cardiorenal Events on HT Waitlist
4.4. Clinical Implications
4.5. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Leiro, M.G.; Metra, M.; Lund, L.H.; Milicic, D.; Costanzo, M.R.; Filippatos, G.; Gustafsson, F.; Tsui, S.; Barge-Caballero, E.; De Jonge, N.; et al. Advanced heart failure: A position statement of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018, 20, 1505–1535. [Google Scholar] [CrossRef] [PubMed]
- Agence de la Biomédecine Résumé d’activité de Greffe Cardiaque. 2019; 1–34.
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef] [PubMed]
- Forrester, J.S.; Waters, D.D. Hospital treatment of congestive heart failure. Management according to hemodynamic profile. Am. J. Med. 1978, 65, 173–180. [Google Scholar] [CrossRef]
- Mehra, M.R.; Canter, C.E.; Hannan, M.M.; Semigran, M.J.; Uber, P.A.; Baran, D.A.; Danziger-Isakov, L.; Kirklin, J.K.; Kirk, R.; Kushwaha, S.S.; et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: A 10-year update. J. Heart Lung Transplant. 2016, 35, 1–23. [Google Scholar] [CrossRef]
- Jasseron, C.; Legeai, C.; Jacquelinet, C.; Leprince, P.; Cantrelle, C.; Audry, B.; Porcher, R.; Bastien, O.; Dorent, R. Prediction of Waitlist Mortality in Adult Heart Transplant Candidates. J. Heart Lung Transplant. 2017, 36, S114. [Google Scholar] [CrossRef]
- Kellum, J.A.; Lameire, N.; Aspelin, P.; Barsoum, R.S.; Burdmann, E.A.; Goldstein, S.L.; Herzog, C.A.; Joannidis, M.; Kribben, A.; Levey, A.S.; et al. Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604. [Google Scholar] [CrossRef]
- Nohria, A.; Tsang, S.W.; Fang, J.C.; Lewis, E.F.; Jarcho, J.A.; Mudge, G.H.; Stevenson, L.W. Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure. J. Am. Coll. Cardiol. 2003, 41, 1797–1804. [Google Scholar] [CrossRef] [Green Version]
- Cooper, L.B.; Mentz, R.J.; Stevens, S.R.; Felker, G.M.; Lombardi, C.; Metra, M.; Stevenson, L.W.; O’Connor, C.M.; Milano, C.A.; Patel, C.B.; et al. Hemodynamic Predictors of Heart Failure Morbidity and Mortality: Fluid or Flow? J. Card. Fail. 2016, 22, 182–189. [Google Scholar] [CrossRef] [Green Version]
- Chioncel, O.; Mebazaa, A.; Maggioni, A.P.; Harjola, V.P.; Rosano, G.; Laroche, C.; Piepoli, M.F.; Crespo-Leiro, M.G.; Lainscak, M.; Ponikowski, P.; et al. Acute heart failure congestion and perfusion status–impact of the clinical classification on in-hospital and long-term outcomes; insights from the ESC-EORP-HFA Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2019, 21, 1338–1352. [Google Scholar] [CrossRef] [PubMed]
- Ibe, T.; Wada, H.; Sakakura, K.; Ugata, Y.; Maki, H.; Yamamoto, K.; Seguchi, M.; Taniguchi, Y.; Jinnouchi, H.; Fujita, H. Cardiac index predicts long-term outcomes in patients with heart failure. PLoS ONE 2021, 16, e0252833. [Google Scholar] [CrossRef] [PubMed]
