Autonomic Dysfunction during Acute SARS-CoV-2 Infection: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy
2.3. Selection Process
2.4. Data Extraction
2.5. Quality Assessment
3. Results
3.1. Study Selection
3.2. Study Characteristics
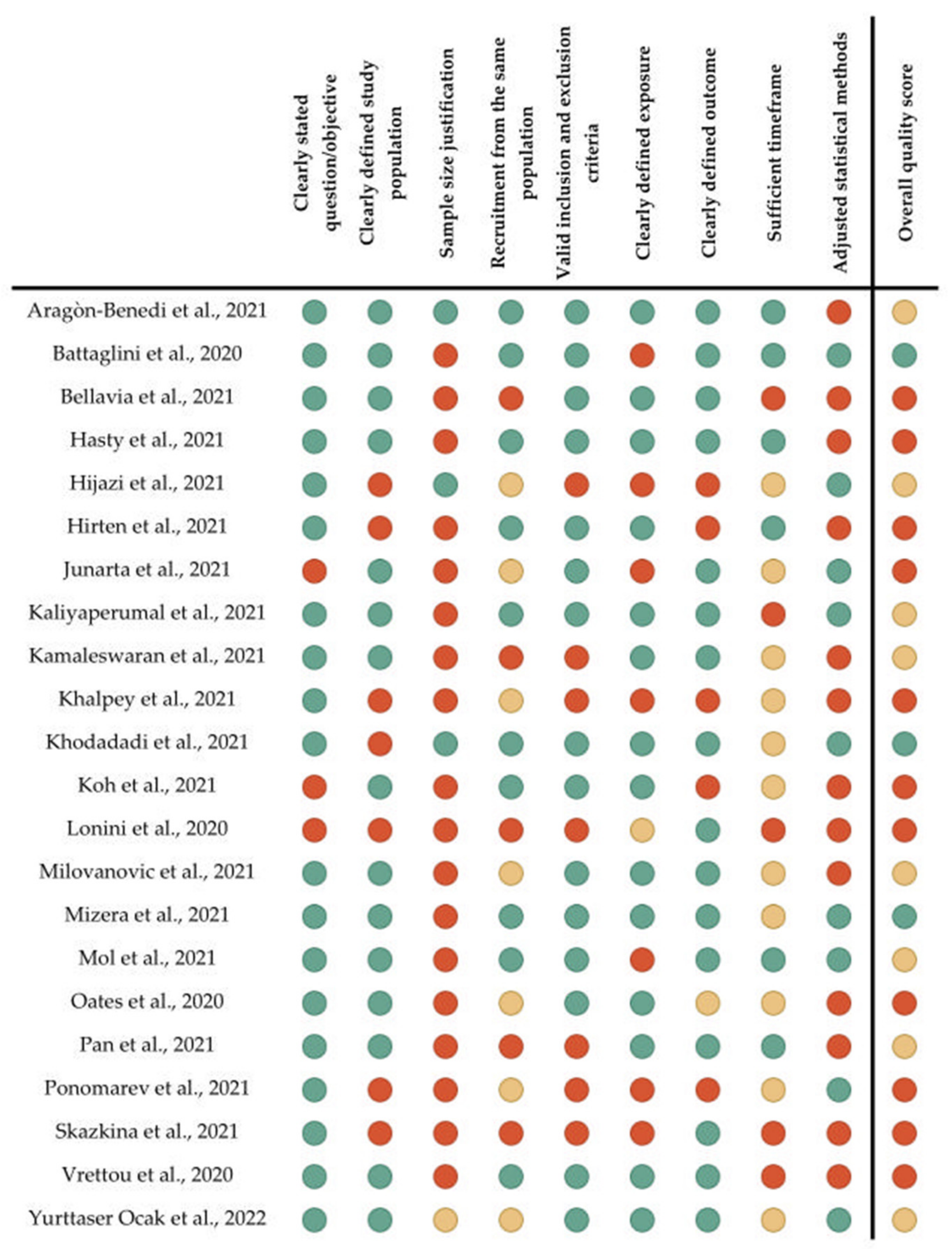
3.3. Quality Assessment
3.4. Outcomes
3.4.1. Characterization of Autonomic Involvement Associated with SARS-CoV-2
Heart Rate Variability (HRV)
Dynamic and Static Pupillometric Parameters
Other Autonomic Parameters
3.4.2. Effects of Autonomic Alterations on SARS-CoV-2 Infection Outcome
3.4.3. Role of Autonomic Parameters in Predicting SARS-CoV-2 Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cucinotta, D.; Vanelli, M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020, 91, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Berlin, D.A.; Gulick, R.M.; Martinez, F.J. Severe COVID-19. N. Engl. J. Med. 2020, 383, 2451–2460. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, R.T.; Lynch, J.B.; Del Rio, C. Mild or Moderate COVID-19. N. Engl. J. Med. 2020, 383, 1757–1766. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef] [Green Version]
- Favas, T.T.; Dev, P.; Chaurasia, R.N.; Chakravarty, K.; Mishra, R.; Joshi, D.; Mishra, V.N.; Kumar, A.; Singh, V.K.; Pandey, M.; et al. Neurological manifestations of COVID-19: A systematic review and meta-analysis of proportions. Neurol. Sci. 2020, 41, 3437–3470. [Google Scholar] [CrossRef]
- Frisullo, G.; Scala, I.; Bellavia, S.; Broccolini, A.; Brunetti, V.; Morosetti, R.; Della Marca, G.; Calabresi, P. COVID-19 and stroke: From the cases to the causes. Rev. Neurosci. 2021, 32, 659–669. [Google Scholar] [CrossRef]
- Luigetti, M.; Iorio, R.; Bentivoglio, A.R.; Tricoli, L.; Riso, V.; Marotta, J.; Piano, C.; Primiano, G.; Zileri Del Verme, L.; Lo Monaco, M.R.; et al. Assessment of neurological manifestations in hospitalized patients with COVID-19. Eur. J. Neurol. 2020, 27, 2322–2328. [Google Scholar] [CrossRef]
- Balcom, E.F.; Nath, A.; Power, C. Acute and chronic neurological disorders in COVID-19: Potential mechanisms of disease. Brain 2021, 144, 3576–3588. [Google Scholar] [CrossRef]
- Carod-Artal, F.J. Infectious diseases causing autonomic dysfunction. Clin. Auton. Res. 2018, 28, 67–81. [Google Scholar] [CrossRef]
- Mattei, J.; Teyssier, G.; Pichot, V.; Barthelemy, J.C.; Achour, E.; Pillet, S.; Bourlet, T.; Patural, H. Autonomic dysfunction in 2009 pandemic influenza A (H1N1) virus-related infection: A pediatric comparative study. Auton. Neurosci. 2011, 162, 77–83. [Google Scholar] [CrossRef]
- Osztovits, J.; Horvath, T.; Abonyi, M.; Toth, T.; Visnyei, Z.; Beko, G.; Csak, T.; Lakatos, P.L.; Littvay, L.; Feher, J.; et al. Chronic hepatitis C virus infection associated with autonomic dysfunction. Liver Int. 2009, 29, 1473–1478. [Google Scholar] [CrossRef]
- Stock, C.; Teyssier, G.; Pichot, V.; Goffaux, P.; Barthelemy, J.C.; Patural, H. Autonomic dysfunction with early respiratory syncytial virus-related infection. Auton. Neurosci. 2010, 156, 90–95. [Google Scholar] [CrossRef]
- Zhu, Z.; Lian, X.; Su, X.; Wu, W.; Marraro, G.A.; Zeng, Y. From SARS and MERS to COVID-19: A brief summary and comparison of severe acute respiratory infections caused by three highly pathogenic human coronaviruses. Respir. Res. 2020, 21, 224. [Google Scholar] [CrossRef]
- Lo, Y.L.; Leong, H.N.; Hsu, L.Y.; Tan, T.T.; Kurup, A.; Fook-Chong, S.; Tan, B.H. Autonomic dysfunction in recovered severe acute respiratory syndrome patients. Can. J. Neurol. Sci. 2005, 32, 264. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Shin, H.S.; Park, H.Y.; Kim, J.L.; Lee, J.J.; Lee, H.; Won, S.D.; Han, W. Depression as a Mediator of Chronic Fatigue and Post-Traumatic Stress Symptoms in Middle East Respiratory Syndrome Survivors. Psychiatry Investig. 2019, 16, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Rogers, J.P.; Chesney, E.; Oliver, D.; Pollak, T.A.; McGuire, P.; Fusar-Poli, P.; Zandi, M.S.; Lewis, G.; David, A.S. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: A systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry 2020, 7, 611–627. [Google Scholar] [CrossRef]
- Novak, P.; Mukerji, S.S.; Alabsi, H.S.; Systrom, D.; Marciano, S.P.; Felsenstein, D.; Mullally, W.J.; Pilgrim, D.M. Multisystem Involvement in Post-Acute Sequelae of Coronavirus Disease 19. Ann. Neurol. 2022, 91, 367–379. [Google Scholar] [CrossRef]
- Abrams, R.M.C.; Simpson, D.M.; Navis, A.; Jette, N.; Zhou, L.; Shin, S.C. Small fiber neuropathy associated with SARS-CoV-2 infection. Muscle Nerve 2022, 65, 440–443. [Google Scholar] [CrossRef]
- Hinduja, A.; Moutairou, A.; Calvet, J.H. Sudomotor dysfunction in patients recovered from COVID-19. Neurophysiol. Clin. 2021, 51, 193–196. [Google Scholar] [CrossRef]
- Buoite Stella, A.; Furlanis, G.; Frezza, N.A.; Valentinotti, R.; Ajcevic, M.; Manganotti, P. Autonomic dysfunction in post-COVID patients with and witfhout neurological symptoms: A prospective multidomain observational study. J. Neurol. 2022, 269, 587–596. [Google Scholar] [CrossRef]
- Eldokla, A.M.; Mohamed-Hussein, A.A.; Fouad, A.M.; Abdelnaser, M.G.; Ali, S.T.; Makhlouf, N.A.; Sayed, I.G.; Makhlouf, H.A.; Shah, J.; Aiash, H. Prevalence and patterns of symptoms of dysautonomia in patients with long-COVID syndrome: A cross-sectional study. Ann. Clin. Transl. Neurol. 2022, 9, 778–785. [Google Scholar] [CrossRef]
- Jamal, S.M.; Landers, D.B.; Hollenberg, S.M.; Turi, Z.G.; Glotzer, T.V.; Tancredi, J.; Parrillo, J.E. Prospective Evaluation of Autonomic Dysfunction in Post-Acute Sequela of COVID-19. J. Am. Coll. Cardiol. 2022, 79, 2325–2330. [Google Scholar] [CrossRef]
- Bitirgen, G.; Korkmaz, C.; Zamani, A.; Iyisoy, M.S.; Kerimoglu, H.; Malik, R.A. Abnormal quantitative pupillary light responses following COVID-19. Int. Ophthalmol. 2022, 1–8. [Google Scholar] [CrossRef]
- Asarcikli, L.D.; Hayiroglu, M.I.; Osken, A.; Keskin, K.; Kolak, Z.; Aksu, T. Heart rate variability and cardiac autonomic functions in post-COVID period. J. Interv. Card. Electrophysiol. 2022, 63, 715–721. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Study Quality Assessment Tools | NHLBI, NIH. Available online: https://www.nhlbi.nih.gov/health-topics/study-qualityassessment-tools (accessed on 9 May 2022).
