Methods for Determination of Individual PEEP for Intraoperative Mechanical Ventilation Using a Decremental PEEP Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
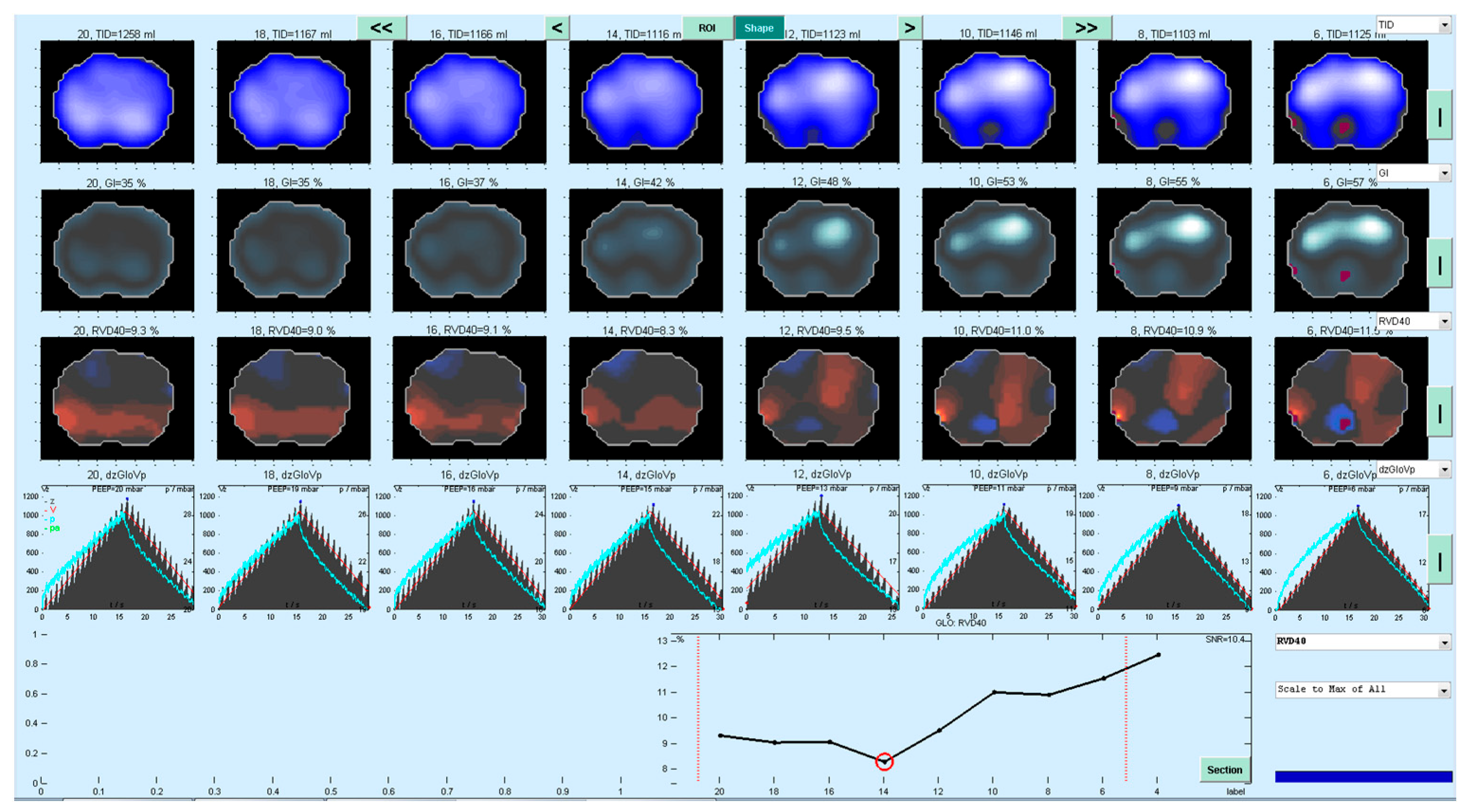
2.2. PEEP Titration and Determination of PEEPRVD
2.3. Comparative Analysis of Different Methods to Determine PEEPIND
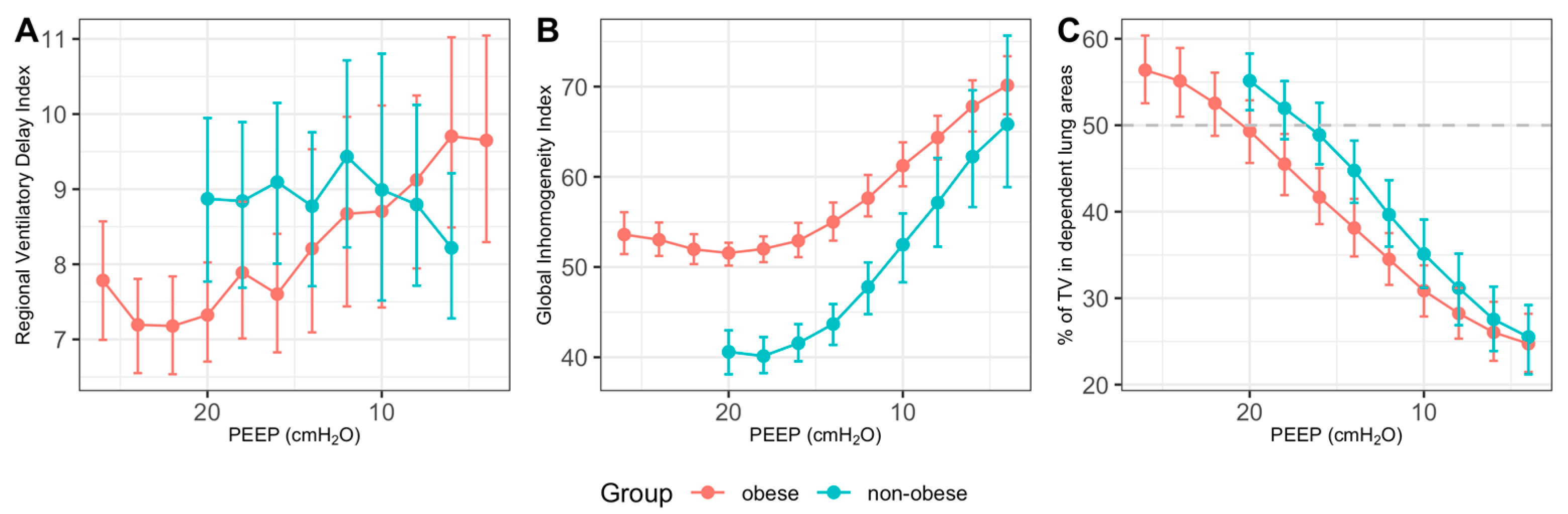
2.3.1. EIT Derived Parameters (PEEPGI, PEEP EIT VT)
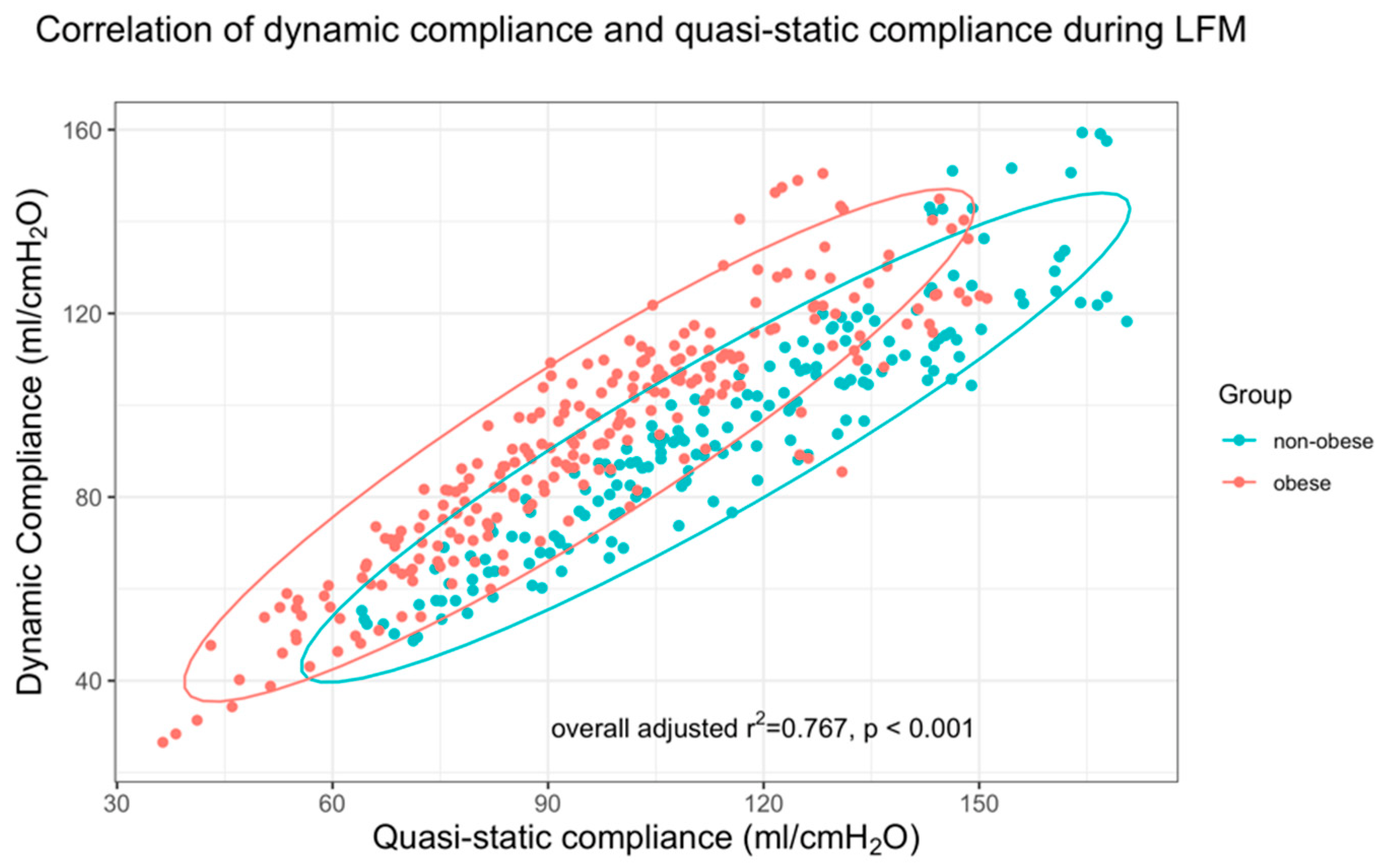
2.3.2. Global Parameters of the Respiratory System (PEEP Cdyn, PEEP CQstat)
2.4. Statistical Analysis
3. Results
3.1. Determination of PEEPIND Using EIT-Based Parameters
3.2. Compliance-Based Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maracajá-Neto, L.F.; Verçosa, N.; Roncally, A.C.; Giannella, A.; Bozza, F.A.; Lessa, M.A. Beneficial effects of high positive end-expiratory pressure in lung respiratory mechanics during laparoscopic surgery. Acta Anaesthesiol. Scand. 2009, 53, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Meininger, D.; Byhahn, C.; Mierdl, S.; Westphal, K.; Zwissler, B. Positive end-expiratory pressure improves arterial oxygenation during prolonged pneumoperitoneum. Acta Anaesthesiol. Scand. 2005, 49, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Writing Committee for the PROBESE Collaborative Group of the PROtective VEntilation Network (PROVEnet) for the Clinical Trial Network of the European Society of Anaesthesiology; Bluth, T.; Serpa Neto, A.; Schultz, M.J.; Pelosi, P.; Gama de Abreu, M.; PROBESE Collaborative Group; Bluth, T.; Bobek, I.; Canet, J.C.; et al. Effect of intraoperative high positive end-expiratory pressure (PEEP) with recruitment maneuvers vs low PEEP on postoperative pulmonary complications in obese patients: A randomized clinical trial. JAMA 2019, 321, 2292–2305. [Google Scholar] [CrossRef] [PubMed]
- Girrbach, F.; Petroff, D.; Schulz, S.; Hempel, G.; Lange, M.; Klotz, C.; Scherz, S.; Giannella-Neto, A.; Beda, A.; Jardim-Neto, A.; et al. Individualised positive end-expiratory pressure guided by electrical impedance tomography for robot-assisted laparoscopic radical prostatectomy: A prospective, randomised controlled clinical trial. Br. J. Anaesth. 2020, 125, 373–382. [Google Scholar] [CrossRef]
