Clinical Characteristics and Healthcare Resource Utilization among Patients with Obstructive Hypertrophic Cardiomyopathy Treated in a Range of Settings in the United States
Abstract
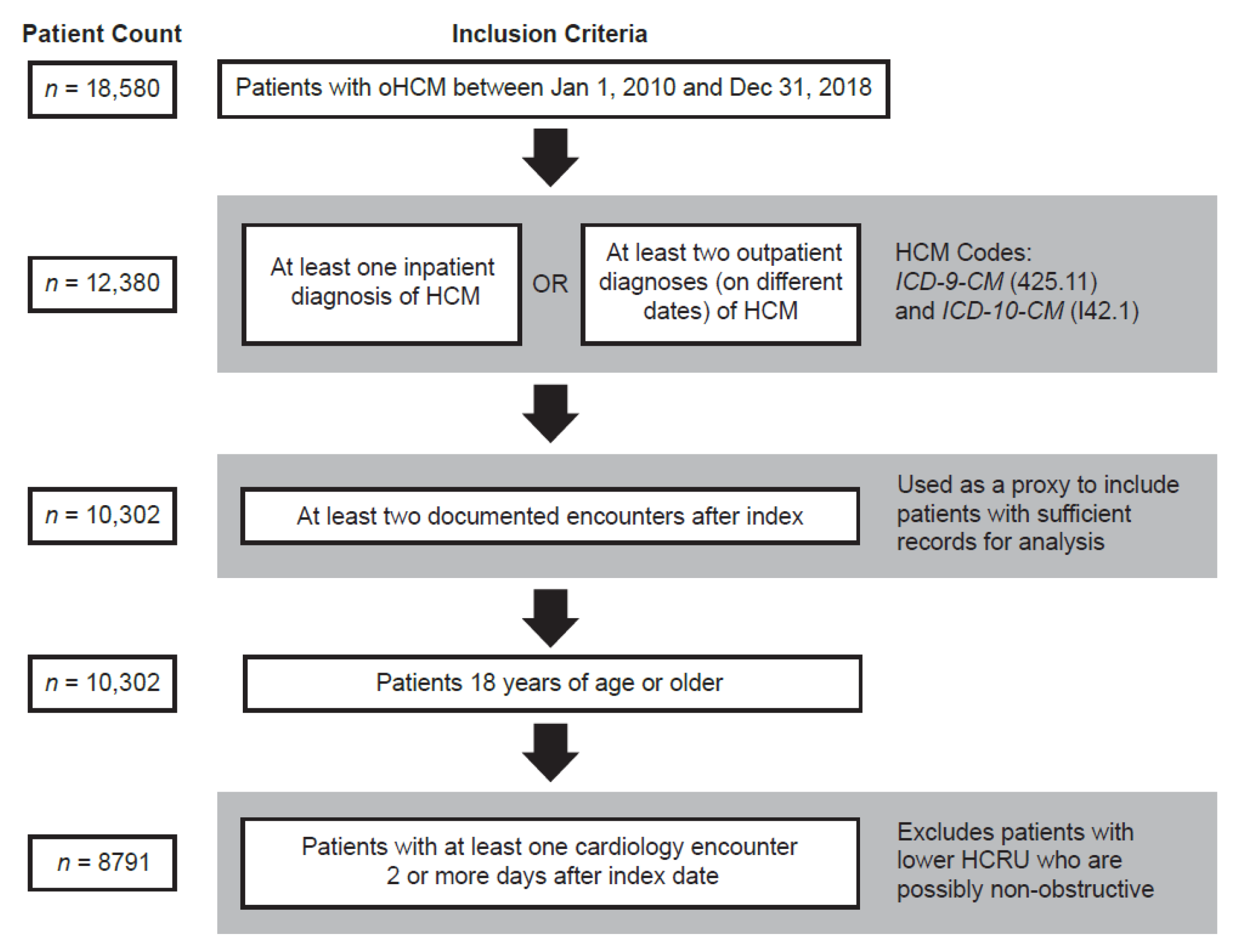
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Source
2.3. Study Measures and Data Analysis
3. Results
3.1. Baseline Characteristics
3.2. Outcomes and Healthcare Resource Utilization
3.3. Septal Reduction Therapy
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

Appendix B
| Characteristic | Categories |
|---|---|
| Age categories, years | 18–39, 40–54, 55–64, 65–74, 75+ |
| Ethnicity | Hispanic, non-Hispanic, other ethnicity, declined, unknown, and missing |
| Race | African American, Asian, White, Hispanic Latino, multi-racial, other, unknown, refused to classify, and missing |
| Insurance type | Medicare, Medicaid, private, self-pay, other public, other, unknown, and missing |
| Geographic region | Northeast, Midwest, South, West, Puerto Rico, and missing |
| Comorbidities | Coronary artery disease, pulmonary hypertension, obstructive sleep apnea, hypertension, type 2 diabetes, obesity/overweight, and conduction disorders |
| HCM-related outcomes | Atrial fibrillation, congestive heart failure, ventricular and supraventricular arrhythmias, and cardiac arrest |
| Diagnostic procedures | Coronary angiography, myocardial imaging, exercise/pharmacologic stress testing, electrocardiography, and genetic testing for HCM |
| Prescription medications | Beta-blockers, CCBs, ACEIs, ARBs, anticoagulation/antiplatelet therapy/thrombolytics, and antiarrhythmic therapy (class I, class III, and other) |
| Surgical procedures | Septal myectomy, alcohol septal ablation, radiofrequency ablation, pulmonary vein ablation, other ablation, coronary revascularization, valve surgery, pacemaker, implantable cardioverter defibrillator, and heart transplantation |
| Reintervention | Reintervention is defined as requiring a septal myectomy, alcohol septal ablation, pacemaker, or implantable cardioverter defibrillator after initial septal myectomy or alcohol septal ablation |
References
- Maron, B.J.; Desai, M.Y.; Nishimura, R.A.; Spirito, P.; Rakowski, H.; Towbin, J.A.; Dearani, J.A.; Rowin, E.J.; Maron, M.S.; Sherrid, M.V. Management of hypertrophic cardiomyopathy: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2022, 79, 390–414. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.Y.; Pozios, I.; Haileselassie, B.; Ventoulis, I.; Liu, H.; Sorensen, L.L.; Canepa, M.; Phillip, S.; Abraham, M.R.; Abraham, T.P. Clinical outcomes in patients with nonobstructive, labile, and obstructive hypertrophic cardiomyopathy. J. Am. Heart Assoc. 