- Pellicori, P.; Shah, P.; Cuthbert, J.; Urbinati, A.; Zhang, J.; Kallvikbacka-Bennett, A.; Clark, A.L.; Cleland, J.G.F. Prevalence, pattern and clinical relevance of ultrasound indices of congestion in outpatients with heart failure. Eur. J. Heart Fail. 2019, 21, 904–916. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Gracia, J.; Demissei, B.G.; ter Maaten, J.M.; Cleland, J.G.; O’Connor, C.M.; Metra, M.; Ponikowski, P.; Teerlink, J.R.; Cotter, G.; Davison, B.A.; et al. Prevalence, predictors and clinical outcome of residual congestion in acute decompensated heart failure. Int. J. Cardiol. 2018, 258, 185–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivas-Lasarte, M.; Álvarez-García, J.; Fernández-Martínez, J.; Maestro, A.; López-López, L.; Solé-González, E.; Pirla, M.J.; Mesado, N.; Mirabet, S.; Fluvià, P.; et al. Lung ultrasound-guided treatment in ambulatory patients with heart failure: A randomized controlled clinical trial (LUS-HF study). Eur. J. Heart Fail. 2019, 21, 1605–1613. [Google Scholar] [CrossRef]
- Aalders, K. Comparison of Hemodynamic Factors Predicting Prognosis in Heart Failure: A Systematic Review. J. Clin. Med. 2019, 8, 1757. [Google Scholar] [CrossRef] [Green Version]
- Damman, K.; van Deursen, V.M.; Navis, G.; Voors, A.A.; van Veldhuisen, D.J.; Hillege, H.L. Increased Central Venous Pressure Is Associated With Impaired Renal Function and Mortality in a Broad Spectrum of Patients With Cardiovascular Disease. J. Am. Coll. Cardiol. 2009, 53, 582–588. [Google Scholar] [CrossRef] [Green Version]
- Nohria, A.; Hasselblad, V.; Stebbins, A.; Pauly, D.F.; Fonarow, G.C.; Shah, M.; Yancy, C.W.; Califf, R.M.; Stevenson, L.W.; Hill, J.A. Cardiorenal Interactions. Insights From the ESCAPE Trial. J. Am. Coll. Cardiol. 2008, 51, 1268–1274. [Google Scholar] [CrossRef] [Green Version]
- Baudry, G.; Sebbag, L.; Bourdin, J.; Hugon-Vallet, E.; Jobbe Duval, A.; Mewton, N.; Pozzi, M.; Rossignol, P.; Girerd, N. Haemodynamic parameters associated with renal function prior to and following heart transplantation. ESC Heart Fail. 2021, 8, 4944–4954. [Google Scholar] [CrossRef]
- Mullens, W.; Abrahams, Z.; Francis, G.S.; Sokos, G.; Taylor, D.O.; Starling, R.C.; Young, J.B.; Tang, W.H.W. Importance of Venous Congestion for Worsening of Renal Function in Advanced Decompensated Heart Failure. J. Am. Coll. Cardiol. 2009, 53, 589–596. [Google Scholar] [CrossRef] [Green Version]
- Guven, G.; Brankovic, M.; Constantinescu, A.A.; Brugts, J.J.; Hesselink, D.A.; Akin, S.; Struijs, A.; Birim, O.; Ince, C.; Manintveld, O.C.; et al. Preoperative right heart hemodynamics predict postoperative acute kidney injury after heart transplantation. Intensive Care Med. 2018, 44, 588–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, P.; Drazner, M.H.; Kato, M.; Lakdawala, N.; Palardy, M.; Nohria, A.; Stevenson, L.W. Mismatch of right- and left-sided filling pressures in chronic heart