Risks in Induction of Platelet Aggregation and Enhanced Blood Clot Formation in Platelet Lysate Therapy: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects and Platelet Lysates
2.2. Platelet Aggregation Assay
2.3. Rotational Thromboelastometry Assay
2.4. Statistical Analysis
3. Results
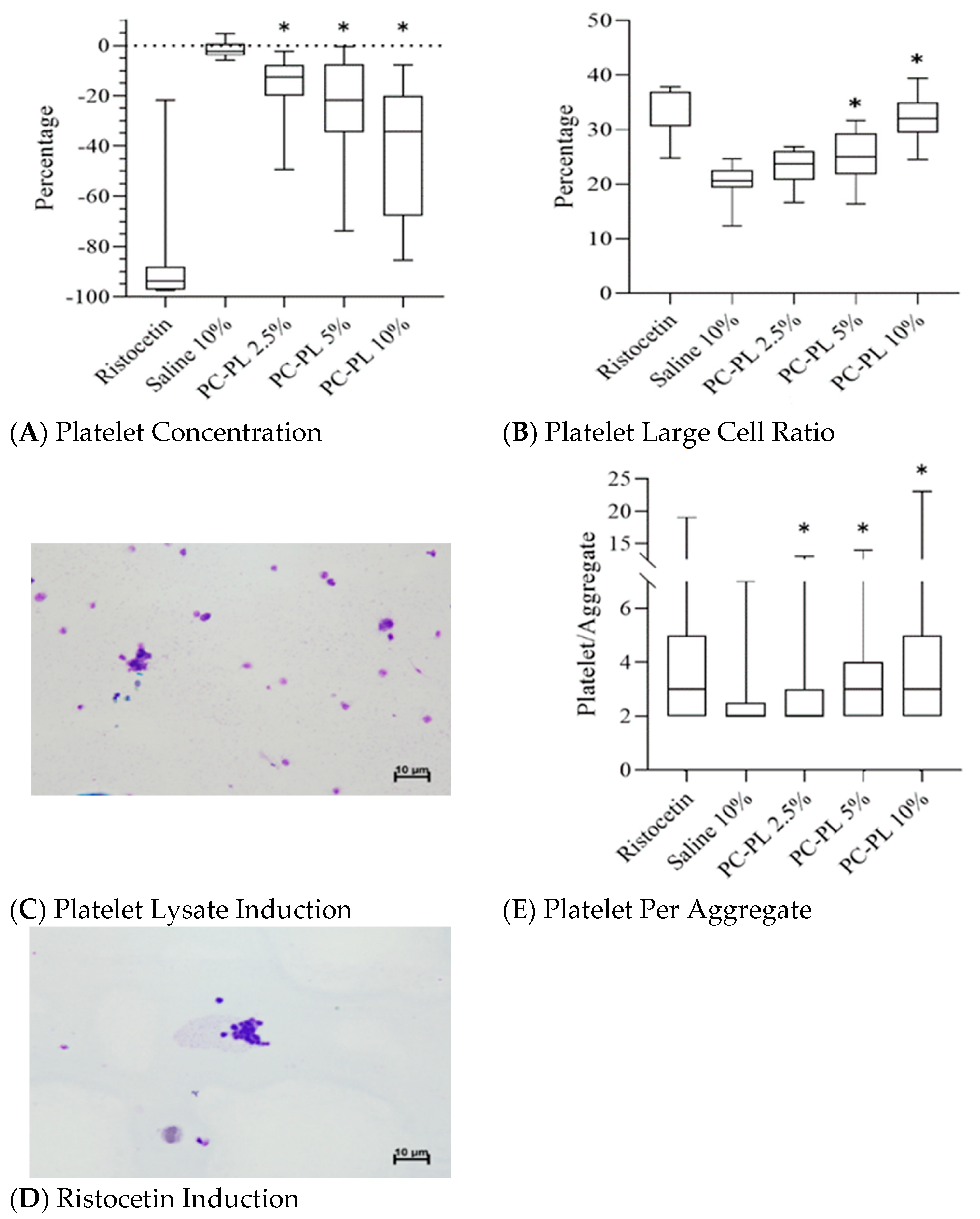
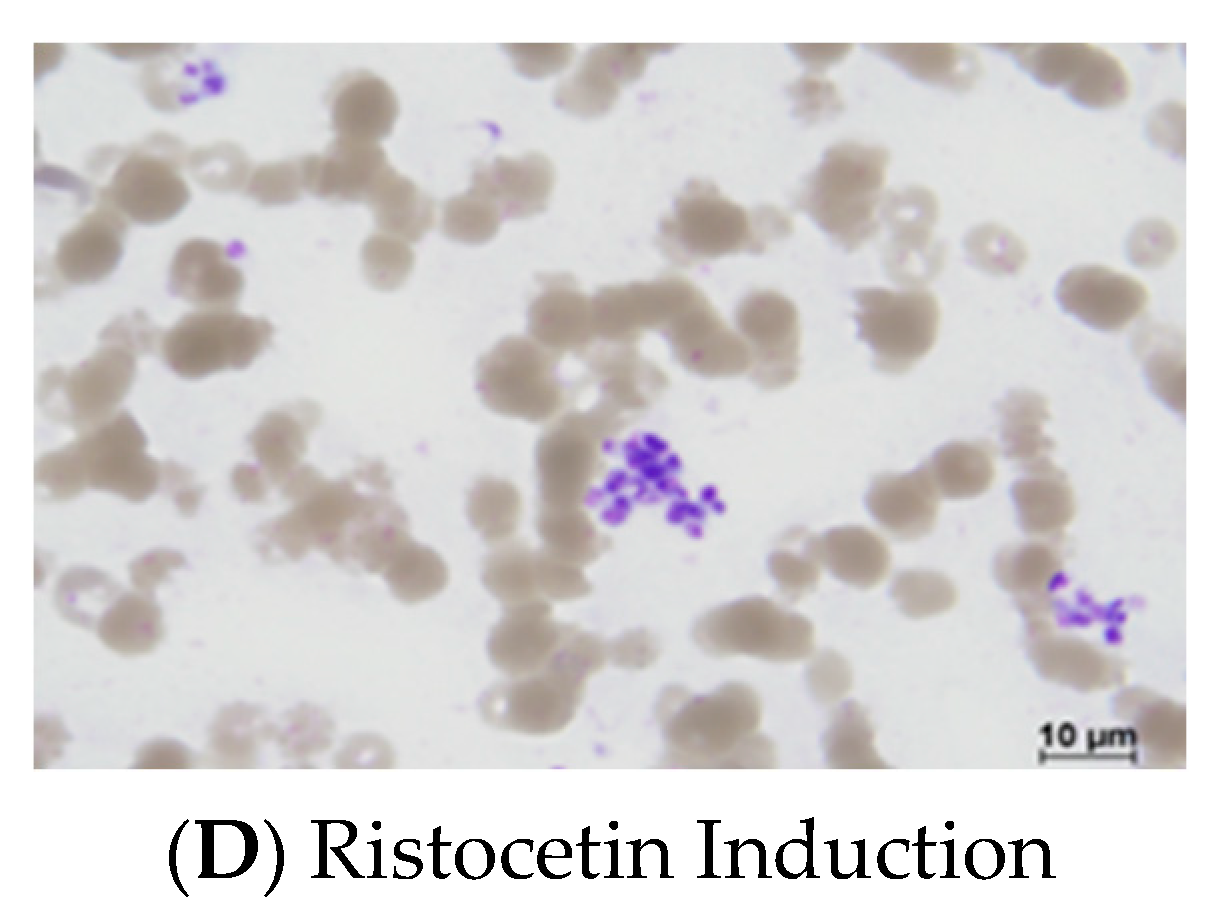
3.1. Platelet Lysate Enhances Platelet Aggregation in Platelet-Rich Plasma
3.2. Platelet Lysate Enhances Platelet Aggregation in Whole Blood
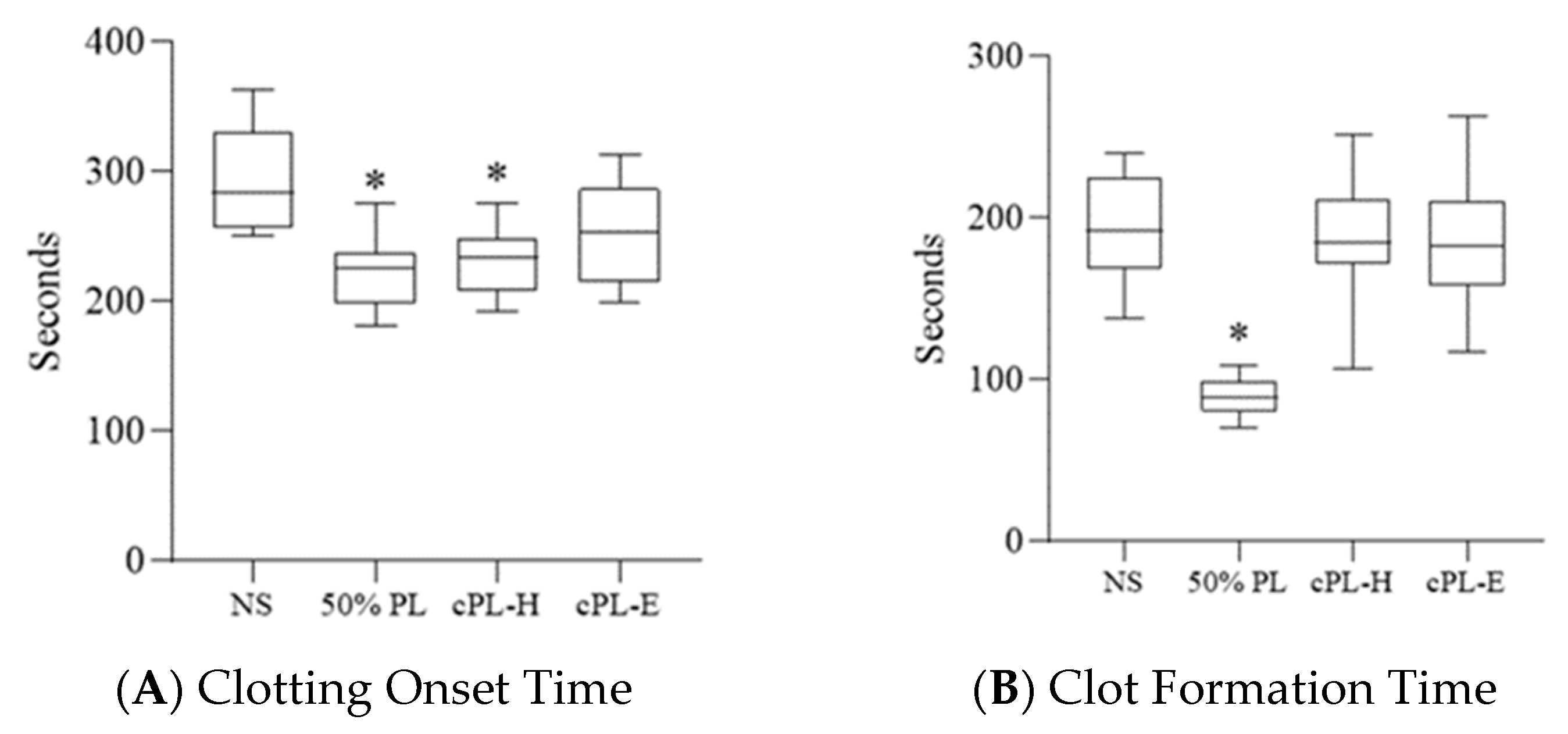
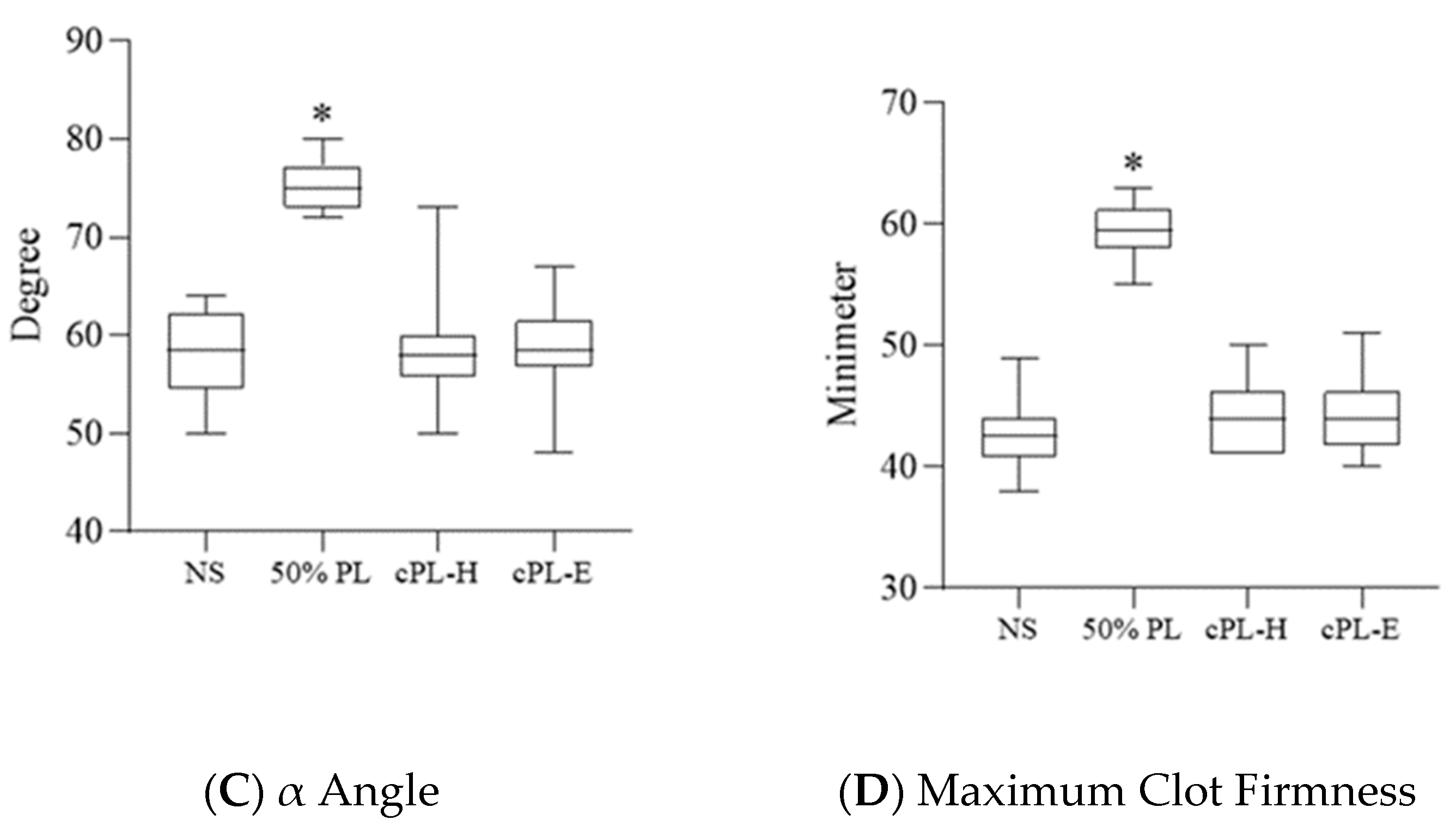
3.3. Platelet Lysate Enhances Blood Clot Formation
3.4. Platelet Lysate-Induced Hypercoagulability Is Prevented by Fibrinogen Depletion or Addition of an Anticoagulant
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dohan Ehrenfest, D.M.; Andia, I.; Zumstein, M.A.; Zhang, C.Q.; Pinto, N.R.; Bielecki, T. Classification of platelet concentrates (Platelet-Rich Plasma-PRP, Platelet-Rich Fibrin-PRF) for topical and infiltrative use in orthopedic and sports medicine: Current consensus, clinical implications and perspectives. Muscles Ligaments Tendons J. 2014, 4, 3–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, J.; An, N.; Ouyang, X. Quantification of growth factors in different platelet concentrates. Platelets 2017, 28, 774–778. [Google Scholar] [CrossRef] [PubMed]
- Alsousou, J.; Thompson, M.; Hulley, P.; Noble, A.; Willett, K. The biology of platelet-rich plasma and its application in trauma and orthopaedic surgery: A review of the literature. J. Bone Joint. Surg. Br. 2009, 91, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, J.; Bulsara, M.; Zheng, M.H. The Effectiveness of Platelet-Rich Plasma in the Treatment of Tendinopathy: A Meta-analysis of Randomized Controlled Clinical Trials. Am. J. Sports Med. 2017, 45, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Yuan, T.; Chen, S.; Xie, X.; Zhang, C. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: Systematic review and meta-analysis of randomized controlled trials. J. Orthop. Surg. Res. 2017, 12, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oudelaar, B.W.; Peerbooms, J.C.; Huis In ‘t Veld, R.; Vochteloo, A.J.H. Concentrations of Blood Components in Commercial Platelet-Rich Plasma Separation Systems: A Review of the Literature. Am. J. Sports Med. 2019, 47, 479–487. [Google Scholar] [CrossRef]
- Coppinger, J.A.; Cagney, G.; Toomey, S.; Kislinger, T.; Belton, O.; McRedmond, J.P.; Cahill, D.J.; Emili, A.; Fitzgerald, D.J.; Maguire, P.B. Characterization of the proteins released from activated platelets leads to localization of novel platelet proteins in human atherosclerotic lesions. Blood 2004, 103, 2096–2104. [Google Scholar] [CrossRef] [Green Version]
- Görmeli, G.; Görmeli, C.A.; Ataoglu, B.; Çolak, C.; Aslantürk, O.; Ertem, K. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: A randomized, double-blind, placebo-controlled trial. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 958–965. [Google Scholar] [CrossRef]
- British Committee For Standards in Haematology; Blood Transfusion Task Force. Guidelines for the use of platelet transfusions. Br. J. Haematol. 2003, 122, 10–23. [Google Scholar] [CrossRef] [Green Version]
- Wen, Y.H.; Lin, W.Y.; Lin, C.J.; Sun, Y.C.; Chang, P.Y.; Wang, H.Y.; Lu, J.J.; Yeh, W.L.; Chiueh, T.S. Sustained or higher levels of growth factors in platelet-rich plasma during 7-day storage. Clin. Chim. Acta 2018, 483, 89–93. [Google Scholar] [CrossRef]
