Does Ionized Magnesium Offer a Different Perspective Exploring the Association between Magnesemia and Targeted Cardiovascular Risk Factors?
Abstract
:1. Introduction
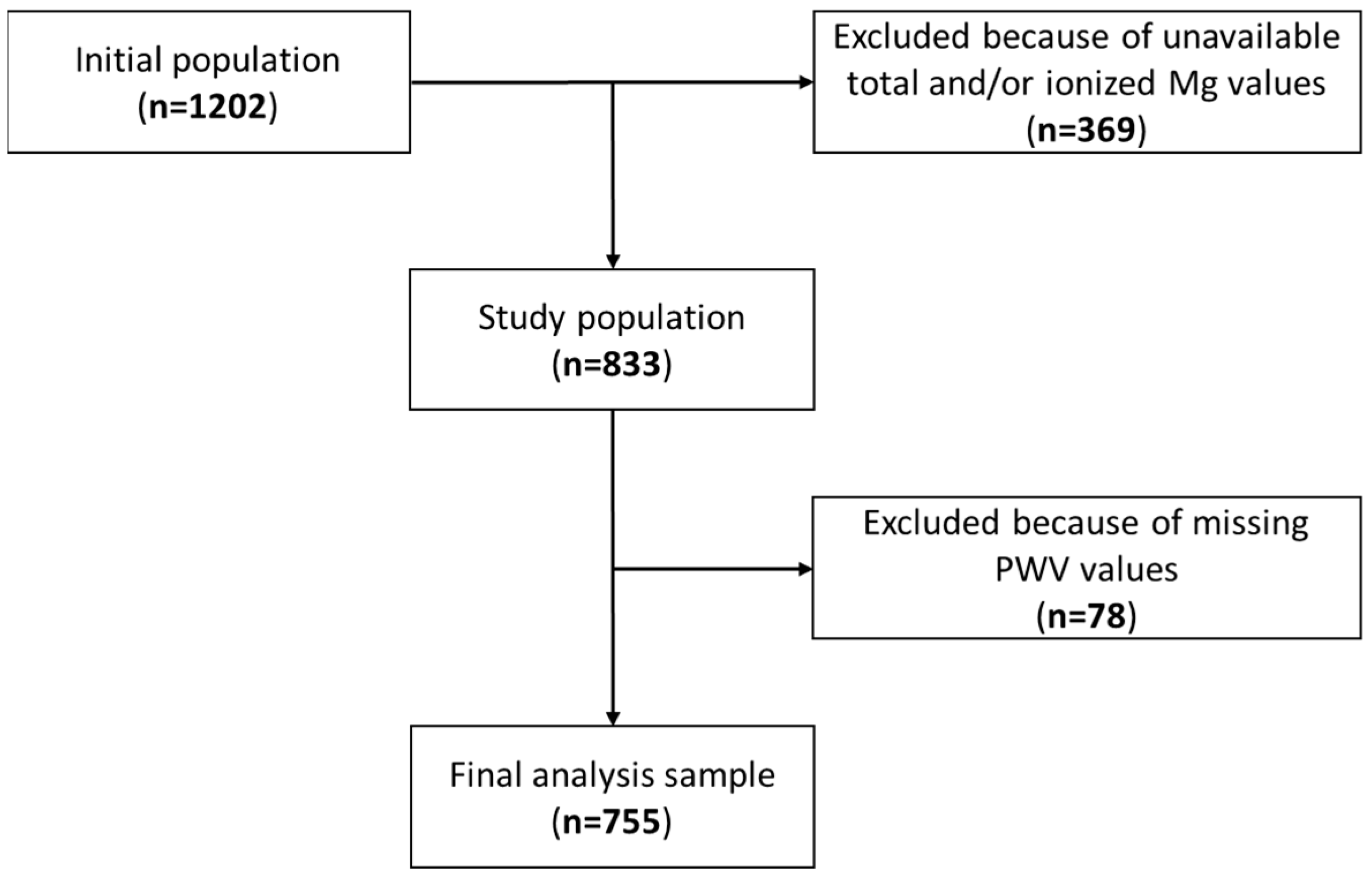
2. Materials and Methods
3. Results
3.1. Patient’s Characteristics
3.2. Magnesium and PWV
3.3. Magnesium: 24 h ABPM Minimum and Maximum Values
3.4. Magnesium, BMI, and Body Composition
3.5. Patients’ Reclassification Using Ionized Magnesium
3.6. Correlations between Blood Mg Levels and Measured Parameters under Alternative Intervals
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


| Tot-Mg ≤ 0.70 (n = 10) | 0.71 ≤ Tot-Mg ≤ 0.80 (n = 224) | 0.81 ≤ Tot-Mg ≤ 0.90 (n = 439) | Tot-Mg ≥ 0.91 (n = 82) | Ion-Mg ≤ 0.45 (n = 10) | 0.46 ≤ Ion-Mg ≤ 0.50 (n = 199) | 0.51 ≤ Ion-Mg ≤ 0.55 (n = 399) | Ion-Mg ≥ 0.56 (n = 147) | Overall Population (n = 755) | |
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 58 ± 17 63 (32–75) | 52 ± 13 53 (21–87) | 55 ± 14 55 (21–91) | 57 ± 11 56 (36–86) | 61 ± 11 61 (42–76) | 52 ± 13 52 (21–86) | 55 ± 13 55 (21–91) | 56 ± 12 55 (21–86) | 54 ± 13 54 (21–91) |
| Female | 6 (60%) | 139 (62%) | 244 (55.6%) | 46 (56%) | 8 (80%) | 130 (65.3%) | 227 (57%) | 69 (47%) | 435 (57.6%) |
| Male | 4 (40%) | 85 (38%) | 195 (44.4%) | 36 (44%) | 2 (20%) | 69 (34.7%) | 171 (43%) | 78 (53%) | 320 (42.4%) |
| Underweight, BMI < 18.5 (kg/m2) | 0 (0%) | 10 (4.5%) | 10 (2.3%) | 1 (1.2%) | 0 (0%) | 6 (3%) | 12 (3%) | 3 (2%) | 21 (2.8%) |
| Normal weight, 18.5 ≤ BMI ≤ 24.9 (kg/m2) | 3 (30%) | 115 (51.3%) | 235 (53.5%) | 50 (61%) | 5 (50%) | 116 (58.3%) | 204 (51.2%) | 78 (53.1%) | 403 (53.4%) |
| Pre-obesity, 25.0 ≤ BMI ≤ 29.9 (kg/m2) | 4 (40%) | 65 (29%) | 146 (33.3%) | 26 (31.7%) | 2 (20%) | 51 (25.6%) | 139 (34.8%) | 49 (33.3%) | 241 (31.9%) |
| Obese, BMI ≥ 30.0 (kg/m2) | 3 (30%) | 34 (15.2%) | 48 (10.9%) | 5 (6.1%) | 3 (30%) | 26 (13.1%) | 44 (11%) | 17 (11.6%) | 90 (11.9%) |
| FFM (kg) | 56.6 ± 10.0 52.9 (44.3–73.1) | 53.3 ± 12.0 49.4 (35.6–98.6) | 53.0 ± 10.4 50.1 (34.8–90.4) | 52.3 ± 10.4 48.8 (38.0–82.2) | 50.6 ± 10.4 46.4 (40.8–73.1) | 52.9 ± 11.4 49.1 (35.6–98.6) | 52.9 ± 10.6 49.3 (36.2–82.7) | 54.2 ± 11.0 53.8 (34.8–90.4) | 53.1 ± 10.9 49.6 (34.8 -98.6) |
| FFM (%) | 71.4 ± 8.5 72.5 (56.6–87.0) | 75.2 ± 8.1 76.1 (49.0–97.6) | 75.1 ± 7.7 75.5 (51.8–97.2) | 75.3 ± 7.4 75.7 (57.9–95.0) | 72.5 ± 8.4 72.8 (56.6–85.0) | 75.3 ± 7.9 76.0 (49.0–97.2) | 74.9 ± 7.5 75.5 (51.3–95.7) | 75.4 ± 8.4 75.5 (54.2–97.6) | 24.9 ± 7.8 75.6 (49.0 -97.6) |
| FM (kg) | 23.1 ± 8.9 21.8 (10.3–41.2) | 18.3 ± 9.1 16.4 (2.0–57.6) | 18.1 ± 8.0 16.9 (2.0–68.6) | 17.4 ± 6.7 16.9 (3.5–34.8) | 20.4 ± 10.0 19.1 (7.2–41.2) | 18.0 ± 8.7 16.0 (2.0–57.6) | 18.2 ± 7.7 17.0 (2.6–54.1) | 18.3 ± 8.8 17.2 (2.0–68.6) | 18.2 ± 8.2 16.8 (2.0 -68.6) |
| FM (%) | 28.6 ± 8.5 27.5 (13.0–43.4) | 24.8 ± 8.1 23.9 (2.4–51.0) | 24.9 ± 7.7 24.5 (2.8–48.2) | 24.7 ± 7.4 24.3 (5.8–42.1) | 27.5 ± 8.4 27.3 (15.0–43.4) | 24.7 ± 7.9 24.0 (2.8–51.0) | 25.1 ± 7.5 24.5 (4.3–48.7) | 24.6 ± 8.4 24.5 (2.4–45.8) | 24.9 ± 7.8 24.4 (2.4 -51.0) |
| Total Mg (mmol/L) | 0.67 ± 0.03 0.67 (0.62–0.70) | 0.77 ± 0.02 0.77 (0.71–0.80) | 0.85 ± 0.03 0.85 (0.81–0.90) | 0.93 ± 0.03 0.92 (0.91–1.09) | 0.70 ± 0.05 0.69 (0.62–0.77) | 0.78 ± 0.04 0.78 (0.66–0.92) | 0.84 ± 0.04 0.84 (0.73–0.98) | 0.89 ± 0.05 0.89 (0.77–1.09) | 0.83 ± 0.06 0.83 (0.62–1.09) |
| Ionized Mg (mmol/L) | 0.46 ± 0.02 0.46 (0.41–0.49) | 0.5 ± 0.02 0.5 (0.45–0.58) | 0.53 ± 0.03 0.53 (0.47–0.63) | 0.57 ± 0.03 0.56 (0.49–0.66) | 0.44 ± 0.01 0.45 (0.41–0.45) | 0.49 ± 0.01 0.49 (0.46–0.50) | 0.53 ± 0.01 0.53 (0.51–0.55) | 0.58 ± 0.02 0.58 (0.56–0.66) | 0.53 ± 0.3 0.52 (0.41–0.66) |