- Aragon-Benedi, C.; Oliver-Fornies, P.; Galluccio, F.; Yamak Altinpulluk, E.; Ergonenc, T.; El Sayed Allam, A.; Salazar, C.; Fajardo-Perez, M. Is the heart rate variability monitoring using the analgesia nociception index a predictor of illness severity and mortality in critically ill patients with COVID-19? A pilot study. PLoS ONE 2021, 16, e0249128. [Google Scholar] [CrossRef]
- Battaglini, D.; Santori, G.; Chandraptham, K.; Iannuzzi, F.; Bastianello, M.; Tarantino, F.; Ball, L.; Giacobbe, D.R.; Vena, A.; Bassetti, M.; et al. Neurological Complications and Noninvasive Multimodal Neuromonitoring in Critically Ill Mechanically Ventilated COVID-19 Patients. Front. Neurol. 2020, 11, 602114. [Google Scholar] [CrossRef]
- Bellavia, S.; Scala, I.; Luigetti, M.; Brunetti, V.; Gabrielli, M.; Zileri Dal Verme, L.; Servidei, S.; Calabresi, P.; Frisullo, G.; Della Marca, G. Instrumental Evaluation of COVID-19 Related Dysautonomia in Non-Critically-Ill Patients: An Observational, Cross-Sectional Study. J. Clin. Med. 2021, 10, 5861. [Google Scholar] [CrossRef]
- Hasty, F.; Garcia, G.; Davila, C.H.; Wittels, S.H.; Hendricks, S.; Chong, S. Heart Rate Variability as a Possible Predictive Marker for Acute Inflammatory Response in COVID-19 Patients. Mil. Med. 2020, 186, e34–e38. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, H.; Abu Talib, M.; Hasasneh, A.; Bou Nassif, A.; Ahmed, N.; Nasir, Q. Wearable Devices, Smartphones, and Interpretable Artificial Intelligence in Combating COVID-19. Sensors 2021, 21, 8424. [Google Scholar] [CrossRef] [PubMed]
- Hirten, R.P.; Danieletto, M.; Tomalin, L.; Choi, K.H.; Zweig, M.; Golden, E.; Kaur, S.; Helmus, D.; Biello, A.; Pyzik, R.; et al. Use of Physiological Data From a Wearable Device to Identify SARS-CoV-2 Infection and Symptoms and Predict COVID-19 Diagnosis: Observational Study. J. Med. Internet Res. 2021, 23, e26107. [Google Scholar] [CrossRef] [PubMed]
- Junarta, J.; Riley, J.M.; Pavri, B.B. Describing heart rate variability in patients with chronic atrial fibrillation during hospitalization for COVID-19. J. Arrhythmia 2021, 37, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Kaliyaperumal, D.; Rk, K.; Alagesan, M.; Ramalingam, S. Characterization of cardiac autonomic function in COVID-19 using heart rate variability: A hospital based preliminary observational study. J. Basic Clin. Physiol. Pharmacol. 2021, 32, 247–253. [Google Scholar] [CrossRef]
- Kamaleswaran, R.; Sadan, O.; Kandiah, P.; Li, Q.; Coopersmith, C.M.; Buchman, T.G. Altered Heart Rate Variability Early in ICU Admission Differentiates Critically Ill Coronavirus Disease 2019 and All-Cause Sepsis Patients. Crit. Care Explor. 2021, 3, e0570. [Google Scholar] [CrossRef]
- Khodadadi, F.; Punait, S.; Kolacz, J.; Zand, F.; Foroutan, A.; Lewis, G.F. Use of heart rate variability to predict hospital length of stay for COVID-19 patients: A prospective observational study. Int. J. Crit. Illn. Inj. Sci. 2021, 11, 134–141. [Google Scholar] [CrossRef]
- Koh, J.S.; De Silva, D.A.; Quek, A.M.L.; Chiew, H.J.; Tu, T.M.; Seet, C.Y.H.; Hoe, R.H.M.; Saini, M.; Hui, A.C.; Angon, J.; et al. Neurology of COVID-19 in Singapore. J. Neurol. Sci. 2020, 418, 117118. [Google Scholar] [CrossRef]
- Lonini, L.; Shawen, N.; Botonis, O.; Fanton, M.; Jayaraman, C.; Mummidisetty, C.K.; Shin, S.Y.; Rushin, C.; Jenz, S.; Xu, S.; et al. Rapid Screening of Physiological Changes Associated With COVID-19 Using Soft-Wearables and Structured Activities: A Pilot Study. IEEE J. Transl. Eng. Health Med. 2021, 9, 4900311. [Google Scholar] [CrossRef]
- Milovanovic, B.; Djajic, V.; Bajic, D.; Djokovic, A.; Krajnovic, T.; Jovanovic, S.; Verhaz, A.; Kovacevic, P.; Ostojic, M. Assessment of Autonomic Nervous System Dysfunction in the Early Phase of Infection With SARS-CoV-2 Virus. Front. Neurosci. 2021, 15, 640835. [Google Scholar] [CrossRef]
- Mizera, L.; Rath, D.; Schoellmann, A.; Petersen-Uribe, A.; Avdiu, A.; Zdanyte, M.; Jaeger, P.; Heinzmann, D.; Muller, K.; Gawaz, M.; et al. Deceleration capacity is associated with acute respiratory distress syndrome in COVID-19. Heart Lung 2021, 50, 914–918. [Google Scholar] [CrossRef]
- Mol, M.B.A.; Strous, M.T.A.; van Osch, F.H.M.; Vogelaar, F.J.; Barten, D.G.; Farchi, M.; Foudraine, N.A.; Gidron, Y. Heart-rate-variability (HRV), predicts outcomes in COVID-19. PLoS ONE 2021, 16, e0258841. [Google Scholar] [CrossRef]
- Oates, C.P.; Turagam, M.K.; Musikantow, D.; Chu, E.; Shivamurthy, P.; Lampert, J.; Kawamura, I.; Bokhari, M.; Whang, W.; Miller, M.A.; et al. Syncope and presyncope in patients with COVID-19. Pacing Clin. Electrophysiol. 2020, 43, 1139–1148. [Google Scholar] [CrossRef]
- Pan, Y.; Yu, Z.; Yuan, Y.; Han, J.; Wang, Z.; Chen, H.; Wang, S.; Wang, Z.; Hu, H.; Zhou, L.; et al. Alteration of Autonomic Nervous System Is Associated With Severity and Outcomes in Patients With COVID-19. Front. Physiol. 2021, 12, 630038. [Google Scholar] [CrossRef]
- Skazkina, V.V.; Krasikova, N.S.; Borovkova, E.I.; Ishbulatov, Y.M.; Gorshkov, A.Y.; Korolev, A.I.; Dadaeva, V.A.; Fedorovich, A.A.; Kuligin, A.V.; Drapkina, O.M.; et al. Synchronization Of Autonomic Control Loops Of Blood Circulation In Patients With COVID-19. ROMJ 2021, 10, 307. [Google Scholar] [CrossRef]
- Vrettou, C.S.; Korompoki, E.; Sarri, K.; Papachatzakis, I.; Theodorakopoulou, M.; Chrysanthopoulou, E.; Andrianakis, I.A.; Routsi, C.; Zakynthinos, S.; Kotanidou, A. Pupillometry in critically ill patients with COVID-19: A prospective study. Clin. Auton. Res. 2020, 30, 563–565. [Google Scholar] [CrossRef]
- Yurttaser Ocak, S.; Ozturan, S.G.; Bas, E. Pupil responses in patients with COVID-19. Int. Ophthalmol. 2022, 42, 385–391. [Google Scholar] [CrossRef]
- Kuznik, B.I.; Smolyakov, Y.N.; Shapovalov, Y.K.; Nolfin, N.A.; Parts, D.S. Hemodynamics and heart rate variability in seriously ill COVID-19 patients at the height of the disease and in the process of rehabilitation. Tromboz. Gemostaz Reol. 2021, 84, 31–39. [Google Scholar]