- Kalmar, A.F.; Foubert, L.; Hendrickx, J.F.A.; Mottrie, A.; Absalom, A.; Mortier, E.P.; Struys, M.M.R.F. Influence of steep trendelenburg position and CO2 Pneumoperitoneum on cardiovascular, cerebrovascular, and respiratory homeostasis during robotic prostatectomy. Br. J. Anaesth. 2010, 104, 433–439. [Google Scholar] [CrossRef] [Green Version]
- Nestler, C.; Simon, P.; Petroff, D.; Hammermüller, S.; Kamrath, D.; Wolf, S.; Dietrich, A.; Camilo, L.M.; Beda, A.; Carvalho, A.R.; et al. Individualized positive end-expiratory pressure in obese patients during general anaesthesia: A randomized controlled clinical trial using electrical impedance tomography. Br. J. Anaesth. 2017, 119, 1194–1205. [Google Scholar] [CrossRef] [Green Version]
- Simon, P.; Girrbach, F.; Petroff, D.; Schliewe, N.; Hempel, G.; Lange, M.; Bluth, T.; Gama de Abreu, M.; Beda, A.; Schultz, M.J.; et al. Individualized versus fixed positive end-expiratory pressure for intraoperative mechanical ventilation in obese patients: A secondary analysis. Anesthesiology 2021, 134, 887–900. [Google Scholar] [CrossRef]
- Fernandez-Bustamante, A.; Sprung, J.; Parker, R.A.; Bartels, K.; Weingarten, T.N.; Kosour, C.; Thompson, B.T.; Vidal Melo, M.F. Individualized PEEP to optimise respiratory mechanics during abdominal surgery: A pilot randomised controlled trial. Br. J. Anaesth. 2020, 125, 383–392. [Google Scholar] [CrossRef]
- Ferrando, C.; Mugarra, A.; Gutierrez, A.; Carbonell, J.A.; García, M.; Soro, M.; Tusman, G.; Belda, F.J. Setting individualized positive end-expiratory pressure level with a positive end-expiratory pressure decrement trial after a recruitment maneuver improves oxygenation and lung mechanics during one-lung ventilation. Anesth. Analg. 2014, 118, 657–665. [Google Scholar] [CrossRef] [Green Version]
- Karsten, J.; Voigt, N.; Gillmann, H.-J.; Stueber, T. Determination of optimal positive end-expiratory pressure based on respiratory compliance and electrical impedance tomography: A pilot clinical comparative trial. Biomed. Eng. Biomed. Technol. 2019, 64, 135–145. [Google Scholar] [CrossRef]
