Influence of Exposure Parameters and Implant Position in Peri-Implant Bone Assessment in CBCT Images: An In Vitro Study
Abstract
:1. Introduction
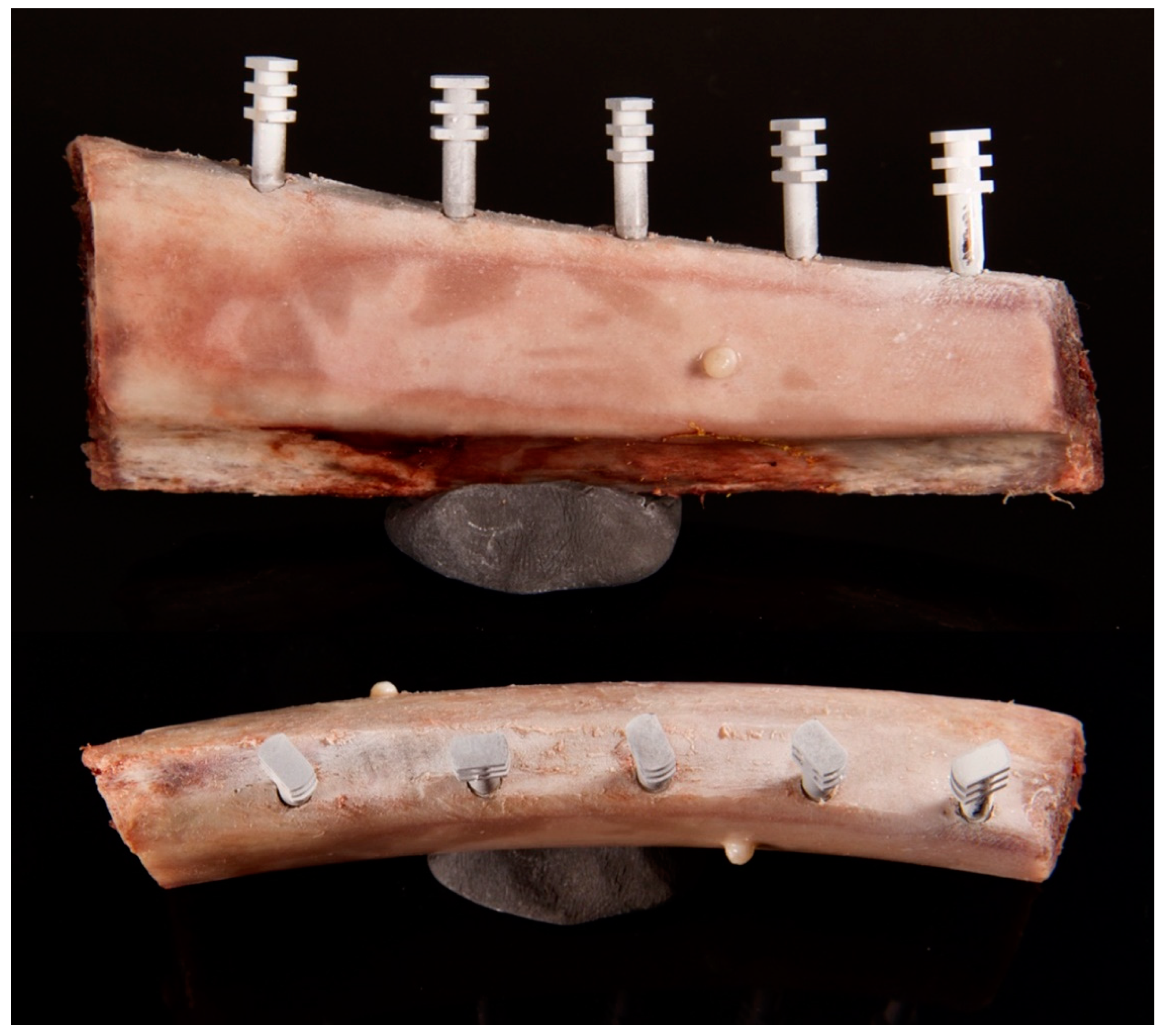
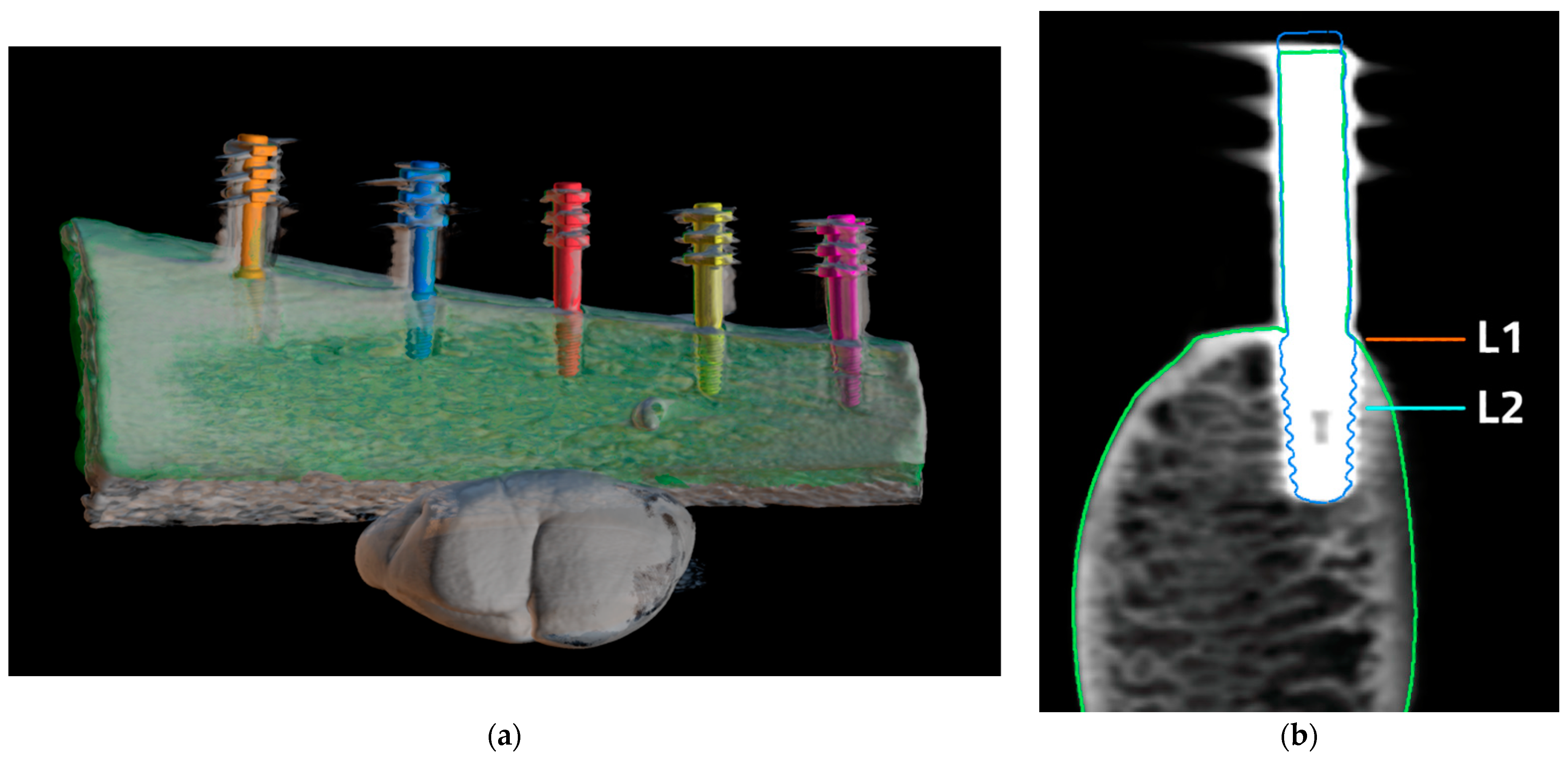
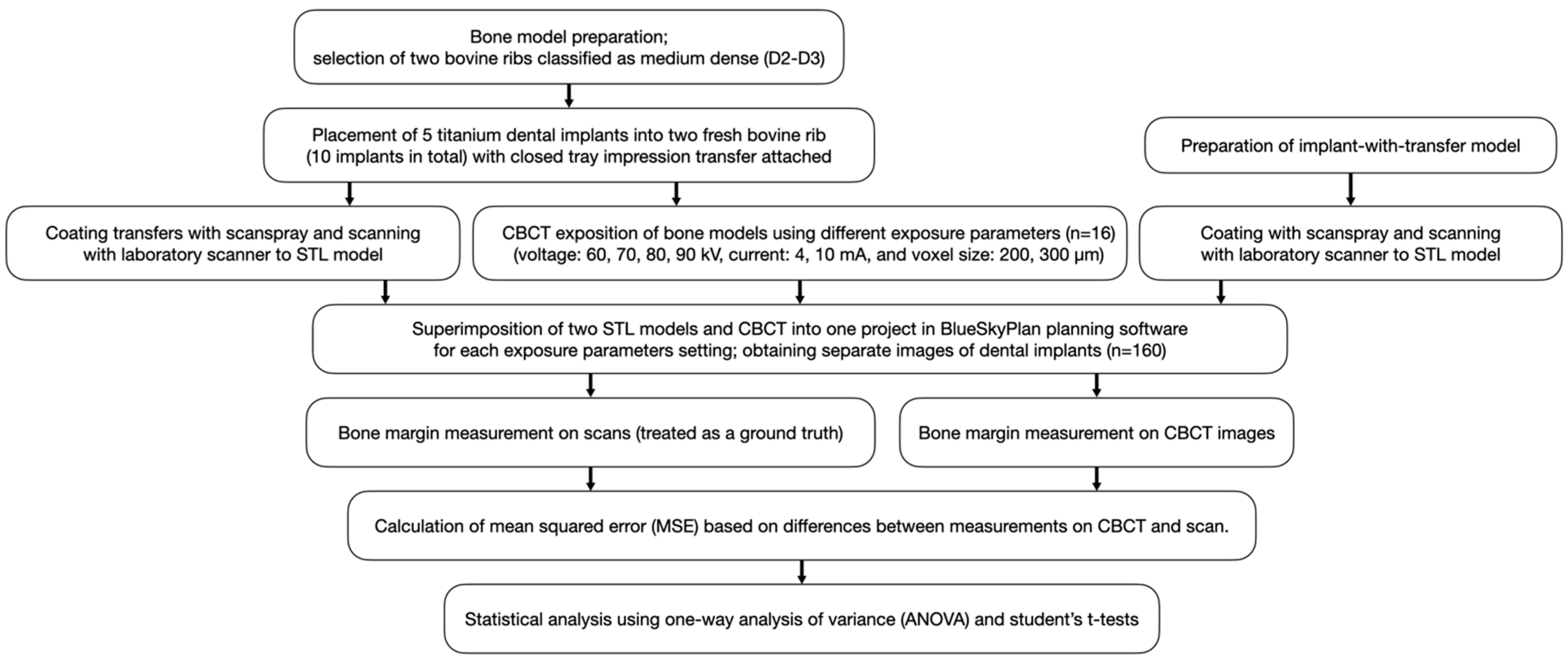
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- An, X.; Oh, J.-H.; Jeong, S.-M.; Choi, B.-H. Natural bone healing in compromised sockets after tooth extraction: Digital measurement methods with cone-beam computed tomography. J. Oral Implant. 2021, 47, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Chappuis, V.; Engel, O.; Reyes, M.; Shahim, K.; Nolte, L.P.; Buser, D. Ridge alterations post-extraction in the esthetic zone: A 3D analysis with CBCT. J. Dent. Res. 2013, 92, 195s–201s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araujo, M.G.; Sukekava, F.; Wennstrom, J.L.; Lindhe, J. Ridge alterations following implant placement in fresh extraction sockets: An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Avila-Ortiz, G.; Chambrone, L.; Vignoletti, F. Effect of alveolar ridge preservation interventions following tooth extraction: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, 195–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buser, D.; Janner, S.F.M.; Wittneben, J.-G.; Brägger, U.; Ramseier, C.A.; Salvi, G.E. 10-year survival and success rates of 511 titanium implants with a sandblasted and acid-etched surface: A retrospective study in 303 partially edentulous patients. Clin. Implant Dent. Relat. Res. 2012, 14, 839–851. [Google Scholar] [CrossRef]
- Monje, A.; Chappuis, V.; Monje, F.; Muñoz, F.; Wang, H.-L.; Urban, I.; Buser, D. The critical peri-implant buccal bone wall thickness revisited: An experimental study in the beagle dog. Int. J. Oral Maxillofac. Implant. 2019, 34, 1328–1336. [Google Scholar] [CrossRef]
- Maier, F.-M. Initial crestal bone loss after implant placement with flapped or flapless surgery—A prospective cohort study. Int. J. Oral Maxillofac. Implant. 2016, 31, 876–883. [Google Scholar] [CrossRef] [Green Version]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S313–S318. [Google Scholar] [CrossRef]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S173–S182. [Google Scholar] [CrossRef] [Green Version]
