Correlation Analysis between Intraocular Pressure and Extraocular Muscles Based on Orbital Magnetic Resonance T2 Mapping in Thyroid-Associated Ophthalmopathy Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Measurement of Orbital MRI-T2 Mapping Parameters
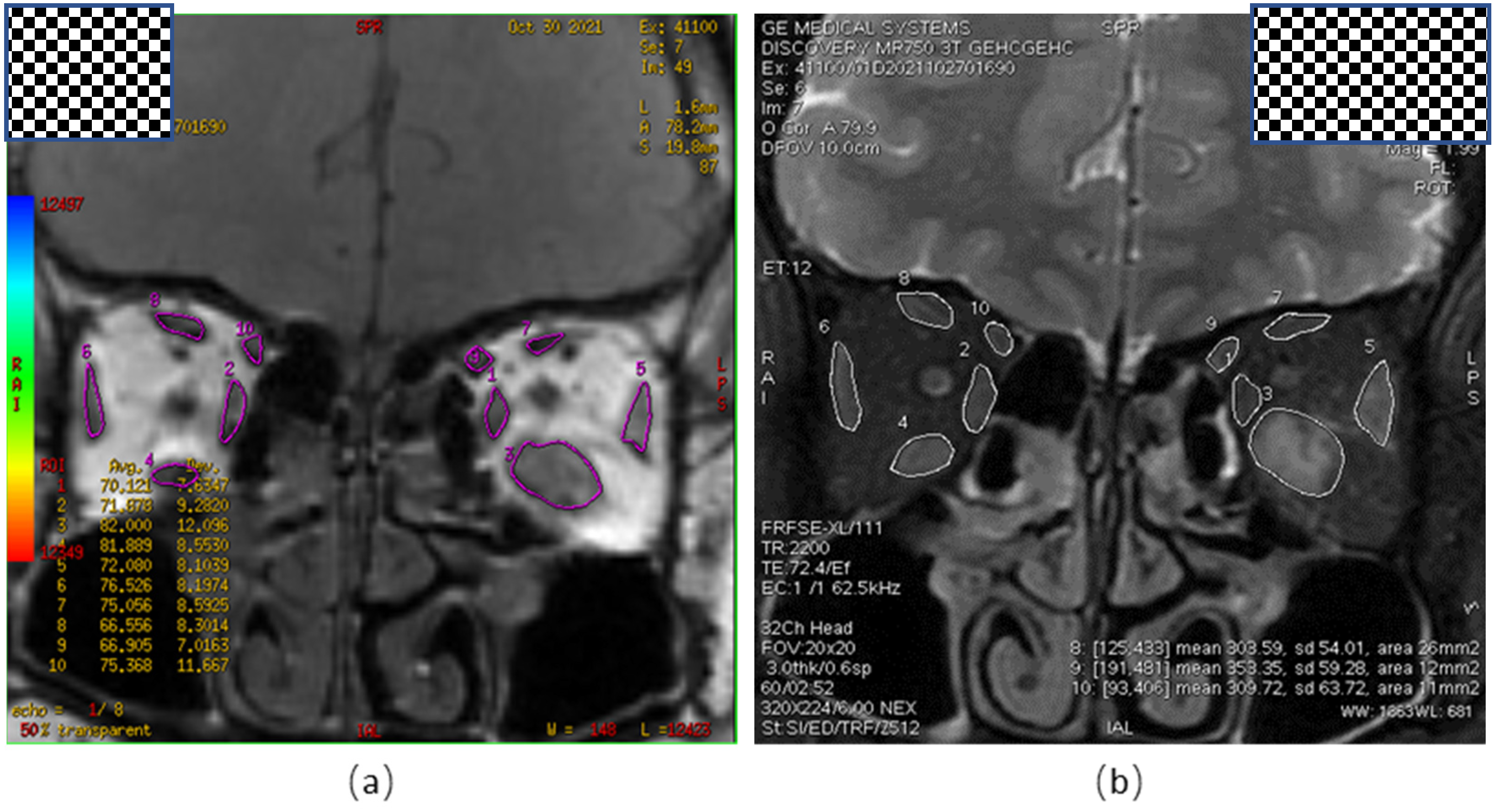
2.3. Image Analysis
2.4. Statistical Analysis
3. Results
3.1. Correlation Analysis
3.2. Multiple Linear Regression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hiromatsu, Y.; Eguchi, H.; Tani, J.; Kasaoka, M.; Teshima, Y. Graves’ ophthalmopathy: Epidemiology and natural history. Intern. Med. 2014, 53, 353–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallika, P.; Tan, A.; Aziz, S.; Alwi, S.S.; Chong, M.; Vanitha, R.; Intan, G. Thyroid associated ophthalmopathy-a review. Malays. Fam. Physician 2009, 4, 8–14. [Google Scholar] [PubMed]
- Burch, H.B.; Wartofsky, L. Graves’ ophthalmopathy: Current concepts regarding pathogenesis and management. Endocr. Rev. 1993, 14, 747–793. [Google Scholar] [PubMed] [Green Version]
- Kalmann, R.; Mourits, M.P. Prevalence and management of elevated intraocular pressure in patients with Graves’ orbitopathy. Br. J. Ophthalmol. 1998, 82, 754–757. [Google Scholar] [CrossRef] [Green Version]
- Cockerham, K.P.; Pal, C.; Jani, B.; Wolter, A.; Kennerdell, J.S. The prevalence and implications of ocular hypertension and glaucoma in thyroid-associated orbitopathy. Ophthalmology 1997, 104, 914–917. [Google Scholar] [CrossRef]
- Gomi, C.F.; Yates, B.; Kikkawa, D.O.; Levi, L.; Weinreb, R.N.; Granet, D.B. Effect on intraocular pressure of extraocular muscle surgery for thyroid-associated ophthalmopathy. Am. J. Ophthalmol. 2007, 144, 654–657. [Google Scholar] [CrossRef]
- Konuk, O.; Onaran, Z.; Oktar, S.O.; Yucel, C.; Unal, M. Intraocular pressure and superior ophthalmic vein blood flow velocity in Graves’ orbitopathy: Relation with the clinical features. Graefe’s Arch. Clin. Exp. Ophthalmol. 2009, 247, 1555–1559. [Google Scholar] [CrossRef]
- Duncan, K.G.; Jumper, M.D.; Ribeiro, R.C.J.; Bailey, K.R.; Yen, P.; Sugawara, A.; Patel, A.; Stern, R.; Chin, W.W.; Baxter, J.D.; et al. Human trabecular meshwork cells as a thyroid hormone target tissue: Presence of functional thyroid hormone receptors. Graefe’s Arch. Clin. Exp. Ophthalmol. 1999, 237, 231–240. [Google Scholar] [CrossRef]