2018, 7, e006657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ommen, S.R.; Mital, S.; Burke, M.A.; Day, S.M.; Deswal, A.; Elliott, P.; Evanovich, L.L.; Hung, J.; Joglar, J.A.; Kantor, P.; et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2020, 142, e558–e631. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Rowin, E.J.; Casey, S.A.; Link, M.S.; Lesser, J.R.; Chan, R.H.; Garberich, R.F.; Udelson, J.E.; Maron, M.S. Hypertrophic cardiomyopathy in adulthood associated with low cardiovascular mortality with contemporary management strategies. J. Am. Coll. Cardiol. 2015, 65, 1915–1928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desai, M.Y.; Bhonsale, A.; Smedira, N.G.; Naji, P.; Thamilarasan, M.; Lytle, B.W.; Lever, H.M. Predictors of long-term outcomes in symptomatic hypertrophic obstructive cardiomyopathy patients undergoing surgical relief of left ventricular outflow tract obstruction. Circulation 2013, 128, 209–216. [Google Scholar] [CrossRef] [Green Version]
- Ommen, S.R.; Maron, B.J.; Olivotto, I.; Maron, M.S.; Cecchi, F.; Betocchi, S.; Gersh, B.J.; Ackerman, M.J.; McCully, R.B.; Dearani, J.A.; et al. Long-term effects of surgical septal myectomy on survival in patients with obstructive hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2005, 46, 470–476. [Google Scholar] [CrossRef] [Green Version]
- Maron, M.S.; Rowin, E.J.; Wessler, B.S.; Mooney, P.J.; Fatima, A.; Patel, P.; Koethe, B.C.; Romashko, M.; Link, M.S.; Maron, B.J. Enhanced American College of Cardiology/American Heart Association strategy for prevention of sudden cardiac death in high-risk patients with hypertrophic cardiomyopathy. JAMA Cardiol. 2019, 4, 644–657. [Google Scholar] [CrossRef] [Green Version]
- Maron, B.J.; Ommen, S.R.; Semsarian, C.; Spirito, P.; Olivotto, I.; Maron, M.S. Hypertrophic cardiomyopathy: Present and future, with translation into contemporary cardiovascular medicine. J. Am. Coll. Cardiol. 2014, 64, 83–99. [Google Scholar] [CrossRef] [Green Version]
- Rastegar, H.; Boll, G.; Rowin, E.J.; Dolan, N.; Carroll, C.; Udelson, J.E.; Wang, W.; Carpino, P.; Maron, B.J.; Maron, M.S.; et al. Results of surgical septal myectomy for obstructive hypertrophic cardiomyopathy: The Tufts experience. Ann. Cardiothorac. Surg. 2017, 6, 353–363. [Google Scholar] [CrossRef] [Green Version]
- Rowin, E.J.; Maron, M.S.; Bhatt, V.; Gillam, L.; Maron, B.J. Hypertrophic cardiomyopathy in “real-world” community cardiology practice. Am. J. Cardiol. 2020, 125, 1398–1403. [Google Scholar] [CrossRef]