- Abrahamsson, I.; Soldini, C. Probe penetration in periodontal and peri-implant tissues: An experimental study in the beagle dog. Clin. Oral Implant. Res. 2006, 17, 601–605. [Google Scholar] [CrossRef]
- Bohner, L.; Habor, D.; Tortamano, P.; Radermacher, K.; Wolfart, S.; Marotti, J. Assessment of buccal bone surrounding dental implants using a high-frequency ultrasound scanner. Ultrasound Med. Biol. 2019, 45, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Shujaat, S.; Vasconcelos, K.D.F.; Huang, Y.; Politis, C.; Lambrichts, I.; Jacobs, R. Diagnostic accuracy of CBCT versus intraoral imaging for assessment of peri-implant bone defects. BMC Med. Imaging 2021, 21, 23. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R.; Mark, R.; Gundappa, M.; Balaji, M.D.S.; Vijay, V.; Umayal, M. Comparative evaluation of periodontal osseous defects using direct digital radiography and cone-beam computed tomography. J. Pharm. Bioallied Sci. 2021, 13, S306–S311. [Google Scholar] [CrossRef] [PubMed]
- Ruetters, M.; Hagenfeld, D.; ElSayed, N.; Zimmermann, N.; Gehrig, H.; Kim, T.-S. Ex vivo comparison of CBCT and digital periapical radiographs for the quantitative assessment of periodontal defects. Clin. Oral Investig. 2020, 24, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Palkovics, D.; Mangano, F.G.; Nagy, K.; Windisch, P. Digital three-dimensional visualization of intrabony periodontal defects for regenerative surgical treatment planning. BMC Oral Health 2020, 20, 351. [Google Scholar] [CrossRef]
- Woelber, J.P.; Fleiner, J.; Rau, J.; Ratka-Krüger, P.; Hannig, C. Accuracy and usefulness of CBCT in periodontology: A systematic review of the literature. Int. J. Periodontics Restor. Dent. 2018, 38, 289–297. [Google Scholar] [CrossRef] [Green Version]
- Golubovic, V.; Mihatovic, I.; Becker, J.; Schwarz, F. Accuracy of cone-beam computed tomography to assess the configuration and extent of ligature-induced peri-implantitis defects. A pilot study. Oral Maxillofac. Surg. 2012, 16, 349–354. [Google Scholar] [CrossRef]