failure. J. Card. Fail. 2011, 17, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Drazner, M.H.; Velez-Martinez, M.; Ayers, C.R.; Reimold, S.C.; Thibodeau, J.T.; Mishkin, J.D.; Mammen, P.P.A.; Markham, D.W.; Patel, C.B. Relationship of right- to left-sided ventricular filling pressures in advanced heart failure insights from the escape trial. Circ. Heart Fail. 2013, 6, 264–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drazner, M.H.; Brown, R.N.; Kaiser, P.A.; Cabuay, B.; Lewis, N.P.; Semigran, M.J.; Torre-Amione, G.; Naftel, D.C.; Kirklin, J.K. Relationship of right- and left-sided filling pressures in patients with advanced heart failure: A 14-year multi-institutional analysis. J. Heart Lung Transplant. 2012, 31, 67–72. [Google Scholar] [CrossRef]
- Sokolska, J.M.; Sokolski, M.; Zymliński, R.; Biegus, J.; Siwołowski, P.; Nawrocka-Millward, S.; Swoboda, K.; Gajewski, P.; Jankowska, E.A.; Banasiak, W.; et al. Distinct clinical phenotypes of congestion in acute heart failure: Characteristics, treatment response, and outcomes. ESC Heart Fail. 2020, 7, 3830–3840. [Google Scholar] [CrossRef]
- Coiro, S.; Rossignol, P.; Ambrosio, G.; Carluccio, E.; Alunni, G.; Murrone, A.; Tritto, I.; Zannad, F.; Girerd, N. Prognostic value of residual pulmonary congestion at discharge assessed by lung ultrasound imaging in heart failure. Eur. J. Heart Fail. 2015, 17, 1172–1181. [Google Scholar] [CrossRef]
- Damman, K.; Voors, A.A.; Hillege, H.L.; Navis, G.; Lechat, P.; Van Veldhuisen, D.J.; Dargie, H.J. Congestion in chronic systolic heart failure is related to renal dysfunction and increased mortality. Eur. J. Heart Fail. 2010, 12, 974–982. [Google Scholar] [CrossRef]
- Girerd, N.; Seronde, M.-F.; Coiro, S.; Chouihed, T.; Bilbault, P.; Braun, F.; Kenizou, D.; Maillier, B.; Nazeyrollas, P.; Roul, G.; et al. Integrative Assessment of Congestion in Heart Failure Throughout the Patient Journey. JACC. Heart Fail. 2018, 6, 273–285. [Google Scholar] [CrossRef]
- Pellicori, P.; Platz, E.; Dauw, J.; ter Maaten, J.M.; Martens, P.; Pivetta, E.; Cleland, J.G.F.; McMurray, J.J.V.; Mullens, W.; Solomon, S.D.; et al. Ultrasound imaging of congestion in heart failure: Examinations beyond the heart. Eur. J. Heart Fail. 2020, 23, 703–712. [Google Scholar] [CrossRef]
- Dauw, J.; Martens, P.; Tersalvi, G.; Schouteden, J.; Deferm, S.; Gruwez, H.; De Moor, B.; Nijst, P.; Dupont, M.; Mullens, W. Diuretic response and effects of diuretic omission in ambulatory heart failure patients on chronic low-dose loop diuretic therapy. Eur. J. Heart Fail. 2021, 23, 1110–1119. [Google Scholar] [CrossRef]
- See, E.J.; Jayasinghe, K.; Glassford, N.; Bailey, M.; Johnson, D.W.; Polkinghorne, K.R.; Toussaint, N.D.; Bellomo, R. Long-term risk of adverse outcomes after acute kidney injury: A systematic review and meta-analysis of cohort studies using consensus definitions of exposure. Kidney Int. 2019, 95, 160–172. [Google Scholar] [CrossRef]
