Bacteria and Dry Eye: A Narrative Review
Abstract
:1. Introduction
1.1. Ocular Surface Flora in General Population
1.2. Ocular Surface Flora in Patients with Dry Eye
1.3. Dry eye and Antibiotic Therapy
1.4. Possible Mechanisms of Bacteria in the Development of Dry Rye
1.4.1. Destruction of Antibacterial Barrier on the Ocular Surface
1.4.2. Effects of Infectious Diseases and Available Treatments
1.4.3. Contact Lens Wear
1.4.4. Microbiome Homeostasis
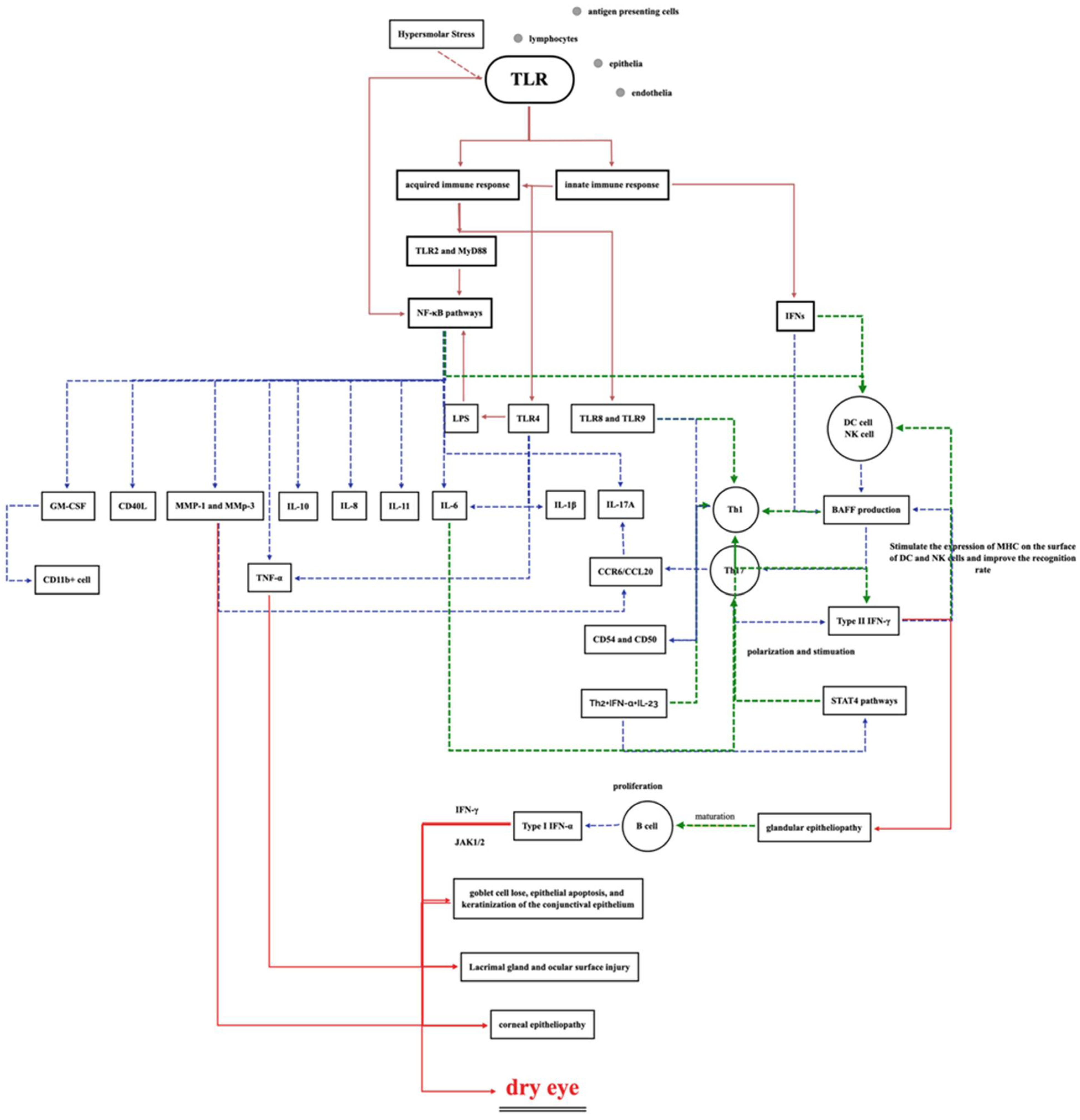
1.4.5. Ocular Surface Toll-like Receptor (TLR) Activation and Inflammatory Factors
1.4.6. Vitamin Deficiency
Vitamin B Deficiency
Vitamin D Deficiency
Other Vitamin Deficiencies
2. Potential Treatment Targets
3. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.-K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Xia, W.; Wang, M.; Chang, X.; Wang, J.; Jin, S.; Wang, J.; Wei, W.; Rudan, I. Variations of dry eye disease prevalence by age, sex and geographic characteristics in China: A systematic review and meta-analysis. J. Glob. Health 2018, 8, 020503. [Google Scholar] [CrossRef] [PubMed]
- Barabino, S. Is dry eye disease the same in young and old patients? A narrative review of the literature. BMC Ophthalmol. 2022, 22, 85. [Google Scholar] [CrossRef] [PubMed]
- Rouen, P.A.W.M. Dry Eye Disease: Prevalence, Assessment, and Management. Home Healthc. Now 2018, 36, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.A.R.; Rocha, E.M. Dry eye disease caused by viral infection: Review. Arq. Bras. Oftalmol. 2013, 76, 129–132. [Google Scholar] [CrossRef] [Green Version]
- Rajalakshmy, A.R.M.J.; Madhavan, H.N.; Bhaskar, S.; Iyer, G.K. Patients with dry eye without hepatitis C virus infection possess the viral RNA in their tears. Cornea 2015, 34, 28–31. [Google Scholar] [CrossRef]
- Groden, L.R.; Murphy, B.; Rodnite, J.; Genvert, G.I. Lid flora in blepharitis. Cornea 1991, 10, 50–53. [Google Scholar] [CrossRef]
- Huang, T.; Wang, Y.; Liu, Z.; Wang, T.; Chen, J. Investigation of tear film change after recovery from acute conjunctivitis. Cornea 2007, 26, 778–781. [Google Scholar] [CrossRef]
- O’Callaghan, R.J.; Girgis, D.O.; Dajcs, J.J.; Sloop, G.D. Host defense against bacterial keratitis. Ocul. Immunol. Inflamm. 2003, 11, 171–181. [Google Scholar] [CrossRef]
- Qi, Y.; Wan, Y.; Li, T.; Zhang, M.; Song, Y.; Hu, Y.; Sun, Y.; Li, L. Comparison of the Ocular Microbiomes of Dry Eye Patients with and without Autoimmune Disease. Front. Cell. Infect. Microbiol. 2021, 11, 716867. [Google Scholar] [CrossRef]
- Baudouin, C.; de Lunardo, C. Short-term comparative study of topical 2% carteolol with and without benzalkonium chloride in healthy volunteers. Br. J. Ophthalmol. 1998, 82, 39–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suto, C.; Morinaga, M.; Yagi, T.; Tsuji, C.; Toshida, H. Conjunctival sac bacterial flora isolated prior to cataract surgery. Infect. Drug. Resist. 2012, 5, 37–41. [Google Scholar] [PubMed] [Green Version]
- Hsu, H.Y.; Lind, J.T.; Tseng, L.; Miller, D. Ocular Flora and Their Antibiotic Resistance Patterns in the Midwest: A Prospective Study of Patients Undergoing Cataract Surgery. Am. J. Ophthalmol. 2013, 155, 36–44.e2. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.E.; Moore, J.E.; Jiru, X.; Moore, J.E.; Goodall, E.A.; Dooley, J.S.; Hayes, V.E.; Dartt, D.A.; Downes, C.S.; Moore, T.C. Ocular pathogen or commensal: A PCR-based study of surface bacterial flora in normal and dry eyes. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5616–5623. [Google Scholar] [CrossRef]