- Stoyanova, N.S.; Konareva-Kostianeva, M.; Mitkova-Hristova, V.; Angelova, I. Correlation between Intraocular Pressure and Thickness of Extraocular Muscles, the Severity and Activity of Thyroid-associated Orbitopathy. Folia Med. 2019, 61, 90–96. [Google Scholar] [CrossRef]
- Yokoyama, N.; Nagataki, S.; Uetani, M.; Ashizawa, K.; Eguchi, K. Role of magnetic resonance imaging in the assessment of disease activity in thyroid-associated ophthalmopathy. Thyroid 2002, 12, 223–227. [Google Scholar] [CrossRef]
- Ohnishi, T.; Noguchi, S.; Murakami, N.; Tajiri, J.; Harao, M.; Kawamoto, H.; Hoshi, H.; Jinnouchi, S.; Jinnouchi, S.; Nagamachi, S. Extraocular muscles in Graves ophthalmopathy: Usefulness of T2 relaxation time measurements. Radiology 1994, 190, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.J.; Rochtchina, E.; Wang, J.J.; Healey, P.R.; Mitchell, P. Open-angle glaucoma and systemic thyroid disease in an older population: The Blue Mountains Eye Study. Eye 2004, 18, 600–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartalena, L.; Baldeschi, L.; Dickinson, A.; Eckstein, A.; Kendall-Taylor, P.; Marcocci, C.; Mourits, M.; Perros, P.; Boboridis, K.; Boschi, A.; et al. Consensus statement of the European Group on Graves’ orbitopathy (EUGOGO) on management of GO. Eur. J. Endocrinol. 2008, 158, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Shin, W.B.; Bae, H.W.; Yoon, J.S. Effects of Orbital Decompression on Lamina Cribrosa Depth in Patients with Graves’ Orbitopathy. Korean J. Ophthalmol. 2019, 33, 436–445. [Google Scholar] [CrossRef] [Green Version]
- Eckstein, A.K.; Johnson, K.T.M.; Thanos, M.; Esser, J.; Ludgate, M.J.H. Current insights into the pathogenesis of Graves’ orbitopathy. Horm. Metab. Res. 2009, 41, 456–464. [Google Scholar] [CrossRef]
- Perros, P.; Kendall-Taylor, P. Pathogenetic mechanisms in thyroid-associated ophthalmopathy. J. Intern. Med. 1992, 231, 205–211. [Google Scholar] [CrossRef]
- Saeed, P.; Rad, S.T.; Bisschop, P. Dysthyroid Optic Neuropathy. Ophthalmic Plast. Reconstr. Surg. 2018, 34, S60–S67. [Google Scholar] [CrossRef]
- Currie, Z.I.; Lewis, S.; Clearkin, L.G. Dysthyroid eye disease masquerading as glaucoma. Ophthalmic Physiol. Opt. 1991, 11, 176–179. [Google Scholar] [CrossRef]