- Kofflard, M.J.; Ten Cate, F.J.; van der Lee, C.; van Domburg, R.T. Hypertrophic cardiomyopathy in a large community-based population: Clinical outcome and identification of risk factors for sudden cardiac death and clinical deterioration. J. Am. Coll. Cardiol. 2003, 41, 987–993. [Google Scholar] [CrossRef] [Green Version]
- Maron, B.J.; Casey, S.A.; Poliac, L.C.; Gohman, T.E.; Almquist, A.K.; Aeppli, D.M. Clinical course of hypertrophic cardiomyopathy in a regional United States cohort. JAMA 1999, 281, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Efthimiadis, G.K.; Parcharidou, D.; Pagourelias, E.D.; Meditskou, S.; Spanos, G.; Hadjimiltiades, S.; Pliakos, C.; Gavrielides, S.; Karvounis, H.; Styliadis, I.H.; et al. Prevalence and clinical outcomes of incidentally diagnosed hypertrophic cardiomyopathy. Am. J. Cardiol. 2010, 105, 1445–1450. [Google Scholar] [CrossRef] [PubMed]
- Nistri, S.; Olivotto, I.; Girolami, F.; Torricelli, F.; Cecchi, F.; Yacoub, M.H. Looking for hypertrophic cardiomyopathy in the community: Why is it important? J. Cardiovasc. Transl. Res. 2009, 2, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, L.M.; Zezulka, A. Hypertrophic cardiomyopathy: A common disease with a good prognosis. Five year experience of a district general hospital. Br. Heart J. 1983, 50, 530–533. [Google Scholar] [CrossRef] [Green Version]
- Petrin, T.J.; Tavel, M.E. Idiopathic hypertrophic subaortic stenosis as observed in a large community hospital: Relation to age and history of hypertension. J. Am. Geriatr. Soc. 1979, 27, 43–46. [Google Scholar] [CrossRef]
- Shah, P.M.; Adelman, A.G.; Wigle, E.D.; Gobel, F.L.; Burchell, H.B.; Hardarson, T.; Curiel, R.; De La Calzada, C.; Oakley, C.M.; Goodwin, J.F. The natural (and unnatural) history of hypertrophic obstructive cardiomyopathy. Circ. Res. 1974, 35 (Suppl. II), 179–195. [Google Scholar]
- Kofflard, M.J.; Waldstein, D.J.; Vos, J.; ten Cate, F.J. Prognosis in hypertrophic cardiomyopathy observed in a large clinic population. Am. J. Cardiol. 1993, 72, 939–943. [Google Scholar] [CrossRef] [Green Version]
- Maron, B.J.; Olivotto, I.; Spirito, P.; Casey, S.A.; Bellone, P.; Gohman, T.E.; Graham, K.J.; Burton, D.A.; Cecchi, F. Epidemiology of hypertrophic cardiomyopathy-related death: Revisited in a large non-referral-based patient population. Circulation 2000, 102, 858–864. [Google Scholar] [CrossRef]
- Maron, B.J.; Peterson, E.E.; Maron, M.S.; Peterson, J.E. Prevalence of hypertrophic cardiomyopathy in an outpatient population referred for echocardiographic study. Am. J. Cardiol. 1994, 73, 577–580. [Google Scholar] [CrossRef]
- The R Core Team. R: A Language and Environment for Statistical Computing, Reference Index, R Foundation for Statistical Computing. Reference Index; Version 2.6.2. 2008. Available online: https://softlibre.unizar.es/manuales/aplicaciones/r/fullrefman.pdf (accessed on 12 May 2022).