- Venugopal, R.; Satpathy, G.; Sangwan, S.; Kapil, A.; Aron, N.; Agarwal, T.; Pushker, N.; Sharma, N. Conjunctival Microbial Flora in Ocular Stevens-Johnson Syndrome Sequelae Patients at a Tertiarty Eye Care Center. Cornea 2016, 35, 1117–1121. [Google Scholar] [CrossRef]
- Rubio, E.F. Climatic influence on conjunctival bacteria of patients undergoing cataract surgery. Eye 2004, 18, 778–784. [Google Scholar] [CrossRef] [Green Version]
- Capriotti, J.A.; Pelletier, J.S.; Shah, M.; Caivano, D.M.; Ritterband, D.C. Normal ocular flora in healthy eyes from a rural population in Sierra Leone. Int. Ophthalmol. 2008, 29, 81–84. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, B.; Li, W. Defining the normal core microbiome of conjunctival microbial communities. Clin. Microbiol. Infect. 2016, 22, 643.e7–643.e12. [Google Scholar] [CrossRef] [Green Version]
- Doan, T.; Akileswaran, L.; Andersen, D.; Johnson, B.; Ko, N.; Shrestha, A.; Shestopalov, V.; Lee, C.S.; Lee, A.Y.; Van Gelder, R.N. Paucibacterial Microbiome and Resident DNA Virome of the Healthy Conjunctiva. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5116–5126. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.D.; He, J.N.; Niu, T.T.; Liu, S.S.; Chan, C.Y.; Ren, C.Y.; Liu, C.; Pang, C.P.; Qu, Y.; Li, R.X.; et al. Effectiveness of meibomian gland massage combined with topical levofloxacin against ocular surface flora in patients before penetrating ocular surgery. Ocul. Surf. 2018, 16, 70–76. [Google Scholar] [CrossRef]
- Jiang, X.; Deng, A.; Yang, J.; Bai, H.; Yang, Z.; Wu, J.; Lv, H.; Li, X.; Wen, T. Pathogens in the Meibomian gland and conjunctival sac: Microbiome of normal subjects and patients with Meibomian gland dysfunction. Infect. Drug Resist. 2018, 11, 1729–1740. [Google Scholar] [CrossRef] [Green Version]
- Cavuoto, K.M.; Banerjee, S.; Miller, D.; Galor, A. Composition and Comparison of the Ocular Surface Microbiome in Infants and Older Children. Transl. Vis. Sci. Technol. 2018, 7, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, X.; Wang, Y.; Wang, W.; Lin, P.; Huang, Y. Composition and Diversity of Bacterial Community on the Ocular Surface of Patients with Meibomian Gland Dysfunction. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4774–4783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Gong, Y.; Chen, S.; Li, S.; Zhang, Y.; Zhong, H.; Wang, Z.; Chen, Y.; Deng, Q.; Jiang, Y.; et al. Comparative portrayal of ocular surface microbe with and without dry eye. J. Microbiol. 2019, 57, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, J.; Nielsen, S.; Diez-Vives, C.; Coroneo, M.; Thomas, T.; Willcox, M. Temporal Stability and Composition of the Ocular Surface Microbiome. Sci. Rep. 2017, 7, 9880. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Zou, X.; Xue, W.; Zhang, P.; Wang, S.; Zou, H. Ocular Surface Microbiota in Diabetic Patients with Dry Eye Disease. Investig. Ophthalmol. Vis. Sci. 2021, 62, 13. [Google Scholar] [CrossRef]
- Zhang, S.D.; He, J.N.; Niu, T.T.; Chan, C.Y.; Ren, C.Y.; Liu, S.S.; Qu, Y.; Chong, K.L.; Wang, H.L.; Tao, J.; et al. Bacteriological profile of ocular surface flora in meibomian gland dysfunction. Ocul. Surf. 2017, 15, 242–247. [Google Scholar] [CrossRef]
- Xue, A.L.; Wang, M.T.M.; Ormonde, S.E.; Craig, J.P. Randomised double-masked placebo-controlled trial of the cumulative treatment efficacy profile of intense pulsed light therapy for meibomian gland dysfunction. Ocul. Surf. 2020, 18, 286–297. [Google Scholar] [CrossRef]
- Watters, G.A.; Turnbull, P.R.; Swift, S.; Petty, A.; Craig, J.P. Ocular surface microbiome in meibomian gland dysfunction. Clin. Exp. Ophthalmol. 2017, 45, 105–111. [Google Scholar] [CrossRef]