- Podzolkov, V.I.; Bragina, A.E.; Tarzimanova, A.I.; Vasileva, L.V.; Batrakova, E.P.; Lobova, N.V.; Bykova, E.E.; Khachuroeva, M.M. Post-covid syndrome and tachycardia: Theoretical base and treatment experience. Ration. Pharmacother. Cardiol. 2021, 17, 256–262. [Google Scholar] [CrossRef]
- Cairo, B.; De Maria, B.; Bari, V.; Gelpi, F.; Minonzio, M.; Barbic, F.; Dalla Vecchia, L.A.; Furlan, R.; Porta, A. Causal Analysis Is Needed to Evaluate Cardiorespiratory Interaction Alterations in Postural Orthostatic Tachycardia Syndrome Patients. In Proceedings of the 2021 Computing in Cardiology (CinC), Brno, Czech Republic, 13–15 September 2021. [Google Scholar]
- Qiao, D.; Zulkernine, F.; Masroor, R.; Rasool, R.; Jaffar, N. Measuring Heart Rate and Heart Rate Variability with Smartphone Camera. In Proceedings of the 22nd IEEE International Conference on Mobile Data Management (MDM), Toronto, ON, Canada, 15–18 June 2021; pp. 248–249. [Google Scholar]
- Skazkina, V.; Borovkova, E.; Krasikova, N.; Kiselev, A.; Gorshkov, A.; Korolev, A.; Dadaeva, V.; Fedorovich, A.; Kuligin, A.; Karavaev, A. Analysis of coupling between autonomic control loops of blood circulation in patients with COVID-19. In Proceedings of the 5th Scientific School Dynamics of Complex Networks and their Applications (DCNA), Kaliningrad, Russia, 13–15 September 2021; pp. 187–189. [Google Scholar]
- Subudhi, D.; Venkatesan, R.K.; Devi, K.; Manivannan, M. Finger Induced Auto-Thermogenesis. In Proceedings of the IEEE 3rd Eurasia Conference on Biomedical Engineering, Healthcare and Sustainability (ECBIOS), Tainan, Taiwan, 28–30 May 2021; pp. 33–37. [Google Scholar]
- Yoshizawa, M.; Sugita, N.; Tanaka, A.; Homma, N.; Yambe, T. A cloud system for extraction of autonomic nervous system indices and blood pressure variabilities from video images. In Proceedings of the 27th International Display Workshops, IDW 2020, Virtual, 9–11 December 2020; pp. 983–984. [Google Scholar]
- Buoite Stella, A.; Filingeri, D.; Ravanelli, N.; Morrison, S.A.; Ajcevic, M.; Furlanis, G.; Manganotti, P. Heat risk exacerbation potential for neurology patients during the COVID-19 pandemic and related isolation. Int. J. Biometeorol. 2021, 65, 627–630. [Google Scholar] [CrossRef]
- Drury, R.L.; Jarczok, M.; Owens, A.; Thayer, J.F. Wireless Heart Rate Variability in Assessing Community COVID-19. Front. Neurosci. 2021, 15, 564159. [Google Scholar] [CrossRef]
- Finsterer, J. Small fiber neuropathy underlying dysautonomia in COVID-19 and in post-SARS-CoV-2 vaccination and long-COVID syndromes. Muscle Nerve 2022, 65, E31–E32. [Google Scholar] [CrossRef]
- Goodman, B.P.; Khoury, J.A.; Blair, J.E.; Grill, M.F. COVID-19 Dysautonomia. Front. Neurol. 2021, 12, 624968. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Yoneyama, K.; Tsuchida, T.; Akashi, Y.J. Post-COVID-19 Postural Orthostatic Tachycardia Syndrome. Intern. Med. 2021, 60, 2345. [Google Scholar] [CrossRef] [PubMed]
- Josephine, M.S.; Lakshmanan, L.; Nair, R.R.; Visu, P.; Ganesan, R.; Jothikumar, R. Monitoring and sensing COVID-19 symptoms as a precaution using electronic wearable devices. Int. J. Pervasive Comput. Commun. 2020, 16, 341–350. [Google Scholar]
- Shouman, K.; Vanichkachorn, G.; Cheshire, W.P.; Suarez, M.D.; Shelly, S.; Lamotte, G.J.; Sandroni, P.; Benarroch, E.E.; Berini, S.E.; Cutsforth-Gregory, J.K.; et al. Autonomic dysfunction following COVID-19 infection: An early experience. Clin. Auton. Res. 2021, 31, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Qusti, S.; Alshammari, E.M.; Gyebi, G.A.; Batiha, G.E. COVID-19-Induced Dysautonomia: A Menace of Sympathetic Storm. ASN Neuro 2021, 13, 17590914211057635. [Google Scholar] [CrossRef]
- Radin, J.M.; Quer, G.; Jalili, M.; Hamideh, D.; Steinhubl, S.R. The hopes and hazards of using personal health technologies in the diagnosis and prognosis of infections. Lancet Digit. Health 2021, 3, e455–e461. [Google Scholar] [CrossRef]
- Beghi, E.; Helbok, R.; Crean, M.; Chou, S.H.; McNett, M.; Moro, E.; Bassetti, C.; Force, E.A.N.N.-C.T. The European Academy of Neurology COVID-19 registry (ENERGY): An international instrument for surveillance of neurological complications in patients with COVID-19. Eur. J. Neurol. 2021, 28, 3303–3323. [Google Scholar] [CrossRef]
- Anudeep, A.; Somu, C.; Kumar, J.S. Clinical profile and outcomes of critically ill COVID-19 patients admitted in a tertiary care hospital. Ann. Trop Med. Public Health 2020, 23, 232141. [Google Scholar] [CrossRef]
- Bajic, D.; Dajic, V.; Milovanovic, B. Entropy Analysis of COVID-19 Cardiovascular Signals. Entropy 2021, 23, 87. [Google Scholar] [CrossRef]
- De Simone, V.; Guardalben, S.; Guarise, P.; Padovani, N.; Giacopelli, D.; Zanotto, G. Home Monitoring trends during COVID-19 infection. J. Arrhythmia 2021, 37, 240–245. [Google Scholar] [CrossRef]
- Hsieh, J.Y.C.; Kan, J.Y.L.; Mattar, S.A.M.; Qin, Y. The clinical implications of sinus tachycardia in mild COVID-19 infection: A retrospective cohort study. SAGE Open Med. 2021, 9, 20503121211054973. [Google Scholar] [CrossRef]
- Kopishinskaia, S.; Lapshova, D.; Sherman, M.; Velichko, I.; Voznesensky, N.; Voznesenskaia, V. Clinical Features in Russian Patients with COVID-Associated Parosmia/Phanthosmia. Psychiatr. Danub. 2021, 33, 130–136. [Google Scholar]
- Maloberti, A.; Ughi, N.; Bernasconi, D.P.; Rebora, P.; Cartella, I.; Grasso, E.; Lenoci, D.; Del Gaudio, F.; Algeri, M.; Scarpellini, S.; et al. Heart Rate in Patients with SARS-CoV-2 Infection: Prevalence of High Values at Discharge and Relationship with Disease Severity. J. Clin. Med. 2021, 10, 5590. [Google Scholar] [CrossRef]
- Martin-Rodriguez, F.; Sanz-Garcia, A.; Melero Guijarro, L.; Ortega, G.J.; Gomez-Escolar Perez, M.; Castro Villamor, M.A.; Santos Pastor, J.C.; Delgado Benito, J.F.; Lopez-Izquierdo, R. Comorbidity-adjusted NEWS predicts mortality in suspected patients with COVID-19 from nursing homes: Multicentre retrospective cohort study. J. Adv. Nurs. 2021, 78, 1618–1631. [Google Scholar] [CrossRef]