- Microsoft Corporation. Microsoft Excel. Available online: https://office.microsoft.com/excel (accessed on 1 April 2022).
- Maron, M.S.; Hellawell, J.L.; Lucove, J.C.; Farzaneh-Far, R.; Olivotto, I. Occurrence of clinically diagnosed hypertrophic cardiomyopathy in the United States. Am. J. Cardiol. 2016, 117, 1651–1654. [Google Scholar] [CrossRef] [PubMed]
- Owens, A.T.; Sutton, M.B.; Gao, W.; Fine, J.T.; Xie, J.; Naidu, S.S.; Desai, N.R. Treatment changes, healthcare resource utilization, and costs among patients with symptomatic obstructive hypertrophic cardiomyopathy: A claims database study. Cardiol. Ther. 2022, 11, 249–267. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.S.; Li, S.S.; Xie, J.; Sutton, M.B.; Fine, J.T.; Edelberg, J.M.; Gao, W.; Spertus, J.A.; Cohen, D.J. Clinical and economic burden of obstructive hypertrophic cardiomyopathy in the United States. J. Med. Econ. 2021, 24, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Butzner, M.; Sarocco, P.; Maron, M.S.; Rowin, E.; Teng, C.C.; Stanek, E.; Tan, H.; Robertson, L.A. Characteristics of patients with obstructive hypertrophic cardiomyopathy in real-world community-based cardiovascular practices. Am. J. Cardiol. 2022, 174, 120–125. [Google Scholar] [CrossRef]
- Butzner, M.; Leslie, D.; Cuffee, Y.; Hollenbeak, C.S.; Sciamanna, C.; Abraham, T.P. Sex differences in clinical outcomes for obstructive hypertrophic cardiomyopathy in the USA: A retrospective observational study of administrative claims data. BMJ Open 2022, 12, e058151. [Google Scholar] [CrossRef]
- Rowin, E.J.; Maron, M.S.; Wells, S.; Patel, P.P.; Koethe, B.C.; Maron, B.J. Impact of sex on clinical course and survival in the contemporary treatment era for hypertrophic cardiomyopathy. J. Am. Heart Assoc. 2019, 8, e012041. [Google Scholar] [CrossRef]
- Meghji, Z.; Nguyen, A.; Fatima, B.; Geske, J.B.; Nishimura, R.A.; Ommen, S.R.; Lahr, B.D.; Dearani, J.A.; Schaff, H.V. Survival differences in women and men after septal myectomy for obstructive hypertrophic cardiomyopathy. JAMA Cardiol. 2019, 4, 237–245. [Google Scholar] [CrossRef]
- Maron, B.J.; Rowin, E.J.; Udelson, J.E.; Maron, M.S. Clinical spectrum and management of heart failure in hypertrophic cardiomyopathy. JACC Heart Fail. 2018, 6, 353–363. [Google Scholar] [CrossRef]
- Liebregts, M.; Vriesendorp, P.A.; Mahmoodi, B.K.; Schinkel, A.F.; Michels, M.; ten Berg, J.M. A systematic review and meta-analysis of long-term outcomes after septal reduction therapy in patients with hypertrophic cardiomyopathy. JACC Heart Fail. 2015, 3, 896–905. [Google Scholar] [CrossRef]