- Legrand, M.; Rossignol, P. Cardiovascular Consequences of Acute Kidney Injury. N. Engl. J. Med. 2020, 382, 2238–2247. [Google Scholar] [CrossRef] [PubMed]
- Mullens, W.; Damman, K.; Testani, J.M.; Martens, P.; Mueller, C.; Lassus, J.; Tang, W.H.W.; Skouri, H.; Verbrugge, F.H.; Orso, F.; et al. Evaluation of kidney function throughout the heart failure trajectory—A position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 584–603. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.P.; Butler, J.; Rossignol, P.; Pitt, B.; Anker, S.D.; Kosiborod, M.; Lund, L.H.; Bakris, G.L.; Weir, M.R.; Zannad, F. Abnormalities of Potassium in Heart Failure: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 2836–2850. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Ronco, F.; McCullough, P.A. A Call to Action to Develop Integrated Curricula in Cardiorenal Medicine. Blood Purif. 2017, 44, 251–259. [Google Scholar] [CrossRef]
- Narang, N.; Chung, B.; Nguyen, A.; Kalathiya, R.J.; Laffin, L.J.; Holzhauser, L.; Ebong, I.A.; Besser, S.A.; Imamura, T.; Smith, B.A.; et al. Discordance Between Clinical Assessment and Invasive Hemodynamics in Patients With Advanced Heart Failure. J. Card. Fail. 2020, 26, 128–135. [Google Scholar] [CrossRef]



| (a) | |||||||
|---|---|---|---|---|---|---|---|
| According to PCWP | Missing Data n (%) | Whole Cohort n = 100 | Warm and pDry n = 29 | Warm and pWet n = 19 | Cold and pDry n = 27 | Cold and pWet n = 25 | p-Value |
| Demographic data | |||||||
| Age, years | 0 (0) | 54 (47–61) | 50 (38–60) | 54 (48–61) | 57 (49–62) | 52 (46–59) | 0.129 |
| Male sex, n (%) | 0 (0) | 72 (72) | 20 (69) | 15 (79) | 17 (63) | 20 (80) | 0.480 |
| Body surface area, m² | 0 (0) | 1.91 (0.23) | 1.86 (0.21) | 1.88 (0.24) | 1.98 (0.22) | 1.96 (0.26) | 0.300 |
| Clinical and functional parameters | |||||||
| Heart rate, beats per min | 0 (0) | 72 (64–85) | 71 (60–90) | 77 (65–86) | 70 (61–79) | 74 (68–86) | 0.326 |
| Systolic blood pressure, mmHg | 0 (0) | 101 (90–110) | 105 (98–114) | 95 (84–108) | 93 (86–112) | 101 (87–112) | 0.128 |
| Diastolic blood pressure, mmHg | 0 (0) | 62 (56–70) | 63 (58–74) | 59 (55–70) | 60 (53–70) | 63 (58–68) | 0.454 |
| NYHA | 0 (0) | 2.76 (0.53) | 2.66 (0.55) | 2.74 (0.65) | 2.89 (0.42) | 2.76 (0.52) | 0.441 |
| VO2max, mL/kg | 30 (30) | 12.6 (4.3) | 13.9 (4.2) | 14.0 (4.3) | 12.1 (4.0) | 11.1 (4.0) | 0.152 |
| Medical history | |||||||
| Cardiovascular risk factors, n (%) | |||||||
| Hypertension | 0 (0) | 22 (22) | 4 (14) | 3 (16) | 8 (30) | 7(28) | 0.391 |
| Diabetes mellitus | 0 (0) | 12 (12) | 3 (10) | 2 (11) | 2 (7) | 5 (20) | 0.538 |
| History of smoking | 0 (0) | 61 (61) | 16 (55) | 13 (68) | 16 (59) | 16 (64) | 0.805 |