- Foulks, G.N.; Borchman, D.; Yappert, M.; Kim, S.-H.; McKay, J.W. Topical Azithromycin Therapy for Meibomian Gland Dysfunction: Clinical Response and Lipid Alterations. Cornea 2010, 29, 781–788. [Google Scholar] [CrossRef] [Green Version]
- Foulks, G.N.; Borchman, D.; Yappert, M.; Kakar, S. Topical Azithromycin and Oral Doxycycline Therapy of Meibomian Gland Dysfunction. Cornea 2013, 32, 44–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, J.; Yan, X. Emerging treatment options for meibomian gland dysfunction. Clin. Ophthalmol. 2013, 7, 1797–1803. [Google Scholar] [PubMed]
- Aghai, Z.; Kode, A.; Saslow, J.; Nakhla, T.; Farhath, S.; Stahl, G.; Eydelman, R.; Strande, L.; Leone, P.; Rahman, I. Azithromycin suppresses activation of nuclear factor-kappa B and synthesis of pro-inflammatory cytokines in tracheal aspirate cells from premature infants. Pediatr. Res. 2007, 62, 483–488. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Kam, W.R.; Ding, J.; Sullivan, D.A. One man’s poison is another man’s meat: Using azithromycin-induced phospholipidosis to promote ocular surface health. Toxicology 2014, 320, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashkouli, M.B.; Fazel, A.J.; Kiavash, V.; Nojomi, M.; Ghiasian, L. Oral azithromycin versus doxycycline in meibomian gland dysfunction: A randomised double-masked open-label clinical trial. Br. J. Ophthalmol. 2015, 99, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.; Albietz, J.M.; Tran, H.; Du Toit, C.; Li, A.H.; Yun, T.; Han, J.; Schmid, K.L. Treatment of contact lens related dry eye with antibacterial honey. Contact Lens Anterior Eye 2017, 40, 389–393. [Google Scholar] [CrossRef]
- Albietz, J.M.; Lenton, L. Effect of antibacterial honey on the ocular flora in tear deficiency and meibomian gland disease. Cornea 2006, 25, 1012–1019. [Google Scholar] [CrossRef]
- Felt, O.; Carrel, A.; Baehni, P.; Buri, P.; Gurny, R. Chitosan as tear substitute: A wetting agent endowed with antimicrobial efficacy. J. Ocul. Pharmacol. Ther. 2000, 16, 261–270. [Google Scholar] [CrossRef]
- McDermott, A.M. Antimicrobial compounds in tears. Exp. Eye Res. 2013, 117, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Alexander, D.B.; Iigo, M.; Yamauchi, K.; Suzui, M.; Tsuda, H. Lactoferrin: An alternative view of its role in human biological fluids. Biochem. Cell Biol. 2012, 90, 279–306. [Google Scholar] [CrossRef]
- Boost, M.; Cho, P.; Wang, Z. Disturbing the balance: Effect of contact lens use on the ocular proteome and microbiome. Clin. Exp. Optom. 2017, 100, 459–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhatem, A.; Cavalcanti, B.; Hamrah, P. In Vivo confocal microscopy in dry eye disease and related conditions. Semin. Ophthalmol. 2012, 27, 138–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villani, E.; Magnani, F.; Viola, F.; Santaniello, A.; Scorza, R.; Nucci, P.; Ratiglia, R. In Vivo Confocal Evaluation of the Ocular Surface Morpho-Functional Unit in Dry Eye. Optom. Vis. Sci. 2013, 90, 576–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stern, M.E.; Gao, J.; Siemasko, K.F.; Beuerman, R.W.; Pflugfelder, S.C. The role of the lacrimal functional unit in the pathophysiology of dry eye. Exp. Eye Res. 2004, 78, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y. Wakayama symposium: Role of canonical Notch signaling in conjucntival goblet cell differentiation and dry eye syndrome. BMC Ophthalmol. 2015, 15 (Suppl. S1), 152. [Google Scholar] [CrossRef] [Green Version]