- Nishimura, R.A.; Seggewiss, H.; Schaff, H.V. Hypertrophic obstructive cardiomyopathy: Surgical myectomy and septal ablation. Circ. Res. 2017, 121, 771–783. [Google Scholar] [CrossRef]
- Woo, A.; Williams, W.G.; Choi, R.; Wigle, E.D.; Rozenblyum, E.; Fedwick, K.; Siu, S.; Ralph-Edwards, A.; Rakowski, H. Clinical and echocardiographic determinants of long-term survival after surgical myectomy in obstructive hypertrophic cardiomyopathy. Circulation 2005, 111, 2033–2041. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Demographic Category | Patients with oHCM (n = 8791) |
|---|---|
| Sex, n (%) | |
| Male | 4134 (47.0) |
| Female | 4657 (53.0) |
| Age at index, years | |
| Mean ± SD | 61.8 ± 15.0 |
| Median (IQR) | 63.0 (21.0) |
| Age categories, years, n (%) | |
| 18–39 | 773 (8.8) |
| 40–54 | 1774 (20.2) |
| 55–64 | 2101 (23.9) |
| 65–74 | 2222 (25.3) |
| 75+ | 1921 (21.9) |
| Ethnicity, n (%) | |
| Hispanic | 300 (3.4) |
| Non-Hispanic | 7769 (88.4) |
| Other | 722 (8.2) |
| Race, * n (%) | |
| African American | 1164 (13.2) |
| Asian | 111 (1.3) |
| White | 7138 (81.2) |
| Hispanic Latino | 33 (0.4) |
| Other | 734 (8.3) |
| Insurance type, * n (%) | |
| Medicare | 4070 (46.3) |
| Medicaid | 712 (8.1) |
| Private | 5189 (59.0) |
| Self-pay | 273 (3.1) |
| Other | 2221 (25.3) |
| Geographic region, * n (%) | |
| Northeast | 783 (8.9) |
| Midwest | 4824 (54.9) |
| South | 2410 (27.4) |
| West | 701 (8.0) |
| Puerto Rico | 2 (0) |
| Missing | 73 (0.8) |
| Cohort enrollment time post-index, months † | |
| Mean ± SD | 41 ± 28 |
| Median ± SD | 38 (44) |
| Patients with enrollment time ≥12 months, n (%) | 7169 (81.5) |
| Patients with enrollment time ≥24 months, n (%) | 5864 (66.7) |
| Patients with oHCM (n = 8791) | 12-Month Follow-Up, n (%) | 24-Month Follow-Up, n (%) | End of Study Period, n (%) |
|---|---|---|---|
| Diagnostic procedures | |||
| Coronary angiography | 812 (9.2) | 995 (11.3) | 1236 (14.1) |
| Myocardial imaging | 6440 (73.3) | 7203 (81.9) | 7682 (87.4) |
| Exercise stress testing | 1726 (19.6) | 2196 (25.0) | 2816 (32.0) |
| Electrocardiography | 5041 (57.3) | 5887 (67.0) | 6456 (73.4) |
| Inpatient hospitalization | 2182 (24.8) | 2493 (28.4) | 2914 (33.1) |
| oHCM comorbidities | |||
| Coronary artery disease | 3123 (35.5) | 3458 (39.3) | 3911 (44.5) |
| Pulmonary hypertension | 509 (5.8) | 651 (7.4) | 1018 (11.6) |
| Obstructive sleep apnea | 1381 (15.7) | 1629 (18.5) | 1979 (22.5) |
| Hypertension | 6457 (73.5) | 6715 (76.4) | 7017 (79.8) |
| Type 2 diabetes | 1852 (21.1) | 2039 (23.2) | 2288 (26.0) |
| Obesity/overweight | 1764 (20.1) | 2092 (23.8) | 2591 (29.5) |