- Natarajan, A.; Su, H.W.; Heneghan, C.; Blunt, L.; O’Connor, C.; Niehaus, L. Measurement of respiratory rate using wearable devices and applications to COVID-19 detection. NPJ Digit. Med. 2021, 4, 136. [Google Scholar] [CrossRef]
- Nathala, P.; Salunkhe, V.; Samanapally, H.; Xu, Q.; Furmanek, S.; Fahmy, O.H.; Deepti, F.; Glynn, A.; McGuffin, T.; Goldsmith, D.C.; et al. Electrocardiographic Features and Outcome: Correlations in 124 Hospitalized Patients With COVID-19 and Cardiovascular Events. J. Cardiothorac. Vasc. Anesth. 2022, 36, 2927–2934. [Google Scholar] [CrossRef]
- Severo Sanchez, A.; Rey, J.R.; Iniesta, A.M.; Merino, J.L.; Castrejon-Castrejon, S.; Lopez-de-Sa, E.; Caro-Codon, J. Heart rate at presentation of COVID-19: Can SARS-CoV-2 be a cause of dysautonomia? Rev. Port. Cardiol. 2022, 41, 355–357. [Google Scholar] [CrossRef]
- Tan, G.P.; Ho, S.; Fan, B.E.; Chotirmall, S.H.; Tan, C.H.; Lew, S.J.W.; Chia, P.Y.; Young, B.E.; Abisheganaden, J.A.; Puah, S.H. Reversible platypnea-orthodeoxia in COVID-19 acute respiratory distress syndrome survivors. Respir. Physiol. Neurobiol. 2020, 282, 103515. [Google Scholar] [CrossRef]
- Vanoli, J.; Marro, G.; Dell’Oro, R.; Facchetti, R.; Quarti-Trevano, F.; Spaziani, D.; Grassi, G. Elevated resting heart rate as independent in-hospital prognostic marker in COVID-19. Cardiol. J. 2022, 29, 181–187. [Google Scholar] [CrossRef]
- Bourdillon, N.; Yazdani, S.; Schmitt, L.; Millet, G.P. Effects of COVID-19 lockdown on heart rate variability. PLoS ONE 2020, 15, e0242303. [Google Scholar] [CrossRef]
- Chao, C.H.; Young, Y.H. Evolution of incidence of audiovestibular disorders during the pandemic COVID-19 period. Eur. Arch. Otorhinolaryngol. 2021, 279, 3341–3345. [Google Scholar] [CrossRef]
- D’Haese, P.F.; Finomore, V.; Lesnik, D.; Kornhauser, L.; Schaefer, T.; Konrad, P.E.; Hodder, S.; Marsh, C.; Rezai, A.R. Prediction of viral symptoms using wearable technology and artificial intelligence: A pilot study in healthcare workers. PLoS ONE 2021, 16, e0257997. [Google Scholar] [CrossRef]
- Diemberger, I.; Vicentini, A.; Cattafi, G.; Ziacchi, M.; Iacopino, S.; Morani, G.; Pisano, E.; Molon, G.; Giovannini, T.; Dello Russo, A.; et al. The Impact of COVID-19 Pandemic and Lockdown Restrictions on Cardiac Implantable Device Recipients with Remote Monitoring. J. Clin. Med. 2021, 10, 5626. [Google Scholar] [CrossRef]
- Ginty, A.T.; Young, D.A.; Tyra, A.T.; Hurley, P.E.; Brindle, R.C.; Williams, S.E. Heart Rate Reactivity to Acute Psychological Stress Predicts Higher Levels of Posttraumatic Stress Disorder Symptoms During the COVID-19 Pandemic. Psychosom. Med. 2021, 83, 351–357. [Google Scholar] [CrossRef]
- Kolacz, J.; Dale, L.P.; Nix, E.J.; Roath, O.K.; Lewis, G.F.; Porges, S.W. Adversity History Predicts Self-Reported Autonomic Reactivity and Mental Health in US Residents During the COVID-19 Pandemic. Front. Psychiatry 2020, 11, 577728. [Google Scholar] [CrossRef] [PubMed]
- Nivethitha, T.; Palanisamy, S.K.; Mohana Prakash, K.; Jeevitha, K. Comparative study of ANN and fuzzy classifier for forecasting electrical activity of heart to diagnose COVID-19. Mater. Today Proc. 2021, 45, 2293–2305. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.L.; Lau, T.; Karsikas, M.; Kinnunen, H.; Chee, M.W.L. A longitudinal analysis of COVID-19 lockdown stringency on sleep and resting heart rate measures across 20 countries. Sci. Rep. 2021, 11, 14413. [Google Scholar] [CrossRef] [PubMed]
- Pla, R.; Bosquet, L.; McGibbon, K.; Mujika, I.; Aubry, A. Heart rate variability in elite swimmers before, during and after COVID-19 lockdown: A brief report on time domain analysis. Appl. Sci. 2021, 11, 8106. [Google Scholar] [CrossRef]
- Rass, V.; Beer, R.; Schiefecker, A.J.; Kofler, M.; Lindner, A.; Mahlknecht, P.; Heim, B.; Limmert, V.; Sahanic, S.; Pizzini, A.; et al. Neurological outcome and quality of life 3 months after COVID-19: A prospective observational cohort study. Eur. J. Neurol. 2021, 28, 3348–3359. [Google Scholar] [CrossRef]
- Sinn, D.I.; Muppidi, S.; Miglis, M.G.; Jaradeh, S. Autonomic function test during the COVID-19 pandemic: The Stanford experience. Clin. Auton. Res. 2021, 31, 127–129. [Google Scholar] [CrossRef]
- Stute, N.L.; Stickford, A.S.L.; Stickford, J.L.; Province, V.M.; Augenreich, M.A.; Bunsawat, K.; Alpenglow, J.K.; Wray, D.W.; Ratchford, S.M. Altered central and peripheral haemodynamics during rhythmic handgrip exercise in young adults with SARS-CoV-2. Exp. Physiol. 2021, 107, 708–721. [Google Scholar] [CrossRef]
- Hou, R.; Tomalin, L.E.; Suarez-Farinas, M. cosinoRmixedeffects: An R package for mixed-effects cosinor models. BMC Bioinform. 2021, 22, 553. [Google Scholar] [CrossRef]
- Eskandar, E.N.; Altschul, D.J.; de la Garza Ramos, R.; Cezayirli, P.; Unda, S.R.; Benton, J.; Dardick, J.; Toma, A.; Patel, N.; Malaviya, A.; et al. Neurologic Syndromes Predict Higher In-Hospital Mortality in COVID-19. Neurology 2021, 96, e1527–e1538. [Google Scholar] [CrossRef]
- Gadaleta, M.; Radin, J.M.; Baca-Motes, K.; Ramos, E.; Kheterpal, V.; Topol, E.J.; Steinhubl, S.R.; Quer, G. Passive detection of COVID-19 with wearable sensors and explainable machine learning algorithms. NPJ Digit. Med. 2021, 4, 166. [Google Scholar] [CrossRef]
- Tanwar, G.; Chauhan, R.; Singh, M.; Singh, D. Pre-Emption of Affliction Severity Using HRV Measurements from a Smart Wearable; Case-Study on SARS-Cov-2 Symptoms. Sensors 2020, 20, 7068. [Google Scholar] [CrossRef]
- Khalpey, Z.I.; Khalpey, A.H.; Modi, B.; Deckwa, J. Autonomic Dysfunction in COVID-19: Early Detection and Prediction Using Heart Rate Variability. JACS 2021, 23, e20–e21. [Google Scholar] [CrossRef]
- Ponomarev, A.; Tyapochkin, K.; Surkova, E.; Smorodnikova, E.; Pravdin, P. Heart Rate Variability as a Prospective Predictor of Early COVID-19 Symptoms. medRxiv 2021. [Google Scholar] [CrossRef]
- COVID-19 and Wearables Open Data Research. [Data Set]. Available online: https://github.com/Welltory/hrv-covid19/blob/master/data/scales_description.csv (accessed on 23 May 2022).