| SBP, 24 h ABPM (mmHg) | 124.0 ± 12.5 125.5 (101.0–138.0) | 119.1 ± 12.1 117.0 (95.0–179.0) | 118.6 ± 11.6 117.0 (93.0–170.0) | 119.2 ± 12.1 119.0 (98.0–161.0) | 122.5 ± 14.3 125.5 (101.0–138.0) | 117.9 ± 11.2 116.0 (98.0–179.0) | 118.7 ± 11.7 117.0 (93.0–170.0) | 120.7 ± 12.8 119.0 (97.0–167.0) | 118.9 ± 11.8 117 (93 -179) |
| DBP, 24 h ABPM (mmHg) | 75.6 ± 8.2 78.0 (60.0–84.0) | 74.2 ± 8.6 73.0 (54.0–99.0) | 73.7 ± 8.4 73.0 (53.0–110.0) | 74.2 ± 9.6 73.0 (56.0–108.0) | 76.4 ± 8.2 80.0 (60.0–84.0) | 73.4 ± 8.5 73.0 (55.0–99.0) | 73.6 ± 8.4 73.0 (53.0–110.0) | 75.1 ± 9.1 73.0 (56.0–108.0) | 73.9 ± 8.6 73 (53–110) |
| ΔSBP, 24 h ABPM (mmHg) | 69.4 ± 25.9 63.5 (35.0–117.0) | 61.3 ± 21.6 57.0 (25.0–158.0) | 59.8 ± 21.7 57.0 (18.0–194.0) | 63.2 ± 22.5 60.0 (30.0–130.0) | 77.6 ± 23.8 81.0 (43.0–117.0) | 60.8 ± 20.2 58.0 (26.0–145.0) | 59.7 ± 20.5 57.0 (18.0–142.0) | 62.5 ± 26.6 59.0 (26.0–194.0) | 60.9 ± 21.8 57 (18–194) |
| ΔDBP, 24 h ABPM (mmHg) | 54.3 ± 19.1 51.5 (33.0–99.0) | 47.6 ± 15.6 44.0 (18.0–130.0) | 47.2 ± 16.6 44.0 (13.0–145.0) | 46.1 ± 14.2 43.0 (20.0–91.0) | 51.3 ± 13.0 54.0 (33.0–69.0) | 47.8 ± 16.0 45.0 (13.0–108.0) | 46.4 ± 15.7 43.0 (20.0–145.0) | 48.6 ± 17.4 44.0 (16.0–128.0) | 47.3 ± 16.1 44 (13–145) |
| Cf-PWV (m/s) | 8.2 ± 1.6 8.9 (5.9–10.0) | 7.2 ± 1.6 6.9 (4.0–12.6) | 7.4 ± 1.7 7.0 (4.4–13.0) | 7.6 ± 1.6 7.3 (5.2–12.7) | 8.4 ± 1.6 8.6 (6.0–10.7) | 7.0 ± 1.6 6.7 (4.6–12.6) | 7.4 ± 1.7 7.1 (4.0–13.0) | 7.6 ± 1.6 7.3 (4.8–12.7) | 7.4 ± 1.7 7.0 (4.0–13.0) |
| O-PWV (m/s) | 8.1 ± 2.2 8.6 (4.9–10.7) | 7.2 ± 1.6 7.0 (4.5–12.6) | 7.5 ± 1.8 7.1 (4.5–12.9) | 7.6 ± 1.7 7.3 (5.3–12.6) | 8.4 ± 1.8 7.9 (5.6–11.0) | 7.1 ± 1.7 6.8 (4.5–12.6) | 7.5 ± 1.8 7.1 (4.5–12.9) | 7.6 ± 1.7 7.3 (4.7–12.5) | 7.4 ± 1.7 7.1 (4.5–12.9) |
| QTc (ms) | 440.4 ± 22.6 443.0 (401.0–467.0) | 423.2 ± 35.1 426.0 (40.0–484.0) | 427.2 ± 21.3 429.0 (367.0–542.0) | 428.2 ± 21.2 426.0 (383.0–513.0) | 429.3 ± 58.1 440.0 (269.0–467.0) | 424.2 ± 34.8 428.0 (40.0–474.0) | 427.5 ± 21.8 428.0 (368.0–542.0) | 425.7 ± 19.9 426.0 (373.0–475.0) | 426.3 ± 26.2 428 (40–542) |
| BPCV (mmHg) | 12.0 ± 3.6 11.7 (7.5–18.0) | 10.8 ± 3.4 10.1 (4.8–25.4) | 10.7 ± 3.6 10.3 (3.1–32.3) | 11.5 ± 3.7 11.1 (5.9–25.7) | 12.9 ± 3.2 13.1 (8.9–17.7) | 11.0 ± 3.5 10.5 (4.8–27.2) | 10.6 ± 3.3 10.1 (3.7–23.4) | 11.2 ± 4.2 10.5 (3.1–32.3) | 10.9 ± 3.5 10.3 (3.1–32.3) |
| Tot-Mg ≤ 0.70 (n = 10) | 0.71 ≤ Tot-Mg ≤ 0.80 (n = 224) | 0.81 ≤ Tot-Mg ≤ 0.90 (n = 439) | Tot-Mg ≥ 0.91 (n = 82) | p-Value | Ion-Mg ≤ 0.45 (n = 10) | 0.46 ≤ Ion-Mg ≤ 0.50 (n = 199) | 0.51 ≤ Ion-Mg ≤ 0.55 (n = 399) | Ion-Mg ≥ 0.56 (n = 147) | p-Value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Cf-PWV | 8.2 ± 1.6 8.9 (5.9–10.0) | 7.2 ± 1.6 6.9 (4.0–12.6) | 7.4 ± 1.7 7.0 (4.4–13.0) | 7.6 ± 1.6 7.3 (5.2–12.7) | 0.056 | 8.4 ± 1.6 8.6 (6.0–10.7) | 7.0 ± 1.6 6.7 (4.6–12.6) | 7.4 ± 1.7 7.1 (4.0–13.0) | 7.6 ± 1.6 7.3 (4.8–12.7) | 0.002 |
| O-PWV | 8.1 ± 2.2 8.6 (4.9–10.7) | 7.2 ± 1.6 7.0 (4.5–12.6) | 7.5 ± 1.8 7.1 (4.5–12.9) | 7.6 ± 1.7 7.3 (5.3–12.6) | 0.057 | 8.4 ± 1.8 7.9 (5.6–11.0) | 7.1 ± 1.7 6.8 (4.5–12.6) | 7.5 ± 1.8 7.1 (4.5–12.9) | 7.6 ± 1.7 7.3 (4.7–12.5) | 0.019 |
| FFM (kg) | 56.6 ± 10.0 52.9 (44.3–73.1) | 53.3 ± 12.0 49.4 (35.6–98.6) | 53.0 ± 10.4 50.1 (34.8–90.4) | 52.3 ± 10.4 48.8 (38.0–82.2) | 0.651 | 50.6 ± 10.4 46.4 (40.8–73.1) | 52.9 ± 11.4 49.1 (35.6–98.6) | 52.9 ± 10.6 49.3 (36.2–82.7) | 54.2 ± 11.0 53.8 (34.8–90.4) | 0.511 |
| FFM (%) | 71.4 ± 8.5 72.5 (56.6–87.0) | 75.2 ± 8.1 76.1 (49.0–97.6) | 75.1 ± 7.7 75.5 (51.8–97.2) | 75.3 ± 7.4 75.7 (57.9–95.0) | 0.497 | 72.5 ± 8.4 72.8 (56.6–85.0) | 75.3 ± 7.9 76.0 (49.0–97.2) | 74.9 ± 7.5 75.5 (51.3–95.7) | 75.4 ± 8.4 75.5 (54.2–97.6) | 0.671 |