- McMonnies, C.W. Conjunctival Tear Layer Temperature, Evaporation, Hyperosmolarity, Inflammation, Hyperemia, Tissue Damage, and Symptoms: A Review of an Amplifying Cascade. Curr. Eye Res. 2017, 42, 1574–1584. [Google Scholar] [CrossRef]
- Khurana, A.K.; Moudgil, S.S.; Parmar, I.P.; Ahluwalia, B.K. Tear Film Flow and Stability in Acute and Chronic Conjunctivitis. Acta Opthalmol. 1987, 65, 303–305. [Google Scholar] [CrossRef]
- Nelson, J.D.; Craig, J.P.; Akpek, E.K.; Azar, D.T.; Belmonte, C.; Bron, A.J.; Clayton, J.A.; Dogru, M.; Dua, H.S.; Foulks, G.N.; et al. TFOS DEWS II Introduction. Ocul. Surf. 2017, 15, 269–275. [Google Scholar] [CrossRef] [Green Version]
- Dogan, A.S.; Gurdal, C.; Arslan, N. Corneal confocal microscopy and dry eye findings in contact lens discomfort patients. Contact Lens Anterior Eye 2018, 41, 101–104. [Google Scholar] [CrossRef]
- Chen, J.; Simpson, T.L. A role of corneal mechanical adaptation in contact lens-related dry eye symptoms. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1200–1205. [Google Scholar] [CrossRef] [Green Version]
- Cerretani, C.; Peng, C.C.; Chauhan, A.; Radke, C.J. Aqueous salt transport through soft contact lenses: An osmotic-withdrawal mechanism for prevention of adherence. Contact Lens Anterior Eye 2012, 35, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Foulks, G.N. What is Dry Eye and What Does It Mean to the Contact Lens Wearer? Eye Contact Lens 2003, 29, S96–S100. [Google Scholar] [CrossRef] [PubMed]
- Markoulli, M.; Kolanu, S. Contact lens wear and dry eyes: Challenges and solutions. Clin. Optom. 2017, 9, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Larkin, D.F.; Silvington, S.; Easty, D.L. Contamination of Contact Lens Storage Cases by Acanthamoeba and Bacteria. Br. J. Ophthalmol. 1990, 74, 133–135. [Google Scholar] [CrossRef] [Green Version]
- Liaqat, I.; Saleem, Q.U.; Tahir, H.M.; Arshad, M.; Arshad, N. Identification of virulence factors in contact lens associated bacteria: A physiological approach. Contact Lens Anterior Eye 2019, 42, 159–164. [Google Scholar] [CrossRef]
- Kilvington, S.; Shovlin, J.; Nikolic, M. Identification and susceptibility to multipurpose disinfectant solutions of bacteria isolated from contact lens storage cases of patients with corneal infiltrative events. Contact Lens Anterior Eye 2013, 36, 294–298. [Google Scholar] [CrossRef]
- Obrubov, A.S.; Slonimskii, A.Y. Contact lens-related keratitis and purulent corneal ulcers. Vestn. Oftalmol. 2018, 134, 17–24. [Google Scholar] [CrossRef]
- Okonkwo, A.; Rimmer, V.; Walkden, A.; Brahma, A.; Carley, F.; McBain, A.J.; Radhakrishnan, H. Next-Generation Sequencing of the Ocular Surface Microbiome: In Health, Contact Lens Wear, Diabetes, Trachoma, and Dry Eye. Eye Contact Lens 2020, 46, 254–261. [Google Scholar] [CrossRef]
- Metruccio, M.M.E.; Wan, S.J.; Horneman, H.; Kroken, A.R.; Sullivan, A.B.; Truong, T.N.; Mun, J.J.; Tam, C.K.P.; Frith, R.; Welsh, L.; et al. A novel murine model for contact lens wear reveals clandestine IL-1R dependent corneal parainflammation and susceptibility to microbial keratitis upon inoculation with Pseudomonas aeruginosa. Ocul. Surf. 2019, 17, 119–133. [Google Scholar] [CrossRef]
- St Leger, A.J.; Caspi, R.R. Visions of Eye Commensals: The Known and the Unknown About How the Microbiome Affects Eye Disease. Bioessays 2018, 40, e1800046. [Google Scholar] [CrossRef]
- Feher, J.; Pinter, E.; Kovacs, I.; Helyes, Z.; Kemeny, A.; Markovics, A.; Plateroti, R.; Librando, A.; Cruciani, F. Irritable eye syndrome: Neuroimmune mechanisms and benefits of selected nutrients. Ocul. Surf. 2014, 12, 134–145. [Google Scholar] [CrossRef] [PubMed]