- Rajendra Acharya, U.; Paul Joseph, K.; Kannathal, N.; Lim, C.M.; Suri, J.S. Heart rate variability: A review. Med. Biol. Eng. Comput. 2006, 44, 1031–1051. [Google Scholar] [CrossRef]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Eur. Heart J. 1996, 17, 354–381. [Google Scholar]
- Hela, E.; Sofien, K.; Kamel, L.; Asma, O.; Dalila, G.; Sondos, K.; Jamel, K. QT interval abnormalities and heart rate variability in patients with cirrhosis. Arab J. Gastroenterol. 2020, 21, 246–252. [Google Scholar] [CrossRef]
- Carter, R., 3rd; Hinojosa-Laborde, C.; Convertino, V.A. Heart rate variability in patients being treated for dengue viral infection: New insights from mathematical correction of heart rate. Front. Physiol. 2014, 5, 46. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, S.; Tejuja, A.; Newman, K.D.; Zarychanski, R.; Seely, A.J. Clinical review: A review and analysis of heart rate variability and the diagnosis and prognosis of infection. Crit. Care 2009, 13, 232. [Google Scholar] [CrossRef] [Green Version]
- Godijk, N.G.; Vos, A.G.; Jongen, V.W.; Moraba, R.; Tempelman, H.; Grobbee, D.E.; Coutinho, R.A.; Deville, W.; Klipstein-Grobusch, K. Heart Rate Variability, HIV and the Risk of Cardiovascular Diseases in Rural South Africa. Glob. Heart 2020, 15, 17. [Google Scholar] [CrossRef] [Green Version]
- Bower, M.M.; Sweidan, A.J.; Xu, J.C.; Stern-Neze, S.; Yu, W.; Groysman, L.I. Quantitative Pupillometry in the Intensive Care Unit. J. Intensive Care Med. 2021, 36, 383–391. [Google Scholar] [CrossRef]
- Scala, I.; Bellavia, S.; Luigetti, M.; Brunetti, V.; Broccolini, A.; Gabrielli, M.; Zileri Dal Verme, L.; Calabresi, P.; Della Marca, G.; Frisullo, G. Autonomic dysfunction in non-critically ill COVID-19 patients during the acute phase of disease: An observational, cross-sectional study. Neurol. Sci. 2022, 1–9. [Google Scholar] [CrossRef]
- Nolan, J.; Batin, P.D.; Andrews, R.; Lindsay, S.J.; Brooksby, P.; Mullen, M.; Baig, W.; Flapan, A.D.; Cowley, A.; Prescott, R.J.; et al. Prospective study of heart rate variability and mortality in chronic heart failure: Results of the United Kingdom heart failure evaluation and assessment of risk trial (UK-heart). Circulation 1998, 98, 1510–1516. [Google Scholar] [CrossRef] [Green Version]
- Tsuji, H.; Larson, M.G.; Venditti, F.J., Jr.; Manders, E.S.; Evans, J.C.; Feldman, C.L.; Levy, D. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation 1996, 94, 2850–2855. [Google Scholar] [CrossRef]
- La Rovere, M.T.; Bigger, J.T., Jr.; Marcus, F.I.; Mortara, A.; Schwartz, P.J. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet 1998, 351, 478–484. [Google Scholar] [CrossRef]
- Bauer, A.; Kantelhardt, J.W.; Barthel, P.; Schneider, R.; Makikallio, T.; Ulm, K.; Hnatkova, K.; Schomig, A.; Huikuri, H.; Bunde, A.; et al. Deceleration capacity of heart rate as a predictor of mortality after myocardial infarction: Cohort study. Lancet 2006, 367, 1674–1681. [Google Scholar] [CrossRef]
- Hu, W.; Jin, X.; Zhang, P.; Yu, Q.; Yin, G.; Lu, Y.; Xiao, H.; Chen, Y.; Zhang, D. Deceleration and acceleration capacities of heart rate associated with heart failure with high discriminating performance. Sci. Rep. 2016, 6, 23617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizera, L.; Boehm, K.; Duckheim, M.; Groga-Bada, P.; Gawaz, M.; Zuern, C.S.; Eick, C. Autonomic Nervous System Activity for Risk Stratification of Emergency Patients With Pneumonia. J. Emerg. Med. 2018, 55, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.L.; Chen, J.H.; Huang, C.C.; Kuo, C.D.; Huang, C.I.; Lee, L.S. Heart rate variability measures as predictors of in-hospital mortality in ED patients with sepsis. Am. J. Emerg. Med. 2008, 26, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, B.E.; Sundman, E.; Terrando, N.; Eriksson, L.I.; Olofsson, P.S. Neural Control of Inflammation: Implications for Perioperative and Critical Care. Anesthesiology 2016, 124, 1174–1189. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, Y.; Qiao, H.; Gao, Z.; Yang, J.; Chuai, X. Hold Breath: Autonomic Neural Regulation of Innate Immunity to Defend Against SARS-CoV-2 Infection. Front. Microbiol. 2021, 12, 819638. [Google Scholar] [CrossRef]
- Williams, D.P.; Koenig, J.; Carnevali, L.; Sgoifo, A.; Jarczok, M.N.; Sternberg, E.M.; Thayer, J.F. Heart rate variability and inflammation: A meta-analysis of human studies. Brain Behav. Immun. 2019, 80, 219–226. [Google Scholar] [CrossRef]
- Grzesiak, E.; Bent, B.; McClain, M.T.; Woods, C.W.; Tsalik, E.L.; Nicholson, B.P.; Veldman, T.; Burke, T.W.; Gardener, Z.; Bergstrom, E.; et al. Assessment of the Feasibility of Using Noninvasive Wearable Biometric Monitoring Sensors to Detect Influenza and the Common Cold Before Symptom Onset. JAMA Netw. Open 2021, 4, e2128534. [Google Scholar] [CrossRef]
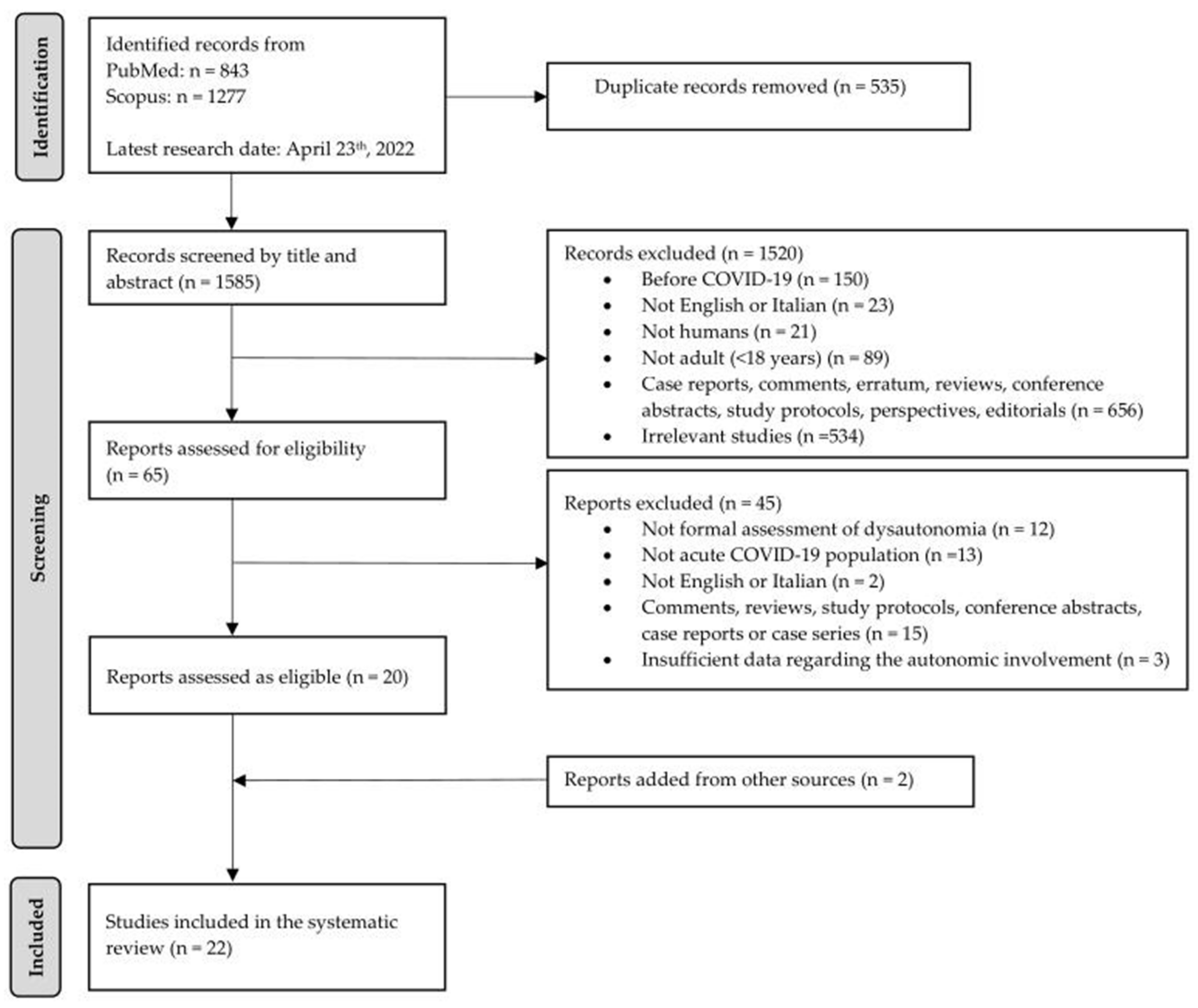
| Inclusion Criteria | Exclusion Criteria | |
|---|---|---|
| Population | Adult (≥18 years) patients with ongoing SARS-CoV-2 infection as diagnosed by a laboratory test, a thorax CT scan, self-reported by study participants, or reported by the authors of the study | Patients recovered from COVID-19, patients without history of SARS-CoV-2 infection, and patients with a dubious diagnosis of COVID-19 |
| Non-human sample | ||
| Intervention | NA | NA |
| Comparison | NA | NA |
| Outcomes | Characterization of autonomic involvement during acute SARS-CoV-2 infection | Absence of a formal evaluation of autonomic parameters |
| Effects of autonomic alterations on the SARS-CoV-2 infection outcome | Lack of sufficient data regarding the autonomic assessment | |
| Role of autonomic parameters in predicting SARS-CoV-2 infection | ||
| Study design | Case–control, cohort, cross-sectional, pre–post studies | Literature reviews, case reports or case series, conference papers, comments, editorials, erratum, study protocols, perspectives |
| Published from 1 December 2020 to 23 April 2022 | Not English or Italian Language |
| Authors, Year | Study Design | Study Population | Demographic Characteristics | COVID-19 Severity | COVID-19 Diagnosis | Dysautonomia Assessment | Study Endpoints | Main Findings |
|---|---|---|---|---|---|---|---|---|
| Bellavia et al., 2021 [31] | Observational, cross-sectional | 20 acute COVID-19 patients vs. 20 healthy controls | COVID+ group: Mean age 56.1 ± 19.2 y; 70% men COVID- group: Mean age 52.6 ± 13.7 y; 65% men | Moderate | RT-PCR | Sudoscan, automated pupillometry (Npi-200), HRV measured from a 10 min long EKG in the lying position and a 3 min long EKG in the standing position, PTT measured with a pulse oximeter | Characterization of the autonomic nervous system involvement in acute COVID-19 patients | Pupillometry: COVID+ group presented higher CV, ACA, BPD, and CH than controls. Sudoscan: COVID+ patients presented feet sudomotor more frequently than controls. No differences between groups in terms of HRV parameters and PTT |