- Plöderl, K.; Strasser, C.; Hennerbichler, S.; Peterbauer-Scherb, A.; Gabriel, C. Development and validation of a production process of platelet lysate for autologous use. Platelets 2011, 22, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Fekete, N.; Gadelorge, M.; Fürst, D.; Maurer, C.; Dausend, J.; Fleury-Cappellesso, S.; Mailänder, V.; Lotfi, R.; Ignatius, A.; Sensebé, L.; et al. Platelet lysate from whole blood-derived pooled platelet concentrates and apheresis-derived platelet concentrates for the isolation and expansion of human bone marrow mesenchymal stromal cells: Production process, content and identification of active components. Cytotherapy 2012, 14, 540–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klatte-Schulz, F.; Schmidt, T.; Uckert, M.; Scheffler, S.; Kalus, U.; Rojewski, M.; Schrezenmeier, H.; Pruss, A.; Wildemann, B. Comparative Analysis of Different Platelet Lysates and Platelet Rich Preparations to Stimulate Tendon Cell Biology: An In Vitro Study. Int. J. Mol. Sci. 2018, 19, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierce, J.; Benedetti, E.; Preslar, A.; Jacobson, P.; Jin, P.; Stroncek, D.F.; Reems, J.A. Comparative analyses of industrial-scale human platelet lysate preparations. Transfusion 2017, 57, 2858–2869. [Google Scholar] [CrossRef]
- Jackson, S.P. Arterial thrombosis--insidious, unpredictable and deadly. Nat. Med. 2011, 17, 1423–1436. [Google Scholar] [CrossRef]
- Shoeib, H.M.; Keshk, W.A.; Foda, A.M.; Abo El Noeman, S. A study on the regenerative effect of platelet-rich plasma on experimentally induced hepatic damage in albino rats. Can. J. Physiol. Pharmacol. 2018, 96, 630–636. [Google Scholar] [CrossRef]