- St Leger, A.J.; Desai, J.V.; Drummond, R.A.; Kugadas, A.; Almaghrabi, F.; Silver, P.; Raychaudhuri, K.; Gadjeva, M.; Iwakura, Y.; Lionakis, M.S.; et al. An Ocular Commensal Protects against Corneal Infection by Driving an Interleukin-17 Response from Mucosal gammadelta T Cells. Immunity 2017, 47, 148–158.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pflugfelder, S.C.; de Paiva, C.S. The Pathophysiology of Dry Eye Disease: What We Know and Future Directions for Research. Ophthalmology 2017, 124, S4–S13. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.; Petznick, A.; Heryati, S.; Rifada, M.; Tong, L. Nuclear Factor-kappaB: Central regulator in ocular surface inflammation and diseases. Ocul. Surf. 2012, 10, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Rolando, M.; Barabino, S. Are there Clinical Ways to Assess Inflammation in Dry Eye Disease? Ocul. Immunol. Inflamm. 2021, 29, 1183–1189. [Google Scholar] [CrossRef]
- Mohammed, I.; Said, D.G.; Dua, H.S. Human antimicrobial peptides in ocular surface defense. Prog. Retin. Eye Res. 2017, 61, 1–22. [Google Scholar] [CrossRef]
- Redfern, R.L.; McDermott, A.M. Toll-like receptors in ocular surface disease. Exp. Eye Res. 2010, 90, 679–687. [Google Scholar] [CrossRef] [Green Version]
- Hamrah, P.; Dana, M.R. Corneal antigen-presenting cells. Chem. Immunol. Allergy 2007, 92, 58–70. [Google Scholar]
- Sun, Y.; Hise, A.G.; Kalsow, C.M.; Pearlman, E. Staphylococcus aureus-induced corneal inflammation is dependent on Toll-like receptor 2 and myeloid differentiation factor 88. Infect. Immun. 2006, 74, 5325–5332. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.K.A.; Gui, J.F.; Yu, F.S. Staphylococcus aureus lipoproteins trigger human corneal epithelial innate response through toll-like receptor-2. Microb. Pathog. 2008, 44, 426–434. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Barrett, R.P.; McClellan, S.A.; Hazlett, L.D. Silencing Toll-like receptor-9 in Pseudomonas aeruginosa keratitis. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4209–4216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Du, W.; McClellan, S.A.; Barrett, R.P.; Hazlett, L.D. TLR4 is required for host resistance in Pseudomonas aeruginosa keratitis. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4910–4916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redfern, R.L.; Patel, N.; Hanlon, S.; Farley, W.; Gondo, M.; Pflugfelder, S.C.; McDermott, A.M. Toll-like receptor expression and activation in mice with experimental dry eye. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1554–1563. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Hattori, T.; Park, E.Y.; Stevenson, W.; Chauhan, S.K.; Dana, R. Expression of toll-like receptor 4 contributes to corneal inflammation in experimental dry eye disease. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5632–5640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reins, R.Y.; Lema, C.; Courson, J.; Kunnen, C.M.E.; Redfern, R.L. MyD88 Deficiency Protects Against Dry Eye-Induced Damage. Investig. Ophthalmol. Vis. Sci. 2018, 59, 2967–2976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawakami, A.; Nakashima, K.; Tamai, M.; Nakamura, H.; Iwanaga, N.; Fujikawa, K.; Aramaki, T. Toll-like receptor in salivary glands from patients with Sjögren’s syndrome: Functional analysis by human salivary gland cell line. J. Rheumatol. 2007, 34, 1019–1026. [Google Scholar]