| Hirten et al., 2021 [34] | Prospective, observational, cohort | 297 healthcare workers reporting data from wearable devices. 13/297 patients (COVID+) | Overall population: Mean age 36.3 ± 9.8 y; 31.6% men | NR | Self-reported RT-PCR test | PRV and HR measured by the PPG signal of wearable devices | Primary endpoint: Differentiation of acute COVID-19 patients from healthy controls through HRV. Secondary endpoints: Evaluation of HRV ability in predicting SARS-CoV-2 infection and in discriminating symptomatic and asymptomatic forms of COVID-19 | Amplitude of SDNN lower in COVID+ than in COVID- groups and higher in uninfected participants than in COVID+ subjects during the 7 days prior and after a COVID-19 diagnosis. No HRV differences between symptomatic and asymptomatic COVID+ subjects |
| Junarta et al., 2021 [35] | Retrospective, observational, pre–post | 38 hospitalized acute COVID-19 patients with chronic atrial fibrillation | Mean age 78.6 ± 11.4 y; 44.7% men | Moderate and severe | NR | HRV measured by EKGs obtained during hospitalization in the pre-COVID period and during admission for acute SARS-CoV-2 infection | Primary endpoint: Presence of HRV changes between the pre-COVID and the COVID period | HRV (SDSD, RMSSD, pNN50) significantly reduced during acute COVID-19 |
| Kaliyaperumal et al., 2021 [36] | Observational, cross-sectional | 63 acute COVID-19 patients vs. 43 age- and sex-matched healthy controls | COVID+: Mean age 48.4 ± 16.3 y; 69.8% men vs. COVID-: Mean age 50.1 ± 10.5 y; 62.8% men | Moderate | RT-PCR | HRV measured by a 5 min long EKG | Comparison of HRV parameters between acute COVID-19 patients and healthy controls | Lower values of HF, LF, and higher values of RMSSD in COVID-19 patients than in controls. Higher parasympathetic overtone (SDNN > 60 and/or RMSSD > 40) in COVID-19 patients than in healthy subjects. No HRV differences between symptomatic and asymptomatic COVID-19 patients |
| Kamaleswaran et al., 2021 [37] | Retrospective, observational, case–control | 141 acute, ICU-admitted COVID-19 patients vs. 208 ICU-admitted patients with sepsis from other causes | COVID+: Mean age 63 ± 16 y; 52% men Septic patients: Mean age 63 ± 16 y; 55% men | Severe | RT-PCR | Average of HRV parameters measured by several 300 s sliding windows obtained from continuous bedside monitoring within 5 days of ICU admission | Secondary endpoint: Comparisons of HRV parameters between COVID-19 patients and patients with sepsis from other causes | COVID-19 patients presented lower median DC, ApEn, SampEn, pNN50, and higher median AC, SD1:SD2, and NN mode than sepsis patients |
| Khalpey et al., 2021 [94] | Retrospective, observational, case–control | 200 patients divided into four groups: symptomatic COVID+, COVID+ with silent hypoxia, symptomatic COVID-, COVID- with silent hypoxia | NR | Not specified (moderate and/or severe) | RT-PCR | HRV measured by an EKG | Determination of HRV changes in patients with COVID-19 pneumonia | RMSSD, SDNN, and HRV triangular index differed between COVID+ and COVID- patients. The same parameters did not differ between symptomatic COVID+ patients and COVID+ subjects with silent hypoxia |
| Koh et al., 2021 [39] | Prospective, observational, cohort | 47,572 acute COVID-19 patients | Overall population: Median age 34 y; age range 1−102 y; 98% men 5/47,572 patients with autonomic symptoms: Mean age 37.8 ± 6.6 y; 100% men | Mild, moderate, and severe | RT-PCR and/or raised IgG-anti SARS-CoV-2 | Tilt table test, sympathetic skin response, and ophthalmological evaluation | Primary endpoint: Definition of neurological symptoms incidence and characterization in acute COVID-19 patients | Dysautonomic symptoms’ incidence in acute COVID-19 patients is 0.01%. Three patients presented pupil abnormalities (one—Adie’s pupil; one—Argyll Robertson; one—inverse Argyll Robertson), two patients presented POTS (one of them also presented Adie’s pupil and the other hyperhidrosis), and one patient presented small fiber neuropathy |
| Lonini et al., 2020 [40] | Observational, cross-sectional | 15 acute COVID-19 patients vs. 14 healthy controls | Demographics are available for 14/15 COVID+ patients and 12/14 controls Overall COVID-19 population: Mean age 52.0 ± 15.2 y; 50% men Healthy controls: Mean age 32.4 ± 6.8 y; 67% men | Mild and moderate | The diagnostic test employed not specified | PRV measured by wearable devices and a sensing platform during periods of rest, walking, and forced coughs | Ability of physiological parameters measured by wearable sensors and sensing platforms in the discrimination between COVID-19 patients and healthy controls | SDNN at rest significantly lower in COVID-19 patients than in controls at baseline (pre-walk). No change in SDNN of COVID-19 patients during and after exercise. Higher post-exercise heart rate in COVID-19 patients than in healthy controls, despite the lower walking cadence |
| Milovanovic et al., 2021 [41] | Retrospective, observational, case–control | 75 acute COVID-19 patients Mild group 30/75 (no pneumonia) Severe group 45/75 (with interstitial pneumonia) vs. 77 age-matched healthy controls | Mild COVID-19 patients: Mean age 41.6 ± 16.7 y; 53% men Severe COVID-19 patients: Mean age 51.3 ± 19.1 y; 53% men Sex- and age-matched controls | Moderate | RT-PCR | CART (Valsalva ratio, deep breathing test, blood pressure response to standing, handgrip test), HRV measured by continuous bedside monitoring, BPV and BRS measured by continuous bedside monitoring | Evaluation of autonomic dysfunction in acute COVID-19 patients and its impact on the cardiovascular system | CART: COVID-19 population presented higher prevalence of combined autonomic dysfunction and sympathetic dysfunction than controls. Parasympathetic dysfunction more frequent in mild cases and lower in severe cases of COVID-19 than in the control group. HRV: LF significantly lower in COVID-19 patients than in the control group. HF significantly lower in mild and LF/HF in severe COVID-19 patients than in controls. SD1 and SD1:SD2 lower in mild COVID-19 patients than in controls. BPV: higher systolic and diastolic HF and diastolic VLF and lower diastolic LF and LF/HF in COVID-19 patients than in controls. BRS: lower BRS in COVID-19 patients than in controls |