| eGFR, n (%) >60 mL/min/1.73 m² <60 mL/min/1.73 m² | 0 (0) | 85 (85) 15 (15) | 28 (97) 1 (3) | 16 (84) 3 (16) | 18 (66) 9 (33) | 23 (92) 2 (8) | 0.011 |
| Previous cardiac surgery, n (%) | 0 (0) | 27 (27) | 9 (31) | 6 (32) | 4 (15) | 8 (32) | 0.425 |
| Treatments and devices | |||||||
| Beta-blocker, n (%) | 0 (0) | 83 (83) | 26 (90) | 15 (79) | 24 (89) | 18 (72) | 0.268 |
| ACE inhibitor or ARB, n (%) | 0 (0) | 46 (46) | 17 (59) | 8 (42) | 8 (29) | 15 (60) | 0.083 |
| ARNI, n (%) | 0 (0) | 41 (41) | 10 (34) | 9 (47) | 16 (59) | 6 (24) | 0.057 |
| Loop diuretic dose mg | 0 (0) | 110 (40–160) | 40 (20–125) | 120 (40–160) | 60 (40–125) | 160 (110–250) | 0.010 |
| MRA, n (%) | 0 (0) | 82 (82) | 25 (86) | 17 (89) | 19 (70) | 21 (84) | 0.307 |
| CRT, n (%) | 0 (0) | 42 (42) | 10 (34) | 8 (42) | 12 (44) | 12 (48) | 0.775 |
| ICD, n (%) | 0 (0) | 86 (86) | 25 (86) | 15 (79) | 26 (96) | 20 (80) | 0.271 |
| Biological data | |||||||
| Creatinine, μmol/L | 0 (0) | 109 (91–132) | 96 (76–115) | 110 (93–146) | 125 (104–147) | 108 (92–121) | 0.017 |
| eGFR mL/min | 0 (0) | 64 (46–79) | 79 (53–100) | 56 (44–83) | 49 (38–66) | 70 (55–74) | 0.002 |
| NT-proBNP, ng/L | 54 (54) | 2723 (974–6231) | 2701 (488–3903) | 3995 (2227–7962) | 1909 (1171–7228) | 3680 (1112–7321) | 0.404 |
| BNP, ng/L | 9 (9) | 744 (351–1586) | 419 (137–904) | 747 (381–1570) | 1020 (494–2196) | 1211 (591–1816) | 0.014 |
| Echography | |||||||
| LVEDD, mm | 6 (6) | 67 (59–75) | 67 (59–75) | 68 (30–74) | 66 (59–75) | 65 (56–79) | 0.998 |
| LVEF, % | 0 (0) | 25 (20–30) | 28 (24–31) | 25 (20–35) | 25 (20–37) | 21 (18–28) | 0.218 |
| Right heart catheterization | |||||||
| Systolic PAP, mmHg | 0 (0) | 40 (30–55) | 29 (23–39) | 55 (47–67) | 31 (28–39) | 56 (45–67) | <0.001 |
| Diastolic PAP, mmHg | 0 (0) | 16 (12–22) | 11 (6–14) | 20 (18–25) | 14 (10–15) | 23 (20–29) | <0.001 |
| Mean PAP, mmHg | 0 (0) | 26 (20–33) | 21 (13–24) | 33 (29–44) | 21 (19–23) | 34 (31–42) | <0.001 |
| PCWP, mmHg | 0 (0) | 17 (12–24) | 13 (6–16) | 23 (21–28) | 13 (11–14) | 26 (22–30) | <0.001 |
| Cardiac index, L/min/m2 | 0 (0) | 2.0 (1.7–2.3) | 2.4 (2.2–2.7) | 2.2 (2.1–2.3) | 1.7 (1.5–1.9) | 1.6 (1.5–1.8) | <0.001 |
| Cardiac out, L/min | 0 (0) | 3.8 (3.2–4.3) | 4.5 (4.0–5.1) | 4.2 (3.8–4.8) | 3.3 (3.0–3.8) | 3.2 (3.0–3.4) | <0.001 |
| Pulmonary vascular resistance, Wood unit | 0 (0) | 2.3 (1.5–3.5) | 1.5 (1.0–2.1) | 2.5 (1.5–3.5) | 2.5 (2.0–3.4) | 2.8 (2.2–4.3) | <0.001 |
| CVP, mmHg | 0 (0) | 6 (3–12) | 5 (1–7) | 9 (6–14) | 5 (3–9) | 12 (6–16) | 0.003 |
| CVP/PCWP | 0 (0) | 0.4 (0.3–0.6) | 0.4 (0.2–0.6) | 0.4 (0.3–0.6) | 0.4 (0.3–0.6) | 0.4 (0.2–0.6) | 0.456 |
| RVSWI | 0 (0) | 9.7 (7.0–12.3) | 8.5 (6.8–10.5) | 14.7 (11.9–16.7) | 7.0 (6.0–8.8) | 10.8 (9.0–12.9) | <0.001 |
| PAPi | 0 (0) | 3.7 (2.2–9.5) | 5.4 (2.7–13.0) | 3.4 (2.2–9.7) | 3.2 (2.5–8.0) | 2.6 (1.7–6.7) | 0.351 |
| (b) | |||||||