| FM (kg) | 23.1 ± 8.9 21.8 (10.3–41.2) | 18.3 ± 9.1 16.4 (2.0–57.6) | 18.1 ± 8.0 16.9 (2.0–68.6) | 17.4 ± 6.7 16.9 (3.5–34.8) | 0.228 | 20.4 ± 10.0 19.1 (7.2–41.2) | 18.0 ± 8.7 16.0 (2.0–57.6) | 18.2 ± 7.7 17.0 (2.6–54.1) | 18.3 ± 8.8 17.2 (2.0–68.6) | 0.828 |
| FM (%) | 28.6 ± 8.5 27.5 (13.0–43.4) | 24.8 ± 8.1 23.9 (2.4–51.0) | 24.9 ± 7.7 24.5 (2.8–48.2) | 24.7 ± 7.4 24.3 (5.8–42.1) | 0.498 | 27.5 ± 8.4 27.3 (15.0–43.4) | 24.7 ± 7.9 24.0 (2.8–51.0) | 25.1 ± 7.5 24.5 (4.3–48.7) | 24.6 ± 8.4 24.5 (2.4–45.8) | 0.667 |
| SBP | 124.0 ± 12.5 125.5 (101.0–138.0) | 119.1 ± 12.1 117.0 (95.0–179.0) | 118.6 ± 11.6 117.0 (93.0–170.0) | 119.2 ± 12.1 119.0 (98.0–161.0) | 0.527 | 122.5 ± 14.3 125.5 (101.0–138.0) | 117.9 ± 11.2 116.0 (98.0–179.0) | 118.7 ± 11.7 117.0 (93.0–170.0) | 120.7 ± 12.8 119.0 (97.0–167.0) | 0.111 |
| DBP | 75.6 ± 8.2 78.0 (60.0–84.0) | 74.2 ± 8.6 73.0 (54.0–99.0) | 73.7 ± 8.4 73.0 (53.0–110.0) | 74.2 ± 9.6 73.0 (56.0–108.0) | 0.791 | 76.4 ± 8.2 80.0 (60.0–84.0) | 73.4 ± 8.5 73.0 (55.0–99.0) | 73.6 ± 8.4 73.0 (53.0–110.0) | 75.1 ± 9.1 73.0 (56.0–108.0) | 0.162 |
| ΔSBP | 69.4 ± 25.9 63.5 (35.0–117.0) | 61.3 ± 21.6 57.0 (25.0–158.0) | 59.8 ± 21.7 57.0 (18.0–194.0) | 63.2 ± 22.5 60.0 (30.0–130.0) | 0.317 | 77.6 ± 23.8 81.0 (43.0–117.0) | 60.8 ± 20.2 58.0 (26.0–145.0) | 59.7 ± 20.5 57.0 (18.0–142.0) | 62.5 ± 26.6 59.0 (26.0–194.0) | 0.048 |
| ΔDBP | 54.3 ± 19.1 51.5 (33.0–99.0) | 47.6 ± 15.6 44.0 (18.0–130.0) | 47.2 ± 16.6 44.0 (13.0–145.0) | 46.1 ± 14.2 43.0 (20.0–91.0) | 0.481 | 51.3 ± 13.0 54.0 (33.0–69.0) | 47.8 ± 16.0 45.0 (13.0–108.0) | 46.4 ± 15.7 43.0 (20.0–145.0) | 48.6 ± 17.4 44.0 (16.0–128.0) | 0.413 |
| QTc | 440.4 ± 22.6 443.0 (401.0–467.0) | 423.2 ± 35.1 426.0 (40.0–484.0) | 427.2 ± 21.3 429.0 (367.0–542.0) | 428.2 ± 21.2 426.0 (383.0–513.0) | 0.072 | 429.3 ± 58.1 440.0 (269.0–467.0) | 424.2 ± 34.8 428.0 (40.0–474.0) | 427.5 ± 21.8 428.0 (368.0–542.0) | 425.7 ± 19.9 426.0 (373.0–475.0) | 0.507 |
| BMI | 28.3 ± 4.7 27.6 (22.9–38.1) | 25.0 ± 4.8 24.4 (16.8–44.7) | 24.9 ± 4.0 24.5 (16.4–45.5) | 24.3 ± 3.7 23.9 (18.3–36.5) | 0.050 | 26.4 ± 6.0 24.5 (19.2–38.1) | 24.8 ± 4.4 24.1 (17.5- 44.7) | 24.9 ± 4.1 24.5 (16.4–40.9) | 25.0 ± 4.3 24.5 (18.0–45.5) | 0.698 |
| BPCV | 12.0 ± 3.6 11.7 (7.5–18.0) | 10.8 ± 3.4 10.1 (4.8–25.4) | 10.7 ± 3.6 10.3 (3.1–32.3) | 11.5 ± 3.7 11.1–(5.9–25.7) | 0.212 | 12.9 ± 3.2 13.1(8.9–17.7) | 11.0 ± 3.5 10.5 (4.8–27.2) | 10.6 ± 3.3 10.1 (3.7–23.4) | 11.2 ± 4.2 10.5 (3.1–32.3) | 0.075 |
| Patients without Total or Ionized Magnesium Data (n = 369) | Patients with Total or Ionized Magnesium (n = 833) | p-Value | |||
|---|---|---|---|---|---|
| Sex * | |||||
| Female | 197 | (53.4%) | 475 | (57.2%) | 0.224 |
| Male | 172 | (46.6%) | 356 | (42.8%) | |
| Age, years | 54.8 | (14.4) | 54.7 | (13.7) | 0.892 |
| BMI (kg/m2) | 25.2 | (4.6) | 25.0 | (4.3) | 0.544 |
| SBP, 24 h ABPM (mmHg) | 119.1 | (11.9) | 119.1 | (11.9) | 0.919 |
| DBP, 24 h ABPM (mmHg) | 74.7 | (9.1) | 74.0 | (8.6) | 0.231 |
| Cf-PWV (m/s) | 7.3 | (1.9) | 7.4 | (1.7) | 0.399 |
| O-PWV (m/s) | 7.4 | (1.9) | 7.4 | (1.7) | 0.932 |
| QTc (ms) | 423.9 | (21.0) | 426.2 | (25.9) | 0.141 |
| Low (Tot Mg < 0.75) (n = 52) | Normal (0.75 ≤ Tot Mg < 0.85) (n = 391) | High (Tot Mg ≥ 0.85) (n = 312) | ||||
|---|---|---|---|---|---|---|
| Age (years) | 53.2 | (13.7) (22–76) | 53.4 | (13.6) (21–90) | 55.93 | (12.8) (22–91) |
| Female | 31 | (59.6%) | 239 | (61.1%) | 165 | (52.9%) |
| Male | 21 | (40.4%) | 152 | (38.9%) | 147 | (47.1%) |
| Underweight, BMI < 18.5 (kg/m2) | 1 | 1.9% | 13 | (3.3%) | 7 | (2.2%) |
| Normal weight, 18.5 ≤ BMI ≤ 24.9 (kg/m2) | 23 | (44.2%) | 213 | (54.5%) | 167 | (53.5%) |