- Takahashi, Y.; Nakamura, Y.; Ichinose, A.; Kakizaki, H. Intraocular Pressure Change with Eye Positions Before and After Orbital Decompression for Thyroid Eye Disease. Ophthalmic Plast. Reconstr. Surg. 2014, 30, 47–50. [Google Scholar] [CrossRef]
- Haefliger, I.O.; von Arx, G.; Pimentel, A.R. Pathophysiology of intraocular pressure increase and glaucoma prevalence in thyroid eye disease: A mini-review. Klin. Mon. Für Augenheilkd. 2010, 227, 292–293. [Google Scholar] [CrossRef]
- Kuebler, A.G.; Wiecha, C.; Reznicek, L.; Klingenstein, A.; Halfter, K.; Priglinger, S.; Hintschich, C. Comparison of different devices to measure the intraocular pressure in thyroid-associated orbitopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 2025–2032. [Google Scholar] [CrossRef] [PubMed]
- Parekh, A.S.; Mansouri, K.; Weinreb, R.N.; Tafreshi, A.; Korn, B.S.; Kikkawa, D.O. Twenty-four-hour intraocular pressure patterns in patients with thyroid eye disease. Clin. Exp. Ophthalmol. 2015, 43, 108–114. [Google Scholar] [CrossRef] [PubMed]

| Demographic Characteristics | |
|---|---|
| Gender (Male/Female) | 21/20 |
| Age (Year) | 44.59 ± 10.77 |
| Thyroid function (euthyroid/hyperthyroidism/hypothyroidism) | 8/32/1 |
| History of glucocorticoid use (No/Yes) | 33/8 |
| Parameters | LIOP Group (n = 41) | HIOP Group (n = 41) | p Value |
|---|---|---|---|
| CAS | 1.90 ± 1.73 | 2.68 ± 1.77 | 0.001 & |
| Exophthalmos | 17.54 ± 2.76 | 19.40 ± 2.74 | <0.0001 & |
| T2RT (MR) | 70.81 ± 11.25 | 74.68 ± 9.39 | 0.015 & |
| Area (MR) | 40.24 ± 18.40 | 46.78 ± 21.82 | 0.002 & |
| T2RT (IR) | 79.55 ± 7.67 | 79.26 ± 9.28 | 0.850 $ |
| Area (IR) | 43.05 ± 16.28 | 68.78 ± 27.89 | <0.0001 & |
| T2RT (LR) | 72.81 ± 6.52 | 74.38 ± 6.75 | 0.251 & |
| Area (LR) | 48.54 ± 13.34 | 56.93 ± 16.52 | <0.0001 & |
| T2RT (SRLC) | 81.87 ± 15.38 | 82.96 ± 14.42 | 0.791 & |
| Area (SRLC) | 57.15 ± 32.20 | 65.17 ± 37.64 | 0.925 & |
| T2RT (SO) | 75.42 ± 6.84 | 75.92 ± 7.67 | 0.506 $ |
| Area (SO) | 18.10 ± 5.03 | 20.08 ± 6.73 | 0.118 & |
| T2RT (OF) | 71.11 ± 14.09 | 70.51 ± 14.29 | 0.667 & |
| Average T2RT | 75.26 ± 6.02 | 76.29 ± 5.95 | 0.168 & |
| Total T2RT | 451.57 ± 36.13 | 457.71 ± 35.72 | 0.168 & |
| Average area | 41.47 ± 13.11 | 51.51 ± 14.01 | <0.0001 & |
| Total area | 206.63 ± 66.42 | 257.54 ± 70.04 | <0.0001 & |