- Liu, R.; Rong, B.; Tu, P.; Tang, Y.; Song, W.; Toyos, R.; Toyos, M.; Yan, X. Analysis of Cytokine Levels in Tears and Clinical Correlations After Intense Pulsed Light Treating Meibomian Gland Dysfunction. Am. J. Ophthalmol. 2017, 183, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Spachidou, M.P.; Bourazopoulou, E.; Maratheftis, C.I.; Kapsogeorgou, E.K.; Moutsopoulos, H.M.; Tzioufas, A.G.; Manoussakis, M.N. Expression of functional Toll-like receptors by salivary gland epithelial cells: Increased mRNA expression in cells derived from patients with primary Sjogren’s syndrome. Clin. Exp. Immunol. 2007, 147, 497–503. [Google Scholar] [CrossRef]
- Gottenberg, J.E.; Cagnard, N.; Lucchesi, C.; Letourneur, F.; Mistou, S.; Lazure, T.; Jacques, S.; Ba, N.; Ittah, M.; Lepajolec, C.; et al. Activation of IFN pathways and plasmacytoid dendritic cell recruitment in target organs of primary Sjogren’s syndrome. Proc. Natl. Acad. Sci. USA 2006, 103, 2770–2775. [Google Scholar] [CrossRef] [Green Version]
- Killedar, S.J.; Eckenrode, S.E.; McIndoe, R.A.; She, J.X.; Nguyen, C.Q.; Peck, A.B.; Cha, S. Early pathogenic events associated with Sjogren’s syndrome (SjS)-like disease of the NOD mouse using microarray analysis. Lab. Investig. 2006, 86, 1243–1260. [Google Scholar] [CrossRef]
- Redfern, R.L.; Barabino, S.; Baxter, J.; Lema, C.; McDermott, A.M. Dry eye modulates the expression of toll-like receptors on the ocular surface. Exp. Eye Res. 2015, 134, 80–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, S.K.; Ramana, K.V. Focus on molecules: Nuclear factor-kappaB. Exp. Eye Res. 2009, 88, 2–3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Wu, X.Y.; Yu, F.S. Inflammatory responses of corneal epithelial cells to Pseudomonas aeruginosa infection. Curr. Eye Res. 2005, 30, 527–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lam, H.; Bleiden, L.; de Paiva, C.S.; Farley, W.; Stern, M.E.; Pflugfelder, S.C. Tear cytokine profiles in dysfunctional tear syndrome. Am. J. Ophthalmol. 2009, 147, 198–205.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, N.W.; Dohlman, T.H.; Foulsham, W.; McSoley, M.; Singh, R.B.; Chen, Y.; Dana, R. The role of Th17 immunity in chronic ocular surface disorders. Ocul. Surf. 2020, 15, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Pflugfelder, S.C.; Corrales, R.M.; de Paiva, C.S. T helper cytokines in dry eye disease. Exp. Eye Res. 2013, 117, 118–125. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Chen, W.; De Paiva, C.S.; Corrales, R.M.; Volpe, E.A.; McClellan, A.J.; Farley, W.J.; Li, D.Q.; Pflugfelder, S.C. Interferon-gamma exacerbates dry eye-induced apoptosis in conjunctiva through dual apoptotic pathways. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6279–6285. [Google Scholar] [CrossRef]
- Ogawa, Y.; Shimizu, E.; Tsubota, K. Interferons and Dry Eye in Sjogren’s Syndrome. Int. J. Mol. Sci. 2018, 19, 3548. [Google Scholar] [CrossRef] [Green Version]
- Takami, Y.; Gong, H.; Amemiya, T. Riboflavin deficiency induces ocular surface damage. Ophthalmic Res. 2004, 36, 156–165. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Y.; Xu, Y.; Li, X.; Fu, J.; Jiang, X.; Chou, Y.; Ma, J.; Hao, R.; Zhang, R.; et al. A new approach of ocular nebulization with vitamin B12 versus oxytocin for the treatment of dry eye disease: An in vivo confocal microscopy study. Drug Des. Devel. Ther. 2019, 13, 2381–2391. [Google Scholar] [CrossRef] [Green Version]