| Pre-obesity, 25.0 ≤ BMI ≤ 29.9 (kg/m2) | 21 | (40.4%) | 111 | (28.4%) | 109 | (34.9%) |
| Obese, BMI ≥ 30.0 (kg/m2) | 7 | (13.5%) | 54 | (13.8%) | 29 | (9.3%) |
| FFM (kg) | 55.5 | (12.5) (39–53) | 52.8 | (11.0) (36–86) | 53.1 | (10.5) (35–90) |
| FFM (%) | 74.1 | (7.4) (57–87) | 75.1 | (7.9) (49–98) | 75.2 | (7.7) (54–96) |
| FM (kg) | 20.1 | (9.6) (9.55) | 18.1 | (8.4) (2–58) | 18 | (7.8) (3–69) |
| FM (%) | 25.9 | (7.4) (13–43) | 24.9 | (7.9) (2–51) | 24.8 | (7.7) (4–46) |
| Total Mg (mmol/L) | 0.7 | (0.03) (0.6–0.7) | 0.8 | (0.03) (0.8–0.9) | 0.9 | (0.03) (0.9–1.1) |
| Ionized Mg (mmol/L) | 0.5 | (0.02) (0.4–0.5) | 0.5 | (0.03) (0.5–0.6) | 0.5 | (0.03) (0.5–0.7) |
| SBP, 24 h ABPM (mmHg) | 118.6 | (10.7) (98–138) | 118.8 | (11.8) (95–179) | 119.1 | (12.2) (93–170) |
| DBP, 24 h ABPM (mmHg) | 74.1 | (8.5) (58–74) | 73.6 | (8.3) (54–99) | 74.2 | (9.0) (53–110) |
| ΔSBP, 24 h ABPM (mmHg) | 61.8 | (19.3) (34–117) | 60.6 | (22.6) (18–194) | 60.8 | (21.3) (21–165) |
| ΔDBP, 24 h ABPM (mmHg) | 52.7 | (18.5) (18–108) | 47.1 | (16.2) (13–145) | 46.6 | (15.3) (16–113) |
| Cf-PWV (m/s) | 7.3 | (1.4) (5–11) | 7.3 | (1.7) (4–13) | 7.5 | (1.7) (4–13) |
| O-PWV (m/s) | 7.3 | (1.7) (5–11) | 7.3 | (1.7) (5–13) | 7.6 | (1.8) (5–13) |
| QTc (ms) | 429.0 | (20.9) (381–467) | 424.6 | (29.8) (40–484) | 428.1 | (21.8) (371–542) |
| BPCV (mmHg) | 11.7 | (3.2) (7–20) | 10.6 | (3.5) (4–27) | 11 | (3.7) (3–32) |
| Low (Tot Mg < 0.85) (n = 443) | Normal (Tot Mg ≥ 0.85) (n = 321) | |||
|---|---|---|---|---|
| Age (years) | 53.4 | (13.6) (21–90) | 55.9 | (12.8) (22–91) |
| Female | 270 | (61.0%) | 165 | (52.9%) |
| Male | 173 | (39.1%) | 147 | (47.1%) |
| Underweight, BMI < 18.5 (kg/m2) | 14 | (3.2%) | 7 | (2.2%) |
| Normal weight, 18.5 ≤ BMI ≤ 24.9 (kg/m2) | 236 | (53.3%) | 167 | (53.5%) |
| Pre-obesity, 25.0 ≤ BMI ≤ 29.9 (kg/m2) | 132 | (29.8%) | 109 | (34.9%) |
| Obese, BMI ≥ 30.0 (kg/m2) | 61 | (13.8%) | 29 | (9.3%) |
| FFM (kg) | 53.1 | (11.2) (36–99) | 53.1 | (10.5) (35–90) |
| FFM (%) | 75.0 | (7.8) (49–98) | 75.2 | (7.7) (54–96) |
| FM (kg) | 18.3 | (8.5) (2–58) | 18.0 | (786) (3–67) |
| FM (%) | 25.0 | (7.8) (2.4–51) | 24.8 | (7.7) (4–46) |
| Total Mg (mmol/L) | 0.8 | (0.04) (0.6–0.9) | 0.9 | (0.03) (0.9–1.1) |
| Ionized Mg (mmol/L) | 0.5 | (0.03) (0.4–0.6) | 0.6 | (0.03) (0.5–0.7) |
| SBP, 24 h ABPM (mmHg) | 118.8 | (11.6) (95–179) | 119.1 | (12.2) (93–170) |
| DBP, 24 h ABPM (mmHg) | 73.7 | (8.3) (54–99) | 74.2 | (9.0) (53–110) |
| ΔSBP, 24 h ABPM (mmHg) | 60.7 | (22.2) (18–194) | 60.8 | (21.3) (21–165) |
| ΔDBP, 24 h ABPM (mmHg) | 47.7 | (16.6) (13–145) | 46.6 | (15.3) (16–113) |
| Cf-PWV (m/s) | 7.3 | (1.7) (4–13) | 7.5 | (1.7) (4–13) |
| O-PWV (m/s) | 7.3 | (1.7) (5–13) | 7.6 | (1.8) (5–13) |
| QTc (ms) | 425.1 | (28.9) (40–484) | 428.1 | (21.8) (371–542) |
| BPCV (mmHg) | 10.8 | (3.5) (4–27) | 11.0 | (3.7) (3–32) |
| Low (Ion Mg < 0.50) (n = 140) | Normal (0.50 ≤ Ion Mg < 0.54) (n = 348) | High (Ion Mg ≥ 0.54) (n = 267) | ||||
|---|---|---|---|---|---|---|
| Age, years | 52.4 | (13.3) (22–80) | 54.3 | (13.6) (21–90) | 55.6 | (12.9) (21–91) |
| Female | 93 | (66.4%) | 209 | (60.06%) | 133 | (49.8%) |
| Male | 47 | (33.6%) | 139 | (39.9%) | 134 | (50.2%) |
| Underweight, BMI < 18.5 (kg/m2) | 4 | (2.9%) | 12 | (3.5%) | 5 | (1.9%) |
| Normal weight, 18.5 ≤ BMI ≤ 24.9 (kg/m2) | 86 | (61.4%) | 178 | (51.2%) | 139 | (52.1%) |
| Pre-obesity, 25.0 ≤ BMI ≤ 29.9 (kg/m2) | 30 | (21.4%) | 122 | (35.1%) | 89 | (33.3%) |
| Obese, BMI ≥ 30.0 (kg/m2) | 20 | (14.3%) | 36 | (10.3%) | 34 | (12.7%) |
| FFM (kg) | 52.8 | (11.7) (36–99 | 52.5 | (10.4) (36–79) | 54.0 | (11.1) (35–90) |