| Oates et al., 2020 [44] | Retrospective, observational, case–control | 37 acute COVID-19 patients with syncope/presyncope vs. 40 acute COVID-19 patients without syncope/presyncope | Overall population: Median age 69 (56–73) y; 55% men Syncope group: Median age 69 (56.5–73) y; 51% men No syncope group: Median age 68 (56–73) y; 57% men | Moderate and severe | RT-PCR | Evaluation of patients’ medical records, including heart rate and blood pressure measurements | Definition of syncope/presyncope incidence, characteristics, and outcomes in acute COVID-19 patients | Syncope/presyncope incidence was 3.7% (37/1000). Syncope/presyncope patients hospitalized less frequently in ICU settings. Within syncope subtypes, 12.5% (4/32) were hypotensive, and 15.6% (5/32) were neurocardiogenic. Two out of four patients with hypotensive syncope presented orthostatic hypotension, while the other two were not tested |
| Skazkina et al., 2021 [46] | Observational, cross-sectional | 32 acute COVID-19 patients vs. 32 healthy controls | COVID+ group: Age range 25–68 y; 56.3% men COVID- group: Age range 17–23 y; 31.3% men | NR | NR | HRV measured by 20 min long EKG and PPG | Comparison of the degree of synchronization of the autonomic control loops of circulation between COVID-19 patients and controls | Mean phase synchronization index was lower in acute COVID-19 patients than in healthy controls |
| Vrettou et al., 2020 [47] | Observational, cross-sectional | 41 patients requiring mechanical ventilation for at least 48 h COVID+ group: 18/41 Respiratory failure from other etiologies: 23/41 | COVID+ group: sedated patients (6/18) median age 68 (55–76) y; 83% men vs. not-sedated patients (12/18) median age 68 (60–78) y; 75% men COVID- group: sedated patients (14/23) median age 65 (51–76) y; 71% men vs. not-sedated patients (9/23) median age 65 (56–80) y; 67% men | Severe | RT-PCR | Automated pupillometry (Npi-200) | Differences in pupillary reactivity between mechanically ventilated ICU patients with COVID-19 and patients with respiratory failure from other etiologies | No significant differences in pupillary reactivity between COVID+ and COVID- groups. BPD, CH, CV, MCV, and DV were higher in awake COVID-19 patients than in sedated COVID-19 group |
| Yurttaser Ocak et al., 2022 [48] | Prospective, observational, pre–post | 58 acute COVID-19 patients | Mean age 47.23 ± 1.1 years; 56.9% men | Moderate | RT-PCR | Automated pupillometry (Sirius Topographer, Phoenix v2.1 software) | Comparison of pupillary reactivity during and three months after COVID-19 infection | Mean mesopic and scotopic diameter significantly lower during the acute phase of the infection than three months later. Mean pupil diameter and average DV significantly lower at different timepoints after the luminous stimulus during the acute phase of infection than after three months |
| Authors, Year | Study Design | Study Population | Demographic Characteristics | COVID-19 Severity | COVID-19 Diagnosis | Dysautonomia Assessment | Study Endpoints | Main Findings |
|---|---|---|---|---|---|---|---|---|
| Aragon-Benedì et al., 2021 [29] | Prospective, observational, cohort | 14 acute ICU-admitted COVID-19 patients Survivors group: 7 Non-survivors group: 7 | Survivors group: median age 64 (60–73) y; 57% men Non-survivors group: median age 71 (57–72) y; 100% men | Severe | RT-PCR | HRV measured by means of analgesia nociception index monitor from 240 s long EKG | Demonstration of an autonomic involvement with a sympathetic predominance and a parasympathetic withdrawal in the most severely ill COVID-19 patients | ANIm and ANIi (indices of the HF component) significantly higher in non-survivors than in survivors. Lower energy (SDNN) correlated with higher SOFA score and, in non-survivors, with fewer survival days. A limit value of 80 for ANIm predicted mortality (sensitivity of 100%; specificity of 85.7%). A limit value of 0.41 ms for energy predicted mortality (sensitivity of 71.4%; specificity of 71.4%) |
| Battaglini et al., 2020 [30] | Retrospective, observational, cohort | 94 acute COVID-19 patients undergoing invasive mechanical ventilation 53/94 (56%) underwent continuous neuromonitoring In 29/94 (31%), pupillary reactivity was tested | Overall population: Mean age 61.6 ± 11.1 y; 78.7% men Patients with neurological complications: Mean age 62.4 ± 8.3 y; 87.2% men Patients without neurological complications: Mean age 60.8 ± 13.3 y; 70.2% men | Severe | RT-PCR | Automated pupillometry (Neurolight Algiscan) | Primary endpoint: Prevalence of neurological complications and their effects on outcome. Secondary endpoint: Role of cerebral hemodynamics changes in predicting outcome and occurrence of neurological complications | Neurological complications incidence was 50%, and they were associated with longer overall and ICU stay. Automated pupillometry evaluation did not discriminate outcome in terms of mortality. Patients with neurological complications presented lower mean CV than patients without neurological symptoms |
| Hasty et al., 2021 [32] | Prospective, observational, cohort study | 16 acute COVID-19 patients requiring HFNO or mechanical ventilation | Mean age 60.5 ± 13.4 y; 71% men | Severe | RT-PCR | HRV measured by 7 min long single-limb EKG traces | Evaluation of a correlation between HRV reduction and CRP increment | A >40% decrease in SDNN predicted a subsequent 50% rise in CRP, with 83.3% sensitivity and 75% specificity |
| Junarta et al., 2021 [35] | Retrospective, observational, pre–post | 38 hospitalized acute COVID-19 patients with chronic atrial fibrillation | Mean age 78.6 ± 11.4 y; 44.7% men | Moderate and severe | NR | HRV measured by EKGs obtained during hospitalization in the pre-COVID period and during admission for acute SARS-CoV-2 infection | Secondary endpoint: Prognostic role of HRV in acute COVID-19 patients, comparing patients with reduced HRV to ones with preserved HRV | Patients with reduced HRV presented higher mortality when stratified for pNN50 |
| Kamaleswaran et al., 2021 [37] | Retrospective, observational, case–control | 141 acute, ICU-admitted COVID-19 patients vs. 208 ICU-admitted patients with sepsis from other causes | COVID+: Mean age 63 ± 16 y; 52% men (survivors: Mean age 59 ± 15 y; 53% men. Non-survivors: Mean age 71 ± 14 y; 52% men) | Severe | RT-PCR | Average of HRV parameters measured by several 300 s sliding windows obtained from continuous bedside monitoring within 5 days of ICU admission | Primary endpoint: Analysis of HRV differences between survivors and non-survivors with acute COVID-19 | SD1:SD2, AC, and pNN50 were higher, and NN, ApEn, SampEN, and DC were lower in COVID-19 non-survivors than in COVID-19 survivors |