- Jacobs, R.; Salmon, B.; Codari, M.; Hassan, B.; Bornstein, M.M. Cone beam computed tomography in implant dentistry: Recommendations for clinical use. BMC Oral Health 2018, 18, 88. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, R.; Vranckx, M.; Vanderstuyft, T.; Quirynen, M.; Salmon, B. CBCT vs other imaging modalities to assess peri-implant bone and diagnose complications: A systematic review. Eur. J. Oral Implantol. 2018, 11, 77–92. [Google Scholar]
- Hussain, R.A.; Miloro, M.; Cohen, J.B. An update on the treatment of periimplantitis. Dent. Clin. N. Am. 2021, 65, 43–56. [Google Scholar] [CrossRef]
- Kayal, R.A. Distortion of digital panoramic radiographs used for implant site assessment. J. Orthod. Sci. 2016, 5, 117–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suphangul, S.; Rattanabanlang, A.; Amornsettachai, P.; Wongsirichat, N. Dimension distortion of digital panoramic radiograph on posterior mandibular regions. Mahidol Dent. J. 2016, 36, 279–286. [Google Scholar]
- Greenstein, G.; Cavallaro, J.S., Jr.; Tarnow, D.P. Clinical pearls for surgical implant dentistry: Part I. Dent. Today 2010, 29, 124–127. [Google Scholar]
- Coelho-Silva, F.; Gaêta-Araujo, H.; Rosado, L.P.L.; Freitas, D.Q.; Haiter-Neto, F.; De-Azevedo-Vaz, S.L. Distortion or magnification? An in vitro cone-beam CT study of dimensional changes of objects with different compositions. Dentomaxillofac. Radiol. 2021, 50, 20210063. [Google Scholar] [CrossRef]
- Schulze, R.K.W.; Berndt, D.; D’Hoedt, B. On cone-beam computed tomography artifacts induced by titanium implants. Clin. Oral Implant. Res. 2010, 21, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, P.; Zawadzki, P.J.; Regulski, P. The Impact of cone-beam computed tomography exposure parameters on peri-implant artifacts: A literature review. Cureus 2022, 14, 23035. [Google Scholar] [CrossRef]
- Commission, E. Cone Beam CT for Dental and Maxillofacial Radiology. In Evidence-Based Guidelines; Radiation Protection No. 172; European Commission: Luxemburg, 2012. [Google Scholar]