- Zhao, Z.; Lee, L.-C.; Chang, M.-Y.; Frerichs, I.; Chang, H.-T.; Gow, C.-H.; Hsu, Y.-L.; Möller, K. The incidence and interpretation of large differences in EIT-based measures for PEEP titration in ARDS patients. J. Clin. Monit. Comput. 2019, 34, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, X.; Hu, S.; Meng, Z.; He, H. Individualized lung protective ventilation vs. conventional ventilation during general anesthesia in laparoscopic total hysterectomy. Exp. Ther. Med. 2020, 19, 3051–3059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.; Chang, M.-Y.; Chang, M.-Y.; Gow, C.-H.; Zhang, J.-H.; Hsu, Y.-L.; Frerichs, I.; Chang, H.-T.; Möller, K. Positive end-expiratory pressure titration with electrical impedance tomography and pressure-volume curve in severe acute respiratory distress syndrome. Ann. Intensive Care 2019, 9, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pintado, M.-C.; de Pablo, R.; Trascasa, M.; Milicua, J.-M.; Sánchez-García, M. Compliance-guided versus FiO2-driven positive-end expiratory pressure in patients with moderate or severe acute respiratory distress syndrome according to the Berlin definition. Med. Intensiva 2017, 41, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Canet, J.; Gallart, L.; Gomar, C.; Paluzie, G.; Vallès, J.; Castillo, J.; Sabaté, S.; Mazo, V.; Briones, Z.; Sanchis, J.; et al. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology 2010, 113, 1338–1350. [Google Scholar] [CrossRef]
- Mazo, V.; Sabaté, S.; Canet, J.; Gallart, L.; de Abreu, M.G.; Belda, J.; Langeron, O.; Hoeft, A.; Pelosi, P. Prospective external validation of a predictive score for postoperative pulmonary complications. Anesthesiology 2014, 121, 219–231. [Google Scholar] [CrossRef]
- Hickling, K.G. Best compliance during a decremental, but not incremental, positive end-expiratory pressure trial is related to open-lung positive end-expiratory pressure: A mathematical model of acute respiratory distress syndrome lungs. Am. J. Respir. Crit. Care Med. 2001, 163, 69–78. [Google Scholar] [CrossRef]