| Khodadadi et al., 2021 [38] | Prospective, observational, cohort | 36 acute COVID-19 patients | NR | Moderate and severe | RT-PCR | LF–HRV, RSA amplitude, heart period, and vagal efficiency measured by a 7–10 min long EKG obtained by means of a Polar H10 heart rate sensor on the first day of admission | Primary endpoint: Definition of the role of demographic, clinical, and HRV parameters in forecasting LOS of COVID-19 patients | A higher vagal efficiency correlated with shorter LOS only in younger patients (<40 y) |
| Mizera et al., 2021 [42] | Prospective, observational, cohort | 60 acute COVID-19 patients with sinus rhythm ARDS group (37/60) vs. non-ARDS group (23/60) | Overall population: Mean age 66.9 ± 13.4 y; 60% men ARDS group: Mean age 69.1 ± 12.8 y; 56.8% men Non-ARDS group: Mean age 63.3 ± 13.9 y; 65.2% men | Moderate and/or severe | RT-PCR | DC measured by 10–30 min long EKG obtained from a 24 h EKG Holter recording | Evaluation of DC’s role in the prediction of ARDS development in acute COVID-19 patients | DC significantly lower in the ARDS group when compared to the non-ARDS group. Patients with ARDS were more likely to show DC < 4.5 ms. Decreased DC was also associated with increased risk of ARDS in the adjusted analysis and presented a good discriminatory capacity for predicting COVID-19 patients at risk of developing ARDS |
| Mol et al., 2021 [43] | Retrospective, observational, cohort | 271 hospitalized, acute COVID-19 patients | Overall population: Mean age 68 y (age range 25 to 95 y); 59% men | Moderate and severe | RT-PCR | HRV measured by a 10 s long EKG performed within 3 days of admission | Primary endpoint: HRV ability to predict overall survival at three weeks from admission. Secondary endpoints: HRV ability to predict ICU referral and impact of HRV on other prognostic factors, such as age | Patients with SDNN > 8 had a lower risk of death than those with SDNN ≤ 8 at three weeks from admission. SDNN ≤ 8 predicted a higher mortality only in older patients (≥70 y). Only patients aged ≥70 y with low HRV were at higher risk of death than younger patients. Lower risk of needing ICU care in patients with SDNN > 8 and RMSSD > 8 |
| Pan et al., 2021 [45] | Prospective, observational, cohort | 34 acute COVID-19 patients Mild group: 13/34 Severe group: 21/34 | Overall population: Mean age 56.2 ± 16.0 y; 32% men Mild group: Mean age 47.5 ± 14.2 y; 23% men Severe group: 61.5 ± 15.0 y; 38% men | Moderate and severe | RT-PCR | HRV measured by a 24 h long Holter EKG | Primary endpoint: Evaluation of HRV ability in prediction of severity and prognosis of acute COVID-19 patients | Lower SDNN, SDANN, and higher LF/HF in severe than in mild patients, and these parameters discriminated the two conditions with a good sensitivity and specificity. Shorter time to viral RNA negative conversion and disease recovery in severe patients with decreased LF/HF than those with increased LF/HF |
| Authors, Year | Study Design | Study Population | Demographic Characteristics | COVID-19 Severity | COVID-19 Diagnosis | Dysautonomia Assessment | Study Endpoints | Main Findings |
|---|---|---|---|---|---|---|---|---|
| Hijazi et al., 2021 [33] | Retrospective, observational, pre–post | 186 acute COVID-19 patients (Weltory study) | Mean age 44 ± 14.1 y; 36% men | NR | Self-reported by study participants | PRV and HR measured by the PPG signal of wearable devices | Evaluation of wearable devices’ role in predicting SARS-CoV-2 infection | SDNN, RMSSD, pNN50 significantly lower, and HR and LF/HF significantly higher during the infection than during the healthy time. The model with five HRV variables (pNN50, RMSSD, LF/HF, SDNN, and HR) and the answer to the question “How do you feel?” ranked the best in the discrimination between infection and healthy time, with an AUC value of 0.938 |
| Lonini et al., 2020 [40] | Observational, cross-sectional | 15 acute COVID-19 patients vs. 14 healthy controls | Demographics are available for 14/15 COVID+ patients and 12/14 controls Overall COVID-19 population: Mean age 52.0 ± 15.2 y; 50% men Healthy controls: Mean age 32.4 ± 6.8 y; 67% men | Mild and moderate | The diagnostic test employed not specified | PRV measured by wearable devices and a sensing platform during periods of rest, walking, and forced coughs | Evaluation of the ability of physiological parameters measured by wearable sensors and sensing platforms in the discrimination between COVID-19 patients and healthy controls | SDNN at rest significantly lower in COVID-19 patients than in controls at baseline (pre-walk). No change in SDNN of COVID-19 patients during and after exercise |
| Ponomarev et al., 2021 [95] | Retrospective, observational, pre–post | 14 acute COVID-19 patients (Weltory study) | Mean age: 44 ± 8.7 y; 64% men | NR | Self-reported by study participants | PRV measured by the PPG signal of wearable devices at the time of day each user took measurements most often | Evaluation of HRV ability in the prediction of COVID-19 infection | No significant differences in HRV parameters before, during, and after acute COVID-19 in the overall population. Analyzing individual users independently, three users presented lower SDNN, one user higher SDNN, and one user lower RMSSD during COVID-19 compared to the period before the infection |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scala, I.; Rizzo, P.A.; Bellavia, S.; Brunetti, V.; Colò, F.; Broccolini, A.; Della Marca, G.; Calabresi, P.; Luigetti, M.; Frisullo, G. Autonomic Dysfunction during Acute SARS-CoV-2 Infection: A Systematic Review. J. Clin. Med. 2022, 11, 3883. https://doi.org/10.3390/jcm11133883
Scala I, Rizzo PA, Bellavia S, Brunetti V, Colò F, Broccolini A, Della Marca G, Calabresi P, Luigetti M, Frisullo G. Autonomic Dysfunction during Acute SARS-CoV-2 Infection: A Systematic Review. Journal of Clinical Medicine. 2022; 11(13):3883. https://doi.org/10.3390/jcm11133883
Chicago/Turabian StyleScala, Irene, Pier Andrea Rizzo, Simone Bellavia, Valerio Brunetti, Francesca Colò, Aldobrando Broccolini, Giacomo Della Marca, Paolo Calabresi, Marco Luigetti, and Giovanni Frisullo. 2022. "Autonomic Dysfunction during Acute SARS-CoV-2 Infection: A Systematic Review" Journal of Clinical Medicine 11, no. 13: 3883. https://doi.org/10.3390/jcm11133883
APA StyleScala, I., Rizzo, P. A., Bellavia, S., Brunetti, V., Colò, F., Broccolini, A., Della Marca, G., Calabresi, P., Luigetti, M., & Frisullo, G. (2022). Autonomic Dysfunction during Acute SARS-CoV-2 Infection: A Systematic Review. Journal of Clinical Medicine, 11(13), 3883. https://doi.org/10.3390/jcm11133883