- Macri, A.; Scanarotti, C.; Bassi, A.M.; Giuffrida, S.; Sangalli, G.; Traverso, C.E.; Iester, M. Evaluation of oxidative stress levels in the conjunctival epithelium of patients with or without dry eye, and dry eye patients treated with preservative-free hyaluronic acid 0.15% and vitamin B12 eye drops. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Yoo, W.S.; Jung, J.H.; Jeong, B.K.; Woo, S.H.; Kim, J.H.; Kim, S.J. Alpha-Lipoic Acid Ameliorates Radiation-Induced Lacrimal Gland Injury through NFAT5-Dependent Signaling. Int. J. Mol. Sci. 2019, 20, 5691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ajith, T.A. Alpha-lipoic acid: A possible pharmacological agent for treating dry eye disease and retinopathy in diabetes. Clin. Exp. Pharmacol. Physiol. 2020, 47, 1883–1890. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, P.G.Y.; Karci, A.A.; Guler, T. Dry eye in vitamin D deficiency: More than an incidental association. Int. J. Rheum. Dis. 2016, 19, 49–54. [Google Scholar] [CrossRef]
- Hwang, J.S.L.Y.; Shin, Y.J. Vitamin D Enhances the Efficacy of Topical Artificial Tears in Patients with Dry Eye Disease. Cornea 2019, 38, 304–310. [Google Scholar] [CrossRef]
- Shetty, R.; Sethu, S.; Chevour, P.; Deshpande, K.; Pahuja, N.; Nagaraja, H.; Pindipapanahalli, N.; Ghosh, A. Lower Vitamin D Level and Distinct Tear Cytokine Profile Were Observed in Patients with Mild Dry Eye Signs but Exaggerated Symptoms. Transl. Vis. Sci. Technol. 2016, 5, 16. [Google Scholar] [CrossRef] [Green Version]
- Yin, Z.; Pintea, V.; Lin, Y.; Hammock, B.D.; Watsky, M.A. Vitamin D enhances corneal epithelial barrier function. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7359–7364. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Dai, Y.; Wu, D.; Xu, J. Calcitriol, the Active Metabolite of Vitamin D3, Inhibits Dry Eye Related Corneal Inflammation In Vivo and In Vitro. Ocul. Immunol. Inflamm. 2019, 27, 257–265. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, J.; Xiang, J.; Li, Y.; Wu, D.; Xu, J. Calcitriol inhibits ROS-NLRP3-IL-1β signaling axis via activation of Nrf2-antioxidant signaling in hyperosmotic stress stimulated human corneal epithelial cells. Redox Biol. 2019, 21, 101093. [Google Scholar] [CrossRef]
- Hsu, H.Y.; Tsai, I.L.; Kuo, L.L.; Tsai, C.Y.; Liou, S.W.; Woung, L.C. Herpetic keratouveitis mixed with bilateral Pseudomonas corneal ulcers in vitamin A deficiency. J. Formos. Med. Assoc. 2015, 114, 184–187. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Geng, Y. Expression and significance of NFκBp65 and NOS2 in cornea of experimental xerophthalmia rabbits. Chin. Ophthalmic Res. 2009, 27, 19–22. [Google Scholar]
- Zhang, W.; Li, W.; Zhang, C.; Zhu, C.; Yi, X.; Zhou, Y.; Lv, Y. Effects of Vitamin A on Expressions of Apoptosis Genes Bax and Bcl-2 in Epithelial Cells of Corneal Tissues Induced by Benzalkonium Chloride in Mice with Dry Eye. Med. Sci. Monit. 2019, 25, 4583–4589. [Google Scholar] [CrossRef] [PubMed]
- Fogagnolo, P.; De Cilla, S.; Alkabes, M.; Sabella, P.; Rossetti, L. A Review of Topical and Systemic Vitamin Supplementation in Ocular Surface Diseases. Nutrients 2021, 13, 1998. [Google Scholar] [CrossRef]
- McCusker, M.M.; Durrani, K.; Payette, M.J.; Suchecki, J. An eye on nutrition: The role of vitamins, essential fatty acids, and antioxidants in age-related macular degeneration, dry eye syndrome, and cataract. Clin. Dermatol. 2016, 34, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, M.; Nakamura, S.; Izuta, Y.; Inoue, S.; Tsubota, K. Dietary Supplementation with a Combination of Lactoferrin, Fish Oil, and Enterococcus faecium WB2000 for Treating Dry Eye: A Rat Model and Human Clinical Study. Ocul. Surf. 2016, 14, 255–263. [Google Scholar] [CrossRef]