- Muders, T.; Luepschen, H.; Zinserling, J.; Greschus, S.; Fimmers, R.; Guenther, U.; Buchwald, M.; Grigutsch, D.; Leonhardt, S.; Putensen, C.; et al. Tidal recruitment assessed by electrical impedance tomography and computed tomography in a porcine model of lung injury. Crit. Care Med. 2012, 40, 903–911. [Google Scholar] [CrossRef]
- Wrigge, H.; Zinserling, J.; Muders, T.; Varelmann, D.; Günther, U.; von der Groeben, C.; Magnusson, A.; Hedenstierna, G.; Putensen, C. Electrical impedance tomography compared with thoracic computed tomography during a slow inflation maneuver in experimental models of lung injury. Crit. Care Med. 2008, 36, 903–909. [Google Scholar] [CrossRef]
- Zhao, Z.; Möller, K.; Steinmann, D.; Frerichs, I.; Guttmann, J. Evaluation of an electrical impedance tomography-based global inhomogeneity index for pulmonary ventilation distribution. Intensive Care Med. 2009, 35, 1900–1906. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef] [PubMed]
- LAS VEGAS investigators Epidemiology. Practice of ventilation and outcome for patients at increased risk of postoperative pulmonary complications: LAS VEGAS—An observational study in 29 countries. Eur. J. Anaesthesiol. 2017, 34, 492–507. [Google Scholar] [CrossRef] [PubMed]
- Ball, L.; Hemmes, S.N.T.; Serpa Neto, A.; Bluth, T.; Canet, J.; Hiesmayr, M.; Hollmann, M.W.; Mills, G.H.; Vidal Melo, M.F.; Putensen, C.; et al. Intraoperative ventilation settings and their associations with postoperative pulmonary complications in obese patients. Br. J. Anaesth. 2018, 121, 899–908. [Google Scholar] [CrossRef]
- Andersson, L.E.; Bååth, M.; Thörne, A.; Aspelin, P.; Odeberg-Wernerman, S. Effect of carbon dioxide pneumoperitoneum on development of atelectasis during anesthesia, examined by spiral computed tomography. Anesthesiology 2005, 102, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Grieco, D.L.; Anzellotti, G.M.; Russo, A.; Bongiovanni, F.; Costantini, B.; D’Indinosante, M.; Varone, F.; Cavallaro, F.; Tortorella, L.; Polidori, L.; et al. Airway closure