- Salem, N.; Helmi, N.; Assaf, N. Renoprotective Effect of Platelet-Rich Plasma on Cisplatin-Induced Nephrotoxicity in Rats. Oxid. Med. Cell Longev. 2018, 2018, 9658230. [Google Scholar] [CrossRef] [Green Version]
- Giusto, G.; Vercelli, C.; Iussich, S.; Tursi, M.; Perona, G.; Gandini, M. Comparison of the effects of platelet-rich or growth factor-rich plasma on intestinal anastomosis healing in pigs. BMC Vet. Res. 2017, 13, 188. [Google Scholar] [CrossRef] [Green Version]
- Sekerci, C.A.; Tanidir, Y.; Sener, T.E.; Sener, G.; Cevik, O.; Yarat, A.; Alev-Tuzuner, B.; Cetinel, S.; Kervancioglu, E.; Sahan, A.; et al. Effects of platelet-rich plasma against experimental ischemia/reperfusion injury in rat testis. J. Pediatr. Urol. 2017, 13, 317.e311–317.e319. [Google Scholar] [CrossRef]
- Chang, Y.; Li, J.; Chen, Y.; Wei, L.; Yang, X.; Shi, Y.; Liang, X. Autologous platelet-rich plasma promotes endometrial growth and improves pregnancy outcome during in vitro fertilization. Int. J. Clin. Exp. Med. 2015, 8, 1286–1290. [Google Scholar]
- Zadehmodarres, S.; Salehpour, S.; Saharkhiz, N.; Nazari, L. Treatment of thin endometrium with autologous platelet-rich plasma: A pilot study. JBRA Assist. Reprod. 2017, 21, 54–56. [Google Scholar] [CrossRef] [PubMed]
- Aghajanova, L.; Houshdaran, S.; Balayan, S.; Manvelyan, E.; Irwin, J.C.; Huddleston, H.G.; Giudice, L.C. In vitro evidence that platelet-rich plasma stimulates cellular processes involved in endometrial regeneration. J. Assist. Reprod. Genet. 2018, 35, 757–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bos-Mikich, A.; de Oliveira, R.; Frantz, N. Platelet-rich plasma therapy and reproductive medicine. J. Assist. Reprod. Genet. 