| FFM (%) | 75.6 | (7.8) (55–92) | 75.2 | (7.8) (49–97) | 74.7 | (7.8) (54–98) |
| FM (kg) | 17.7 | (8.9) (4–55) | 17.9 | (8.0) (2–58) | 18.8 | (8.2) (2–69) |
| FM (%) | 24.4 | (7.8) (8–45) | 24.8 | (7.8) (3–51) | 25.3 | (7.8) (2–46) |
| Total Mg (mmol/L) | 0.8 | (0.0) (0.7–0.9) | 0.8 | (0.01) (0.7–0.9) | 0.9 | (0.01) (0.8–1.1) |
| Ionized Mg (mmol/L) | 0.5 | (0.01) (0.4–0.5) | 0.5 | (0.01) (0.5–0.6) | 0.6 | (0.01) (0.5–0.7) |
| SBP, 24 h ABPM (mmHg) | 117.5 | (11.3) (98–179) | 118.6 | (11.1) (93–159) | 120.1 | (12.9) (96–170) |
| DBP, 24 h ABPM (mmHg) | 72.9 | (8.2) (55–99) | 73.9 | (8.3) (53–99) | 74.4 | (9.2) (56–110) |
| ΔSBP, 24 h ABPM (mmHg) | 60.3 | (19.6) (26–145) | 59.5 | (20.4) (21–133) | 62.6 | (24.6) (18–194) |
| ΔDBP, 24 h ABPM (mmHg) | 47.6 | (15.2) (18–108) | 46.7 | (16.4) (13–145) | 47.8 | (16.1) (16–128) |
| Cf-PWV (m/s) | 7.1 | (1.5) (5–12) | 7.4 | (1.7) (4–13) | 7.5 | (1.6) (4–13) |
| O-PWV (m/s) | 7.1 | (1.6) (5–11) | 7.4 | (1.8) (5–13) | 7.6 | (1.7) (5–13) |
| QTc (ms) | 427.6 | (24.0) (269–467) | 425.5 | (30.8) (40–542) | 426.7 | (20.2) (373–513) |
| BPCV (mmHg) | 10.9 | (3.3) (5–25) | 10.6 | (3.4) (4–27) | 11.1 | (3.8) (3–32) |
| Ion Mg < 0.55 (n = 558) | Ion Mg ≥ 0.55 (n = 197) | |||
|---|---|---|---|---|
| Age (years) | 54.0 | (13.5) (21–90) | 55.61 | (12.80) (21–91) |
| Female | 342 | (61.3%) | 93 | (47.2%) |
| Male | 216 | (38.7%) | 104 | (52.8%) |
| Underweight, BMI < 18.5 (kg/m2) | 17 | (3.1%) | 4 | (2.0%) |
| Normal weight, 18.5 ≤ BMI ≤ 24.9 (kg/m2) | 298 | (53.4%) | 105 | (53.3%) |
| Pre-obesity, 25.0 ≤ BMI ≤ 29.9 (kg/m2) | 179 | (32.1%) | 62 | (31.5%) |
| Obese, BMI ≥ 30.0 (kg/m2) | 64 | (11.5%) | 26 | (13.2%) |
| FFM (kg) | 52.7 | (10.8) (36–99) | 54.3 | (11.0) (35–90) |
| FFM (%) | 75.0 | (7.6) (49.97) | 75.5 | (8.2) (54–98) |
| FM (kg) | 18.1 | (8.0) (2–58) | 18.3 | (8.7) (2–69) |
| FM (%) | 25.1 | (7.6) (3–51) | 24.5 | (8.2) (2–46) |
| Total Mg (mmol/L) | 0.81 | (0.1) (0.6–1.0) | 0.9 | (0.05) (0.8–1.1) |
| Ionized Mg (mmol/L) | 0.51 | (0.02) (0.4–0.5) | 0.6 | (0.02) (0.6–0.7) |
| SBP, 24 h ABPM (mmHg) | 118.4 | (11.4) (93–179) | 120.3 | (13.0) (96–167) |
| DBP, 24 h ABPM (mmHg) | 73.6 | (8.3) (53–99) | 74.7 | (9.3) (56–110) |
| ΔSBP, 24 h ABPM (mmHg) | 60.1 | (20.3) (18–145) | 62.6 | (25.6) (26–194) |
| ΔDBP, 24 h ABPM (mmHg) | 47.0 | (16.0) (13–145) | 47.9 | (16.4) (16–128) |
| Cf-PWV (m/s) | 7.3 | (1.7) (4–13) | 7.6 | (1.6) (5–13) |
| O-PWV (m/s) | 7.4 | (1.8) (5–13) | 7.6 | (1.7) (5–13) |
| QTc (ms) | 426.1 | (27.9) (40–542) | 427.1 | (20.7) (373–513) |
| BPCV (mmHg) | 10.8 | (3.4) (4–27) | 11.2 | (4.0) (3–32) |
References
- Ayuk, J.; Gittoes, N.J. Contemporary view of the clinical relevance of magnesium homeostasis. Ann. Clin. Biochem. Int. J. Lab. Med. 2014, 51 Pt 2, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Baaij, J.H.F.; de Hoenderop, J.G.J.; Bindels, R.J.M. Magnesium in man: Implications for health and disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Fiorentini, D.; Cappadone, C.; Farruggia, G.; Prata, C. Magnesium: Biochemistry, Nutrition, Detection, and Social Impact of Diseases Linked to Its Deficiency. Nutrients 2021, 13, 1136. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Mohammed, A. Magnesium: The Forgotten Electrolyte—A Review on Hypomagnesemia. Med. Sci. 2019, 7, 56. [Google Scholar] [CrossRef] [Green Version]
- Gröber, U.; Schmidt, J.; Kisters, K. Magnesium in Prevention and Therapy. Nutrients 2015, 7, 8199–8226. [Google Scholar] [CrossRef] [Green Version]
- Seo, J.W.; Park, T.J. Magnesium metabolism. Electrolyte Blood Press. 2008, 6, 86–95. [Google Scholar] [CrossRef] [Green Version]