| RUME (y/n) | 11/30 | 26/15 | 0.0008 # |
| RDME (y/n) | 9/32 | 10/31 | 0.794 # |
| RIME (y/n) | 0/41 | 1/40 | >0.999 * |
| ROME (y/n) | 6/35 | 9/32 | 0.392 # |
| Spontaneous retrobulbar pain (y/n) | 9/32 | 19/22 | 0.020 # |
| Pain on attempted upward or downward gaze (y/n) | 9/32 | 14/27 | 0.219 # |
| Redness of eyelids (y/n) | 9/32 | 10/31 | 0.794 # |
| Redness of conjunctiva (y/n) | 12/29 | 16/25 | 0.352 # |
| Swelling of eyelids (y/n) | 26/15 | 30/11 | 0.343 # |
| Swelling of conjunctiva (y/n) | 10/31 | 14/27 | 0.332 # |
| Swelling of caruncle or plica (y/n) | 3/38 | 7/34 | 0.177 # |
| Parameters | Correlation Coefficient with IOP | p Value |
|---|---|---|
| CAS | 0.254 | 0.021 |
| Exophthalmos | 0.402 | <0.001 |
| T2RT (MR) | 0.250 | 0.023 |
| Area (MR) | 0.257 | 0.020 |
| T2RT (IR) | −0.101 | 0.367 |
| Area (IR) | 0.550 | <0.001 |
| T2RT (LR) | 0.093 | 0.406 |
| Area (LR) | 0.340 | 0.002 |
| T2RT (SRLC) | 0.034 | 0.759 |
| Area (SRLC) | 0.033 | 0.765 |
| T2RT (SO) | 0.045 | 0.691 |
| Area (SO) | 0.178 | 0.111 |
| T2RT(OF) | 0.106 | 0.344 |
| Average T2RT | 0.151 | 0.177 |
| Average area | 0.440 | <0.001 |
| B | P | |
|---|---|---|
| Constant | 10.426 | 0.061 |
| Exophthalmos | 0.345 | 0.153 |
| T2RT(MR) | −0.057 | 0.471 |
| Area (IR) | 0.103 | 0.001 |
| Area (LR) | 0.050 | 0.307 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, B.; Wang, W.; Li, X.; Zhang, H.; Zhang, Y.; Hu, W. Correlation Analysis between Intraocular Pressure and Extraocular Muscles Based on Orbital Magnetic Resonance T2 Mapping in Thyroid-Associated Ophthalmopathy Patients. J. Clin. Med. 2022, 11, 3981. https://doi.org/10.3390/jcm11143981
Luo B, Wang W, Li X, Zhang H, Zhang Y, Hu W. Correlation Analysis between Intraocular Pressure and Extraocular Muscles Based on Orbital Magnetic Resonance T2 Mapping in Thyroid-Associated Ophthalmopathy Patients. Journal of Clinical Medicine. 2022; 11(14):3981. https://doi.org/10.3390/jcm11143981
Chicago/Turabian StyleLuo, Ban, Wei Wang, Xinyu Li, Hong Zhang, Yaoli Zhang, and Weikun Hu. 2022. "Correlation Analysis between Intraocular Pressure and Extraocular Muscles Based on Orbital Magnetic Resonance T2 Mapping in Thyroid-Associated Ophthalmopathy Patients" Journal of Clinical Medicine 11, no. 14: 3981. https://doi.org/10.3390/jcm11143981