2018, 35, 753–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarmiento, A.G.C.; Danguilan, J.L.J.; Mariano, Z.M.; Barzaga, M.T.A. Use of autologous platelet rich plasma (PRP) in stopping massive hemoptysis at the Lung Center of the Philippines: A pilot study. J. Vis. Surg. 2017, 3, 111. [Google Scholar] [CrossRef] [Green Version]
- Gunaydin, B.; Acar, M.; Emmez, G.; Akcali, D.; Tokgoz, N. Epidural patch with autologous platelet rich plasma: A novel approach. J. Anesth. 2017, 31, 907–910. [Google Scholar] [CrossRef]
- Karam, E.Z.; Gan, A.; Muci Mendoza, R.; Martinez, E.; Perez, E. Visual Loss after Platelet-rich Plasma Injection into the Face. Neuroophthalmology 2020, 44, 371–378. [Google Scholar] [CrossRef]
- Kalyam, K.; Kavoussi, S.C.; Ehrlich, M.; Teng, C.C.; Chadha, N.; Khodadadeh, S.; Liu, J. Irreversible Blindness Following Periocular Autologous Platelet-Rich Plasma Skin Rejuvenation Treatment. Ophthalmic. Plast. Reconstr. Surg. 2017, 33, S12–S16. [Google Scholar] [CrossRef]
- Broos, K.; Feys, H.B.; De Meyer, S.F.; Vanhoorelbeke, K.; Deckmyn, H. Platelets at work in primary hemostasis. Blood Rev. 2011, 25, 155–167. [Google Scholar] [CrossRef]
- Golebiewska, E.M.; Poole, A.W. Platelet secretion: From haemostasis to wound healing and beyond. Blood Rev. 2015, 29, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Zamani, M.; Yaghoubi, Y.; Movassaghpour, A.; Shakouri, K.; Mehdizadeh, A.; Pishgahi, A.; Yousefi, M. Novel therapeutic approaches in utilizing platelet lysate in regenerative medicine: Are we ready for clinical use? J. Cell. Physiol. 2019, 234, 17172–17186. [Google Scholar] [CrossRef]
- Hirsh, J.; Raschke, R. Heparin and low-molecular-weight heparin: The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004, 126, 188S–203S. [Google Scholar] [CrossRef]