- Jahnen-Dechent, W.; Ketteler, M. Magnesium basics. Clin. Kidney J. 2012, 5 (Suppl. 1), i3–i14. [Google Scholar] [CrossRef] [Green Version]
- Swaminathan, R. Magnesium metabolism and its disorders. Clin. Biochem. Rev. 2003, 24, 47–66. [Google Scholar]
- Ehrenpreis, E.D.; Jarrouj, G.; Meader, R.; Wagner, C.; Ellis, M. A comprehensive review of hypomagnesemia. Disease-A-Month 2021, 68, 101285. [Google Scholar] [CrossRef]
- Lowenstein, F.W.; Stanton, M.F. Serum magnesium levels in the United States, 1971–1974. J. Am. Coll. Nutr. 1986, 5, 399–414. [Google Scholar] [CrossRef]
- Workinger, J.L.; Doyle, R.P.; Bortz, J. Challenges in the Diagnosis of Magnesium Status. Nutrients 2018, 10, 1202. [Google Scholar] [CrossRef] [PubMed]
- Glasdam, S.-M.; Glasdam, S.; Peters, G.H. The Importance of Magnesium in the Human Body; Elsevier: Amsterdam, The Netherlands, 2016; pp. 169–193. [Google Scholar]
- Altura, B.T.; Shirey, T.L.; Young, C.C.; Dellorfano, K.; Hiti, J.; Welsh, R.; Yeh, Q.; Barbour, R.L.; Altura, B.M. Characterization of a New Ion Selective Electrode for Ionized Magnesium in Whole Blood, Plasma, Serum, and Aqueous Samples. Scand. J. Clin. Lab. Investig. 1994, 54, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Greenway, D.C.; Hindmarsh, J.T.; Wang, J.; Khodadeen, J.A.; Hébert, P.C. Reference interval for whole blood ionized magnesium in a healthy population and the stability of ionized magnesium under varied laboratory conditions. Clin. Biochem. 1996, 29, 515–520. [Google Scholar] [CrossRef]
- Banach, W.; Nitschke, K.; Krajewska, N.; Mongiałło, W.; Matuszak, O.; Muszyński, J.; Skrypnik, D. The Association between Excess Body Mass and Disturbances in Somatic Mineral Levels. Int. J. Mol. Sci. 2020, 21, 7306. [Google Scholar] [CrossRef]
- Lecube, A.; Baena-Fustegueras, J.A.; Fort, J.M.; Pelegrí, D.; Hernández, C.; Simó, R. Diabetes Is the Main Factor Accounting for Hypomagnesemia in Obese Subjects. PLoS ONE 2012, 7, e30599. [Google Scholar] [CrossRef] [Green Version]
- Musso, C.G. Magnesium metabolism in health and disease. Int. Urol. Nephrol. 2009, 41, 357–362. [Google Scholar] [CrossRef]
- Kieboom, B.C.T.; Kiefte–de Jong, J.C.; Eijgelsheim, M.; Franco, O.H.; Kuipers, E.J.; Hofman, A.; Zietse, R.; Stricker, B.H.; Hoorn, E.J. Proton Pump Inhibitors and Hypomagnesemia in the General Population: A Population-Based Cohort Study. Am. J. Kidney Dis. 2015, 66, 775–782. [Google Scholar] [CrossRef]
- Gröber, U. Magnesium and Drugs. Int. J. Mol. Sci. 2019, 20, 2094. [Google Scholar] [CrossRef] [Green Version]
- Hashizume, N.; Mori, M. An analysis of hypermagnesemia and hypomagnesemia. Jpn. J. Med. 1990, 29, 368–372. [Google Scholar] [CrossRef] [Green Version]
- Rooney, M.R.; Lutsey, P.L.; Alonso, A.; Selvin, E.; Pankow, J.S.; Rudser, K.D.; Dudley, S.C.; Chen, L.Y. Serum magnesium and burden of atrial and ventricular arrhythmias: The Atherosclerosis Risk in Communities (ARIC) Study. J. Electrocardiol. 2020, 62, 20–25. [Google Scholar] [CrossRef]
- Kieboom, B.C.T.; Niemeijer, M.N.; Leening, M.J.G.; Berg, M.E.V.D.; Franco, O.H.; Deckers, J.W.; Hofman, A.; Zietse, R.; Stricker, B.H.; Hoorn, E.J. Serum Magnesium and the Risk of Death From Coronary Heart Disease and Sudden Cardiac Death. J. Am. Hear. Assoc. 2016, 5, e002707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Chen, C.; Duan, P.; Thapaliya, S.; Gao, L.; Dong, Y.; Yin, X.; Yang, X.; Zhang, R.; Tan, R.; et al. The ECG Characteristics of Patients with Isolated Hypomagnesemia. Front. Physiol. 2021, 11, 617374. [Google Scholar] [CrossRef] [PubMed]