during surgical pneumoperitoneum in obese patients. Anesthesiology 2019, 131, 58–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shono, A.; Katayama, N.; Fujihara, T.; Böhm, S.H.; Waldmann, A.D.; Ugata, K.; Nikai, T.; Saito, Y. Positive end-expiratory pressure and distribution of ventilation in pneumoperitoneum combined with steep trendelenburg position. Anesthesiology 2020, 132, 476–490. [Google Scholar] [CrossRef] [PubMed]
- Tharp, W.G.; Murphy, S.; Breidenstein, M.W.; Love, C.; Booms, A.; Rafferty, M.N.; Friend, A.F.; Perrapato, S.; Ahern, T.P.; Dixon, A.E.; et al. Body habitus and dynamic surgical conditions independently impair pulmonary mechanics during robotic-assisted laparoscopic surgery. Anesthesiology 2020, 133, 750–763. [Google Scholar] [CrossRef]
- Valenza, F.; Vagginelli, F.; Tiby, A.; Francesconi, S.; Ronzoni, G.; Guglielmi, M.; Zappa, M.; Lattuada, E.; Gattinoni, L. Effects of the beach chair position, positive end-expiratory pressure, and pneumoperitoneum on respiratory function in morbidly obese patients during anesthesia and paralysis. Anesthesiology 2007, 107, 725–732. [Google Scholar] [CrossRef] [Green Version]
- Mazzinari, G.; Diaz-Cambronero, O.; Alonso-Iñigo, J.M.; Garcia-Gregorio, N.; Ayas-Montero, B.; Ibañez, J.L.; Serpa Neto, A.; Ball, L.; Gama de Abreu, M.; Pelosi, P.; et al. Intraabdominal pressure targeted positive end-expiratory pressure during laparoscopic surgery: An open-label, nonrandomized, crossover, clinical trial. Anesthesiology 2020, 132, 667–677. [Google Scholar] [CrossRef]
- Futier, E.; Constantin, J.-M.; Pelosi, P.; Chanques, G.; Kwiatkoskwi, F.; Jaber, S.; Bazin, J.-E. Intraoperative recruitment maneuver reverses detrimental pneumoperitoneum-induced respiratory effects in healthy weight and obese patients undergoing laparoscopy. Anesthesiology 2010, 113, 1310–1319. [Google Scholar] [CrossRef]