- Whiting, D.; DiNardo, J.A. TEG and ROTEM: Technology and clinical applications. Am. J. Hematol. 2014, 89, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Costa-Almeida, R.; Franco, A.R.; Pesqueira, T.; Oliveira, M.B.; Babo, P.S.; Leonor, I.B.; Mano, J.F.; Reis, R.L.; Gomes, M.E. The effects of platelet lysate patches on the activity of tendon-derived cells. Acta Biomater. 2018, 68, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Sandri, G.; Bonferoni, M.C.; Rossi, S.; Ferrari, F.; Mori, M.; Del Fante, C.; Perotti, C.; Caramella, C. Thermosensitive eyedrops containing platelet lysate for the treatment of corneal ulcers. Int. J. Pharm. 2012, 426, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Rauch, C.; Feifel, E.; Amann, E.M.; Spötl, H.P.; Schennach, H.; Pfaller, W.; Gstraunthaler, G. Alternatives to the use of fetal bovine serum: Human platelet lysates as a serum substitute in cell culture media. Altex 2011, 28, 305–316. [Google Scholar] [CrossRef]
- Henschler, R.; Gabriel, C.; Schallmoser, K.; Burnouf, T.; Koh, M.B.C. Human platelet lysate current standards and future developments. Transfusion 2019, 59, 1407–1413. [Google Scholar] [CrossRef]
- Schallmoser, K.; Henschler, R.; Gabriel, C.; Koh, M.B.C.; Burnouf, T. Production and Quality Requirements of Human Platelet Lysate: A Position Statement from the Working Party on Cellular Therapies of the International Society of Blood Transfusion. Trends Biotechnol. 2020, 38, 13–23. [Google Scholar] [CrossRef]
- Bieback, K.; Fernandez-Muñoz, B.; Pati, S.; Schäfer, R. Gaps in the knowledge of human platelet lysate as a cell culture supplement for cell therapy: A joint publication from the AABB and the International Society for Cell & Gene Therapy. Cytotherapy 2019, 21, 911–924. [Google Scholar] [CrossRef]