- Del Gobbo, L.C.; Imamura, F.; Wu, J.H.; de Oliveira Otto, M.C.; Chiuve, S.E.; Mozaffarian, D. Circulating and dietary magnesium and risk of cardiovascular disease: A systematic review and meta-analysis of prospective studies. Am. J. Clin. Nutr. 2013, 98, 160–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Hu, M.; Yang, L.; Xu, H.; Song, W.; Qian, Y.; Zhao, M. Quantitative Association between Serum/Dietary Magnesium and Cardiovascular Disease/Coronary Heart Disease Risk: A Dose-Response Meta-analysis of Prospective Cohort Studies. J. Cardiovasc. Pharmacol. 2019, 74, 516–527. [Google Scholar] [CrossRef]
- Pham, P.C.T.; Pham, P.A.T.; Pham, S.V.; Pham, P.T.T.; Pham, P.M.T.; Pham, P.T.T. Hypomagnesemia: A clinical perspective. Int. J. Nephrol. Renov. Dis. 2014, 7, 219–230. [Google Scholar] [CrossRef] [Green Version]
- Gragossian, A.; Bashir, K.; Friede, R. Hypomagnesemia; StatPearls Publishing LLC: Treasure Island, FL, USA, 2021. [Google Scholar]
- Fang, X.; Wang, K.; Han, D.; He, X.; Wei, J.; Zhao, L.; Imam, M.U.; Ping, Z.; Li, Y.; Wang, F.; et al. Dietary magnesium intake and the risk of cardiovascular disease, type 2 diabetes, and all-cause mortality: A dose-response meta-analysis of prospective cohort studies. BMC Med. 2016, 14, 210. [Google Scholar] [CrossRef] [Green Version]
- Kass, L.S.; Weekes, J.; Carpenter, L. Effect of magnesium supplementation on blood pressure: A meta-analysis. Eur. J. Clin. Nutr. 2012, 66, 411–418. [Google Scholar] [CrossRef]
- Banjanin, N.; Belojevic, G. Changes of Blood Pressure and Hemodynamic Parameters after Oral Magnesium Supplementation in Patients with Essential Hypertension—An Intervention Study. Nutrients 2018, 10, 581. [Google Scholar] [CrossRef] [Green Version]
- Han, H.; Fang, X.; Wei, X.; Liu, Y.; Jin, Z.; Chen, Q.; Fan, Z.; Aaseth, J.; Hiyoshi, A.; He, J.; et al. Dose-response relationship between dietary magnesium intake, serum magnesium concentration and risk of hypertension: A systematic review and meta-analysis of prospective cohort studies. Nutr. J. 2017, 16, 26. [Google Scholar] [CrossRef]
- Marques, B.C.A.A.; Klein, M.R.S.T.; da Cunha, M.R.; Mattos, S.D.S.; Nogueira, L.D.P.; de Paula, T.; Corrêa, F.M.; Oigman, W.; Neves, M.F. Effects of Oral Magnesium Supplementation on Vascular Function: A Systematic Review and Meta-analysis of Randomized Controlled Trials. High Blood Press. Cardiovasc. Prev. 2019, 27, 19–28. [Google Scholar] [CrossRef]
- Chrysant, S.G.; Chrysant, G.S. Adverse cardiovascular and blood pressure effects of drug-induced hypomagnesemia. Expert Opin. Drug Saf. 2019, 19, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Kostov, K.; Halacheva, L. Role of Magnesium Deficiency in Promoting Atherosclerosis, Endothelial Dysfunction, and Arterial Stiffening as Risk Factors for Hypertension. Int. J. Mol. Sci. 2018, 19, 1724. [Google Scholar] [CrossRef] [Green Version]
- Joris, P.J.; Plat, J.; Bakker, S.J.L.; Mensink, R.P. Long-term magnesium supplementation improves arterial stiffness in overweight and obese adults: Results of a randomized, double-blind, placebo-controlled intervention trial. Am. J. Clin. Nutr. 2016, 103, 1260–1266. [Google Scholar] [CrossRef] [Green Version]
- Afsar, B.; Elsurer, R. The relationship between magnesium and ambulatory blood pressure, augmentation index, pulse wave velocity, total peripheral resistance, and cardiac output in essential hypertensive patients. J. Am. Soc. Hypertens. 2014, 8, 28–35. [Google Scholar] [CrossRef]