| According to CVP | Missing data n (%) | Whole Cohort n = 100 | Warm and sDry n = 35 | Warm and sWet n = 13 | Cold and sDry n = 31 | Cold and sWet n = 21 | p-Value |
| Demographic data | |||||||
| Age, years | 0 (0) | 54 (47–61) | 52 (45–60) | 60 (43–62) | 56 (49–61) | 52(45–61) | 0.483 |
| Male sex, n (%) | 0 (0) | 72 (72) | 26 (74) | 9 (69) | 22 (71) | 15 (71) | 0.984 |
| Body surface area, m² | 0 (0) | 1.91 (0.23) | 1.84 (0.20) | 1.93 (0.25) | 1.95 (0.23) | 1.94 (0.25) | 0.192 |
| Clinical and functional parameters | |||||||
| Heart rate, beats per min | 0 (0) | 72 (64–85) | 69 (60–86) | 81 (73–94) | 67 (63–78) | 80 (71–87) | 0.027 |
| Systolic blood pressure, mmHg | 0 (0) | 101 (90–110) | 101 (92–110) | 108 (85–112) | 105 (88–116) | 92 (85–105) | 0.497 |
| Diastolic blood pressure, mmHg | 0 (0) | 62 (56–70) | 63 (57–71) | 61 (53–72) | 62 (58–71) | 62 (53–67) | 0.635 |
| NYHA | 0 (0) | 2.76 (0.53) | 2.57 (0.55) | 3 (0.58) | 2.81 (0.48) | 2.86 (0.48) | 0.045 |
| VO2max, mL/kg | 30 (30) | 12.6 (4.3) | 14 (4.3) | 11.8 (3.3) | 12.1 (4.2) | 11.0 (4.1) | 0.044 |
| Medical history | |||||||
| Cardiovascular risk factors, n (%) | |||||||
| Hypertension | 0 (0) | 22 (22) | 4 (11) | 3 (23) | 11 (35) | 4 (19) | 0.128 |
| Diabetes mellitus | 0 (0) | 12 (12) | 2 (6) | 3 (23) | 4 (13) | 3 (14) | 0.400 |
| History of smoking | 0 (0) | 61 (61) | 21 (60) | 8 (62) | 23 (74) | 9 (43) | 0.158 |
| GFR, n (%) >60 mL/min/1.73 m² <60 mL/min/1.73 m² | 0 (0) | 85 (85) 15 (15) | 34 (97) 1 (3) | 10 (77) 3 (23) | 25 (81) 6 (19) | 16 (76) 5 (24) | 0.092 |
| Previous cardiac surgery, n (%) | 0 (0) | 27 (27) | 7 (20) | 8 (62) | 5 (16) | 7 (33) | 0.012 |
| Treatments and devices | |||||||
| Beta-blocker, n (%) | 0 (0) | 83 (83) | 31 (89) | 10 (77) | 28 (90) | 14 (66) | 0.100 |
| ACE inhibitor or ARB, n (%) | 0 (0) | 46 (46) | 18 (51) | 7 (54) | 11 (35) | 12 (57) | 0.393 |
| ARNI, n (%) | 0 (0) | 41 (41) | 16 (46) | 3 (23) | 18 (31) | 4 (19) | 0.019 |
| Loop diuretic dose, mg | 0 (0) | 110 (40–160) | 40 (20–125) | 125 (90–330) | 100 (40–160) | 125 (90–205) | 0.058 |
| MRA, n (%) | 0 (0) | 82 (82) | 31 (89) | 11 (85) | 23 (74) | 17 (81) | 0.497 |
| CRT, n (%) | 0 (0) | 42 (42) | 14 (40) | 4 (31) | 16 (52) | 8 (28) | 0.565 |
| ICD, n (%) | 0 (0) | 86 (86) | 33 (94) | 7 (54) | 30(97) | 16 (76) | <0.001 |
| Biological data | |||||||
| Creatinine, μmol/L | 0 (0) | 109 (91–132) | 97 (81–113) | 131 (89–163) | 112 (99–142) | 113 (104–136) | 0.037 |
| GFR mL/min | 0 (0) | 64 (46–79) | 77 (53–89) | 50 (38–90) | 58 (42–73) | 59 (43–73) | 0.013 |
| NT-proBNP, ng/L | 54 (54) | 2723 (974–6231) | 2418 (470–4075) | 4090(3200–11529) | 1856 (973–6118) | 7071 (4829–8135) | 0.040 |
| BNP, ng/L | 9 (9) | 744 (351–1586) | 555 (185–919) | 902 (241–1491) | 744 (417–1475) | 1586 (730–2514) | 0.209 |
| Echography | |||||||
| LVEDD, mm | 6 (6) | 67 (59–75) | 68 (60–75) | 62 (53–71) | 68 (58–80) | 64 (53–78) | 0.550 |