- Aljohani, M.; Yong, S.L.; Bin Rahmah, A. The effect of surgical regenerative treatment for peri-implantitis: A systematic review. Saudi Dent. J. 2020, 32, 109–119. [Google Scholar] [CrossRef]
- Falco, A.; Berardini, M.; Trisi, P. Correlation between implant geometry, implant surface, insertion torque, and primary stability: In vitro biomechanical analysis. Int. J. Oral Maxillofac. Implant. 2018, 33, 824–830. [Google Scholar] [CrossRef]
- Misch, C. Bone character: Second vital implant criterion. Dent. Today 1988, 7, 39–40. [Google Scholar]
- Saberi, B.V.; Khosravifard, N.; Ghandari, F.; Hadinezhad, A. Detection of peri-implant bone defects using cone-beam computed tomography and digital periapical radiography with parallel and oblique projection. Imaging Sci. Dent. 2019, 49, 265–272. [Google Scholar] [CrossRef]
- Schwindling, F.S.; Hilgenfeld, T.; Weber, D.; Kosinski, M.A.; Rammelsberg, P.; Tasaka, A. In vitro diagnostic accuracy of low-dose CBCT for evaluation of peri-implant bone lesions. Clin. Oral Implant. Res. 2019, 30, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Eskandarloo, A.; Saati, S.; Ardakani, M.P.; Jamalpour, M.; Mezerji, N.M.G.; Akheshteh, V. Diagnostic accuracy of three cone beam computed tomography systems and periapical radiography for detection of fenestration around dental implants. Contemp. Clin. Dent. 2018, 9, 376–381. [Google Scholar] [CrossRef] [PubMed]
- de-Azevedo-Vaz, S.L.; Peyneau, P.; Ramirez-Sotelo, L.; de Faria Vasconcelos, K.; Campos, P.S.F.; Haiter-Neto, F. Efficacy of a cone beam computed tomography metal artifact reduction algorithm for the detection of peri-implant fenestrations and dehiscences. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 121, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Yuan, L.; Liu, L.; Qian, Y.; Xia, L.; Ye, N.; Fang, B. Detection of alveolar bone defects with three different voxel sizes of cone-beam computed tomography: An in vitro study. Sci. Rep. 2019, 9, 8146. [Google Scholar] [CrossRef]