- Vermeulen, E.A.; de Jong, H.B.T.; Blomjous, A.G.A.; Eelderink, C.; Hoekstra, T.; Elders, P.J.M.; de Borst, M.H.; Vervloet, M.G.; van Ballegooijen, A.J.; Beulens, J.W. Magnesium intake and vascular structure and function: The Hoorn Study. Eur. J. Nutr. 2021, 61, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shlomo, Y.; Spears, M.; Boustred, C.; May, M.; Anderson, S.G.; Benjamin, E.J.; Boutouyrie, P.; Cameron, J.; Chen, C.; Wilkinson, I.B.; et al. Aortic pulse wave velocity improves cardiovascular event prediction: An individual participant meta-analysis of prospective observational data from 17,635 subjects. J. Am. Coll. Cardiol. 2014, 63, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Del Giorno, R.; Gabutti, S.; Troiani, C.; Stefanelli, K.; Falciano, R.; Graziano, E.; Negro, T.R.; Gabutti, L. Association Between HDL Cholesterol and QTc Interval: A Population-Based Epidemiological Study. J. Clin. Med. 2019, 8, 1527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Giorno, R.; Troiani, C.; Gabutti, S.; Stefanelli, K.; Gabutti, L. Comparing oscillometric and tonometric methods to assess pulse wave velocity: A population-based study. Ann. Med. 2021, 53, 1–16. [Google Scholar] [CrossRef]
- Witte, R.S.; Witte, J.S. Statistics; Wiley: Hoboken, NJ, USA, 2017. [Google Scholar]
- Bm, A. Importance of Ionized Magnesium Measurements in Physiology and Medicine and the Need for Ion-selective Electrodes. J. Clin. Case Stud. 2016, 1, 1–4. [Google Scholar] [CrossRef]
- Rooney, M.R.; Rudser, K.D.; Alonso, A.; Harnack, L.; Saenger, A.K.; Lutsey, P.L. Circulating Ionized Magnesium: Comparisons with Circulating Total Magnesium and the Response to Magnesium Supplementation in a Randomized Controlled Trial. Nutrients 2020, 12, 263. [Google Scholar] [CrossRef] [Green Version]
- Del Giorno, R.; Lavorato Hadjeres, S.; Stefanelli, K.; Allegra, G.; Zapparoli, C.; Predrag, L.; Berwert, L.; Gabutti, L. Consequences of Supraphysiological Dialysate Magnesium on Arterial Stiffness, Hemodynamic Profile, and Endothelial Function in Hemodialysis: A Randomized Crossover Study Followed by a Non-Controlled Follow-Up Phase. Adv. Ther. 2020, 37, 4848–4865. [Google Scholar] [CrossRef] [PubMed]
- Stevens, S.L.; Wood, S.; Koshiaris, C.; Law, K.; Glasziou, P.; Stevens, R.J.; McManus, R.J. Blood pressure variability and cardiovascular disease: Systematic review and meta-analysis. BMJ 2016, 354, i4098. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Ion-Mg ≤ 0.45 | 0.46 ≤ Ion-Mg ≤ 0.50 | 0.51 ≤ Ion-Mg ≤ 0.55 | Ion-Mg ≥ 0.56 | Total | |
|---|---|---|---|---|---|
| Tot-Mg ≤ 0.70 | 5 | 5 | 0 | 0 | 10 |
| 0.71 ≤ Tot-Mg ≤ 0.80 | 5 | 139 | 76 | 4 | 224 |
| 0.81 ≤ Tot-Mg ≤ 0.90 | 0 | 53 | 292 | 94 | 439 |
| Tot-Mg ≥ 0.91 | 0 | 2 | 31 | 49 | 82 |
| Total | 10 | 199 | 399 | 147 | 755 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gagliano, V.; Schäffeler, F.; Del Giorno, R.; Bianchetti, M.; Carvajal Canarte, C.F.; Caballero Regueira, J.J.; Gabutti, L. Does Ionized Magnesium Offer a Different Perspective Exploring the Association between Magnesemia and Targeted Cardiovascular Risk Factors? J. Clin. Med. 2022, 11, 4015. https://doi.org/10.3390/jcm11144015
Gagliano V, Schäffeler F, Del Giorno R, Bianchetti M, Carvajal Canarte CF, Caballero Regueira JJ, Gabutti L. Does Ionized Magnesium Offer a Different Perspective Exploring the Association between Magnesemia and Targeted Cardiovascular Risk Factors? Journal of Clinical Medicine. 2022; 11(14):4015. https://doi.org/10.3390/jcm11144015
Chicago/Turabian StyleGagliano, Vanessa, Fabian Schäffeler, Rosaria Del Giorno, Mario Bianchetti, Cesar Fabian Carvajal Canarte, José Joel Caballero Regueira, and Luca Gabutti. 2022. "Does Ionized Magnesium Offer a Different Perspective Exploring the Association between Magnesemia and Targeted Cardiovascular Risk Factors?" Journal of Clinical Medicine 11, no. 14: 4015. https://doi.org/10.3390/jcm11144015