| Conduction disorders | 1456 (16.6) | 1854 (21.1) | 2663 (30.3) |
| Prescription medication | |||
| Beta-blockers | 6054 (68.9) | 6651 (75.7) | 7078 (80.5) |
| CCBs | 2923 (33.2) | 3406 (38.7) | 4052 (46.1) |
| ACEIs | 1692 (19.2) | 2021 (23.0) | 2431 (27.7) |
| ARBs | 1063 (12.1) | 1289 (14.7) | 1657 (18.8) |
| Anticoagulation/antiplatelet therapy/thrombolytics | 4884 (55.6) | 5590 (63.6) | 6355 (72.3) |
| Antiarrhythmic therapy | |||
| Disopyramide | 151 (1.7) | 174 (2.0) | 212 (2.4) |
| Amiodarone | 781 (8.9) | 907 (10.3) | 1141 (13.0) |
| Surgical procedures | |||
| Septal myectomy | 1690 (19.2) | 1800 (20.5) | 1937 (22.0) |
| Alcohol septal ablation | 34 (0.4) | 40 (0.5) | 48 (0.6) |
| Radiofrequency ablation | 100 (1.1) | 158 (1.8) | 282 (3.2) |
| Pulmonary vein ablation | 79 (0.9) | 112 (1.3) | 196 (2.2) |
| Other ablation | 1363 (15.5) | 1492 (17.0) | 1718 (19.5) |
| Coronary revascularization | 335 (3.8) | 395 (4.5) | 506 (5.8) |
| Valve surgery | 746 (8.5) | 826 (9.4) | 937 (10.7) |
| Pacemaker | 429 (4.9) | 532 (6.1) | 750 (8.5) |
| Implantable cardioverter defibrillator | 567 (6.4) | 708 (8.1) | 984 (11.2) |
| Heart transplantation | 14 (0.2) | 18 (0.2) | 27 (0.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butzner, M.; Rowin, E.; Yakubu, A.; Seale, J.; Robertson, L.A.; Sarocco, P.; Maron, M.S. Clinical Characteristics and Healthcare Resource Utilization among Patients with Obstructive Hypertrophic Cardiomyopathy Treated in a Range of Settings in the United States. J. Clin. Med. 2022, 11, 3898. https://doi.org/10.3390/jcm11133898
Butzner M, Rowin E, Yakubu A, Seale J, Robertson LA, Sarocco P, Maron MS. Clinical Characteristics and Healthcare Resource Utilization among Patients with Obstructive Hypertrophic Cardiomyopathy Treated in a Range of Settings in the United States. Journal of Clinical Medicine. 2022; 11(13):3898. https://doi.org/10.3390/jcm11133898
Chicago/Turabian StyleButzner, Michael, Ethan Rowin, Amin Yakubu, Josiah Seale, Laura A. Robertson, Phil Sarocco, and Martin S. Maron. 2022. "Clinical Characteristics and Healthcare Resource Utilization among Patients with Obstructive Hypertrophic Cardiomyopathy Treated in a Range of Settings in the United States" Journal of Clinical Medicine 11, no. 13: 3898. https://doi.org/10.3390/jcm11133898
APA StyleButzner, M., Rowin, E., Yakubu, A., Seale, J., Robertson, L. A., Sarocco, P., & Maron, M. S. (2022). Clinical Characteristics and Healthcare Resource Utilization among Patients with Obstructive Hypertrophic Cardiomyopathy Treated in a Range of Settings in the United States. Journal of Clinical Medicine, 11(13), 3898. https://doi.org/10.3390/jcm11133898