- Whalen, F.X.; Gajic, O.; Thompson, G.B.; Kendrick, M.L.; Que, F.L.; Williams, B.A.; Joyner, M.J.; Hubmayr, R.D.; Warner, D.O.; Sprung, J. The effects of the alveolar recruitment maneuver and positive end-expiratory pressure on arterial oxygenation during laparoscopic bariatric surgery. Anesth. Analg. 2006, 102, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Young, C.C.; Harris, E.M.; Vacchiano, C.; Bodnar, S.; Bukowy, B.; Elliott, R.R.D.; Migliarese, J.; Ragains, C.; Trethewey, B.; Woodward, A.; et al. Lung-protective ventilation for the surgical patient: International expert panel-based consensus recommendations. Br. J. Anaesth. 2019, 123, 898–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, H.-K.; Kim, B.R.; Yoon, S.; Jeong, Y.H.; Ku, J.H.; Kim, W.H. The effect of ventilation with individualized positive end-expiratory pressure on postoperative atelectasis in patients undergoing robot-assisted radical prostatectomy: A randomized controlled trial. J. Clin. Med. 2021, 10, 850. [Google Scholar] [CrossRef] [PubMed]
- Sahetya, S.K. Searching for the optimal positive end-expiratory pressure for lung protective ventilation. Curr. Opin. Crit. Care 2020, 26, 53–58. [Google Scholar] [CrossRef]
- Blankman, P.; Hasan, D.; Erik, G.; Gommers, D. Detection of “best” positive end-expiratory pressure derived from electrical impedance tomography parameters during a decremental positive end-expiratory pressure trial. Crit. Care Lond. Engl. 2014, 18, R95. [Google Scholar] [CrossRef] [Green Version]
- Muders, T.; Hentze, B.; Kreyer, S.; Wodack, K.H.; Leonhardt, S.; Hedenstierna, G.; Wrigge, H.; Putensen, C. Measurement of electrical impedance tomography-based regional ventilation delay for individualized titration of end-expiratory pressure. J. Clin. Med. 2021, 10, 2933. [Google Scholar] [CrossRef]
- Zhao, Z.; Steinmann, D.; Frerichs, I.; Guttmann, J.; Möller, K. PEEP titration guided by ventilation homogeneity: A feasibility study using electrical impedance tomography. Crit. Care Lond. Engl. 2010, 14, R8. [Google Scholar] [CrossRef] [Green Version]
- Haase, J.; Buchloh, D.C.; Hammermüller, S.; Salz, P.; Mrongowius, J.; Carvalho, N.C.; Beda, A.; Rau, A.; Starke, H.; Spieth, P.M.; et al. Mechanical ventilation strategies targeting different magnitudes of collapse and tidal recruitment in porcine acid aspiration-induced lung injury. J. Clin. Med. 2019, 8, 1250. [Google Scholar] [CrossRef] [Green Version]