| LVEF, % | 0 (0) | 25 (20–30) | 27 (23–30) | 26 (21–50) | 26 (24–31) | 20(17–27) | 0.156 |
| Right heart catheterization | |||||||
| Systolic PAP, mmHg | 0 (0) | 40 (30–55) | 38 (25–47) | 55 (34–66) | 39 (31–48) | 49 (34–62) | 0.004 |
| Diastolic PAP, mmHg | 0 (0) | 16 (12–22) | 12 (8–19) | 20 (17–27) | 15 (12–21) | 22 (15–32) | <0.001 |
| Mean PAP, mmHg | 0 (0) | 26 (20–33) | 22 (13–29) | 33 (25–45) | 23 (20–31) | 33 (24–43) | <0.001 |
| PCWP, mmHg | 0 (0) | 17 (12–24) | 15 (8–21) | 23 (18–27) | 14 (12–21) | 24 (16.5–30) | <0.001 |
| Cardiac index, L/min/m2 | 0 (0) | 2.0 (1.7–2.3) | 2.3 (2.1–2.7) | 2.2 (2.1–2.5) | 1.7 (1.6–1.9) | 1.6 (1.4–1.8) | <0.001 |
| Cardiac out, L/min | 0 (0) | 3.8 (3.2–4.3) | 4.2 (4.0–4.7) | 4.7 (3.8–5.2) | 3.3 (3.0–3.8) | 3.1 (2.7–3.2) | <0.001 |
| Pulmonary vascular resistance, Wood unit | 0 (0) | 2.3 (1.5–3.5) | 1.6 (1.1–2.6) | 2.3 (1.5–3.3) | 2.6 (2.0–3.6) | 2.5 (2.0–4.3) | 0.004 |
| CVP, mmHg | 0 (0) | 6 (3–12) | 5 (1–6) | 14 (11–20) | 5 (2–6) | 14 (12–18) | <0.001 |
| CVP/PCWP | 0 (0) | 0.4 (0.3–0.6) | 0.3 (0.2–0.4) | 0.6 (0.5–0.8) | 0.3 (0.2–0.4) | 0.6 (0.5–0.9) | <0.001 |
| RVSWI | 0 (0) | 9.7 (7.0–12.3) | 10.1 (7.2–12.9) | 15.2 (11.2–16.7) | 8.4 (6.7–10.7) | 9.4 (6.0–11.7) | <0.001 |
| PAPi | 0 (0) | 3.7 (2.2–9.5) | 6.7 (4.0–13.5) | 2.0 (1.4–3.3) | 5.8 (3.2–12.3) | 1.7 (1.0–2.5) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baudry, G.; Bourdin, J.; Mocan, R.; Hugon-Vallet, E.; Pozzi, M.; Jobbé-Duval, A.; Paulo, N.; Rossignol, P.; Sebbag, L.; Girerd, N. Prognosis of Advanced Heart Failure Patients according to Their Hemodynamic Profile Based on the Modified Forrester Classification. J. Clin. Med. 2022, 11, 3663. https://doi.org/10.3390/jcm11133663
Baudry G, Bourdin J, Mocan R, Hugon-Vallet E, Pozzi M, Jobbé-Duval A, Paulo N, Rossignol P, Sebbag L, Girerd N. Prognosis of Advanced Heart Failure Patients according to Their Hemodynamic Profile Based on the Modified Forrester Classification. Journal of Clinical Medicine. 2022; 11(13):3663. https://doi.org/10.3390/jcm11133663
Chicago/Turabian StyleBaudry, Guillaume, Juliette Bourdin, Raluca Mocan, Elisabeth Hugon-Vallet, Matteo Pozzi, Antoine Jobbé-Duval, Nicolas Paulo, Patrick Rossignol, Laurent Sebbag, and Nicolas Girerd. 2022. "Prognosis of Advanced Heart Failure Patients according to Their Hemodynamic Profile Based on the Modified Forrester Classification" Journal of Clinical Medicine 11, no. 13: 3663. https://doi.org/10.3390/jcm11133663
APA StyleBaudry, G., Bourdin, J., Mocan, R., Hugon-Vallet, E., Pozzi, M., Jobbé-Duval, A., Paulo, N., Rossignol, P., Sebbag, L., & Girerd, N. (2022). Prognosis of Advanced Heart Failure Patients according to Their Hemodynamic Profile Based on the Modified Forrester Classification. Journal of Clinical Medicine, 11(13), 3663. https://doi.org/10.3390/jcm11133663