- Chiodo, T.A.; Ziccardi, V.B.; Janal, M.; Sabitini, C. Failure strength of 2.0 locking versus 2.0 conventional Synthes mandibular plates: A laboratory model. J. Oral Maxillofac. Surg. 2006, 64, 1475–1479. [Google Scholar] [CrossRef]
- Choi, B.-H.; Huh, J.-Y.; Suh, C.-H.; Kim, K.-N. An in vitro evaluation of miniplate fixation techniques for fractures of the atrophic edentulous mandible. Int. J. Oral Maxillofac. Surg. 2005, 34, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Bredbenner, T.L.; Haug, R.H. Substitutes for human cadaveric bone in maxillofacial rigid fixation research. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2000, 90, 574–580. [Google Scholar] [CrossRef]
- Vanderstuyft, T.; Tarce, M.; Sanaan, B.; Jacobs, R.; Vasconcelos, K.D.F.; Quirynen, M. Inaccuracy of buccal bone thickness estimation on cone-beam CT due to implant blooming: An ex-vivo study. J. Clin. Periodontol. 2019, 46, 1134–1143. [Google Scholar] [CrossRef]
- Gonzalez-Martin, O.; Oteo, C.; Ortega, R.; Alandez, J.; Sanz, M.; Veltri, M. Evaluation of peri-implant buccal bone by computed tomography: An experimental study. Clin. Oral Implant. Res. 2016, 27, 950–955. [Google Scholar] [CrossRef]
- Wang, D.; Künzel, A.; Golubovic, V.; Mihatovic, I.; John, G.; Chen, Z.; Becker, J.; Schwarz, F. Accuracy of peri-implant bone thickness and validity of assessing bone augmentation material using cone beam computed tomography. Clin. Oral Investig. 2013, 17, 1601–1609. [Google Scholar] [CrossRef]
- Razavi, T.; Palmer, R.M.; Davies, J.; Wilson, R.; Palmer, P.J. Accuracy of measuring the cortical bone thickness adjacent to dental implants using cone beam computed tomography. Clin. Oral Implant. Res. 2010, 21, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Lo Giudice, R.; Sindoni, A.; Tribst, J.P.M.; de Oliveira Dal Piva, A.M.; Lo Giudice, G.; Bellezza, U.; Lo Giudice, G.; Famà, F. Evaluation of zirconia and high-performance polymer abutment surface roughness and stress concentration for implant-supported fixed dental prostheses. Coatings 2022, 12, 238. [Google Scholar] [CrossRef]