| Ristocetin | 10% Saline | Platelet Lysate | |||
|---|---|---|---|---|---|
| 2.50% | 5% | 10% | |||
| Percentage change of platelet concentration | −83.1 ± 27% | −1.5 ± 3% | −16.3 ± 13% * | −24.3 ± 22% * | −39.8 ± 26% * |
| Platelet large cell ratio | 32.6 ± 5% | 20.5 ± 3% | 23.1 ± 3% | 25.0 ± 5% * | 32.1 ± 4% * |
| Platelet per aggregate | 3.9 ± 2.7 | 2.4 ± 0.9 | 2.8 ± 1.8 * | 3.5 ± 2.3 * | 4.3 ± 3.2 * |
| Ristocetin | 50% Saline | Platelet Lysate | |||
|---|---|---|---|---|---|
| 30% | 40% | 50% | |||
| Percentage change of platelet concentration | −41.1 ± 22% | −21.8 ± 19% | −46.1 ± 38% | −45.3 ± 24% * | 47.1 ± 20% * |
| Platelet large cell ratio | 33.4 ± 5% | 20.8 ± 4% | 28.0 ± 4% * | 29.2 ± 5% * | 29.0 ± 5% * |
| Platelet per aggregate | 15.7 ± 10.0 | 1.7 ± 0.9 | 6.0 ± 5.2 * | 5.3 ± 3.9 * | 4.5 ± 3.1 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, Y.-H.; Lee, C.-F.; Chen, Y.-J.; Chang, G.-J.; Chong, K.-Y. Risks in Induction of Platelet Aggregation and Enhanced Blood Clot Formation in Platelet Lysate Therapy: A Pilot Study. J. Clin. Med. 2022, 11, 3972. https://doi.org/10.3390/jcm11143972
Wen Y-H, Lee C-F, Chen Y-J, Chang G-J, Chong K-Y. Risks in Induction of Platelet Aggregation and Enhanced Blood Clot Formation in Platelet Lysate Therapy: A Pilot Study. Journal of Clinical Medicine. 2022; 11(14):3972. https://doi.org/10.3390/jcm11143972
Chicago/Turabian StyleWen, Ying-Hao, Chen-Fang Lee, Yu-Ju Chen, Gwo-Jyh Chang, and Kowit-Yu Chong. 2022. "Risks in Induction of Platelet Aggregation and Enhanced Blood Clot Formation in Platelet Lysate Therapy: A Pilot Study" Journal of Clinical Medicine 11, no. 14: 3972. https://doi.org/10.3390/jcm11143972