| All Patients | Non-Obese | Obese | p-Value | |
|---|---|---|---|---|
| Number | 45 | 20 | 25 | |
| Age (years) | 52.8 (12.7) | 62.6 (7.5) | 44.9 (10.3) | <0.001 * |
| Sex (male/female) | 28/17 | 20/0 | 8/17 | <0.001 * |
| Height (cm) | 177 (11) | 182 (9) | 173 (11) | 0.004 * |
| Weight (kg) | 118.0 (36.4) | 84.4 (12.2) | 145.0 (24.6) | <0.001 * |
| BMI (kg m2) | 38.1 (12.7) | 25.4 (2.3) | 48.2 (7.0) | <0.001 * |
| PEEPIND RVDI (cm H2O) | 16.3 (4.5) | 14.9 (3.1) | 17.4 (5.2) | 0.047 * |
| Method | n | Mean PEEPIND (95% CI) | n with Difference to PEEP RVDI max. 4 cm H2O | Mean Difference to PEEP RVDI (95% CI) | p-Value |
|---|---|---|---|---|---|
| RVDI | 45 | 16.3 (14.9–17.6) | – | – | – |
| EIT GIT | 45 | 18.7 (17.4–20.0) | 35 (78%) | −2.4 (−1.2; −3.6) | 0.010 * |
| EIT VT | 45 | 18.6 (17.3–19.9) | 32 (71%) | −2.3 (−0.9; −3.7) | 0.014 * |
| Cdyn | 42 | 16.8 (15.6–18.0) | 37 (88%) | −0.4 (0.7; −1.5) | 0.57 |
| CQstat | 42 | 16.0 (14.8–17.1) | 39 (93%) | 0.5 (1.5; −0.5) | 0.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girrbach, F.; Zeutzschel, F.; Schulz, S.; Lange, M.; Beda, A.; Giannella-Neto, A.; Wrigge, H.; Simon, P. Methods for Determination of Individual PEEP for Intraoperative Mechanical Ventilation Using a Decremental PEEP Trial. J. Clin. Med. 2022, 11, 3707. https://doi.org/10.3390/jcm11133707
Girrbach F, Zeutzschel F, Schulz S, Lange M, Beda A, Giannella-Neto A, Wrigge H, Simon P. Methods for Determination of Individual PEEP for Intraoperative Mechanical Ventilation Using a Decremental PEEP Trial. Journal of Clinical Medicine. 2022; 11(13):3707. https://doi.org/10.3390/jcm11133707
Chicago/Turabian StyleGirrbach, Felix, Franziska Zeutzschel, Susann Schulz, Mirko Lange, Alessandro Beda, Antonio Giannella-Neto, Hermann Wrigge, and Philipp Simon. 2022. "Methods for Determination of Individual PEEP for Intraoperative Mechanical Ventilation Using a Decremental PEEP Trial" Journal of Clinical Medicine 11, no. 13: 3707. https://doi.org/10.3390/jcm11133707