| Implant | Mean of Two Measurements at Level 1 [mm] | Mean of Two Measurements at Level 2 [mm] |
|---|---|---|
| 1 | 0.00 | 0.17 |
| 2 | 0.27 | 1.21 |
| 3 | 0.77 | 1.83 |
| 4 | 1.09 | 2.31 |
| 5 | 1.20 | 2.54 |
| 6 | 0.25 | 1.26 |
| 7 | 0.55 | 1.55 |
| 8 | 0.61 | 1.68 |
| 9 | 0.43 | 1.71 |
| 10 | 0.95 | 2.19 |
| kV | MSE [mm2] | SD of Error [mm2] |
|---|---|---|
| 60 | 0.27 | 0.35 |
| 70 | 0.18 | 0.22 |
| 80 | 0.18 | 0.19 |
| 90 | 0.14 | 0.14 |
| All | 0.19 | 0.23 |
| Current | Voxel Size | |||||
|---|---|---|---|---|---|---|
| 4 mA | 10 mA | p-Value | 200 µm | 300 µm | p-Value | |
| mean MSE [mm2] | 0.18 | 0.18 | 0.969 | 0.20 | 0.15 | 0.055 |
| Voltage | Bone Margin Thickness for MSE < 0.25 | p-Value | Regression Equation |
|---|---|---|---|
| 60 kV | never | ||
| 70 kV | 0.00–0.72 | 0.161 | 0.17 × d + 0.13 |
| 80 kV | 0.00–1.08 | 0.004 | 0.21 × d + 0.03 |
| 90 kV | 0.00–1.12 | <0.001 | 0.23 × d + −0.01 |
| ALL | 0.00–0.88 | 0.047 | 0.13 × d + 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawicki, P.; Regulski, P.; Winiarski, A.; Zawadzki, P.J. Influence of Exposure Parameters and Implant Position in Peri-Implant Bone Assessment in CBCT Images: An In Vitro Study. J. Clin. Med. 2022, 11, 3846. https://doi.org/10.3390/jcm11133846
Sawicki P, Regulski P, Winiarski A, Zawadzki PJ. Influence of Exposure Parameters and Implant Position in Peri-Implant Bone Assessment in CBCT Images: An In Vitro Study. Journal of Clinical Medicine. 2022; 11(13):3846. https://doi.org/10.3390/jcm11133846
Chicago/Turabian StyleSawicki, Paweł, Piotr Regulski, Artur Winiarski, and Paweł J. Zawadzki. 2022. "Influence of Exposure Parameters and Implant Position in Peri-Implant Bone Assessment in CBCT Images: An In Vitro Study" Journal of Clinical Medicine 11, no. 13: 3846. https://doi.org/10.3390/jcm11133846
APA StyleSawicki, P., Regulski, P., Winiarski, A., & Zawadzki, P. J. (2022). Influence of Exposure Parameters and Implant Position in Peri-Implant Bone Assessment in CBCT Images: An In Vitro Study. Journal of Clinical Medicine, 11(13), 3846. https://doi.org/10.3390/jcm11133846