- Tredici, C.; Fasciani, R.; Villano, A.; Gambini, G.; Caporossi, A. Efficacy of eye drops containing crosslinked hyaluronic acid and CoQ10 in restoring ocular health exposed to chlorinated water. Eur. J. Ophthalmol. 2020, 30, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Wolpert, L.E.; Snieder, H.; Jansonius, N.M.; Utheim, T.P.; Hammond, C.J.; Vehof, J. Medication use and dry eye symptoms: A large, hypothesis-free, population-based study in the Netherlands. Ocul. Surf. 2021, 22, 1–12. [Google Scholar] [CrossRef]
- Gupta, P.K.; Venkateswaran, N. The role of KPI-121 0.25% in the treatment of dry eye disease: Penetrating the mucus barrier to treat periodic flares. Ther. Adv. Ophthalmol. 2021, 13, 25158414211012797. [Google Scholar] [CrossRef]
- Mittal, R.; Patel, S.; Galor, A. Alternative therapies for dry eye disease. Curr. Opin. Ophthalmol. 2021, 32, 348–361. [Google Scholar] [CrossRef]
- Moscovici, B.K.; Holzchuh, R.; Sakassegawa-Naves, F.E.; Hoshino-Ruiz, D.R.; Albers, M.B.; Santo, R.M.; Hida, R.Y. Treatment of Sjogren’s syndrome dry eye using 0.03% tacrolimus eye drop: Prospective double-blind randomized study. Contact Lens Anterior Eye 2015, 38, 373–378. [Google Scholar] [CrossRef]
- Messmer, E.M. Novel current and future therapy options for treatment of dry eye disease. Ophthalmologe 2018, 115, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Jo, K.; Lee, T.G.; Hyun, S.W.; Kim, J.S.; Kim, C.S. Polydatin Inhibits NLRP3 Inflammasome in Dry Eye Disease by Attenuating Oxidative Stress and Inhibiting the NF-kappaB Pathway. Nutrients 2019, 11, 2792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, C.H.; Ryu, J.S.; Moon, J.; Kim, M.K. Association between aging-dependent gut microbiome dysbiosis and dry eye severity in C57BL/6 male mouse model: A pilot study. BMC Microbiol. 2021, 21, 106. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhou, X.; Tan, W.; Zhang, M. Association between Helicobacter pylori infection and Sjogren syndrome: A meta-analysis. Medicine 2018, 97, e13528. [Google Scholar] [CrossRef]
- Trujillo-Vargas, C.M.; Schaefer, L.; Alam, J.; Pflugfelder, S.C.; Britton, R.A.; de Paiva, C.S. The gut-eye-lacrimal gland-microbiome axis in Sjogren Syndrome. Ocul. Surf. 2020, 18, 335–344. [Google Scholar] [CrossRef]

| Authors | Country | n | Microbiome in General Population | Microbiome in Dry Eye Patients |
|---|---|---|---|---|
| Huang et al. [18] (2016) | China | 31 healthy eyes | Corynebacterium (28.22%) Pseudomonas (26.75%) Staphylococcus (5.28%) Acinetobacter (4.74%) Streptococus (2.85%) Millisia (2.16%) Anaerococcus (1.86%) Finegoldia (1.68%) Simosiella (1.48%) Veillonella (1.00%) | |
| Doan et al. [19] (2016) | America | 428 healthy eyes | Coagulase-negative Staphyloccocus (45.3%) Propionibacterium (33.9%) Diphtheroids (15.4%) Streptococcus (3.5%) Coagulase-positive Staphylococcus (2.1%) Micrococcus (2.1%) Bacillus (2.1%) Lactobacillus (0.2%) Rothia (0.2%) Unidentified Gram-negative bacteria (2.5%) Neisseria (0.9%) Hemophilus (0.5%) Escherichia (0.2%) Enterobacter (0.2%) Moraxella (0.2%) | |
| Watters et al. [29] (2016) | New Zealand | 39 | S. aureus (48.7%) P. acnes (25.6%) Corynebact sp. (1.3%) Gm neg. rods inc. Pseudomonas (5.1%) | S. aureus (30.3%) P. acnes (36.8%) Corynebact sp. (3.2%) Streptococcus sp. (3.5%) |
| Zhang et al. [27] (2017) | China | 84 healthy eyes and 201 MGD eyes | Staphylococcus epidermidis (48.6%) Corynebacterium macginleyi (11.4%) Staphylococcus lentus (8.6%) Staphylococcus hominis (5.7%) Staphylococcus lugdunensis (5.7%) | Staphylococcus epidermidis (64.1%) Staphylococcus lentus (12.2%) Staphylococcus aureus (5.1%) Corynebacterium macginleyi (3.8%) Staphylococcus homini (3.2%) Staphylococcus haemolyticus (2.6%) Corynebacterium tuberculostearicum (1.9%) |
| Ozkan et al. [25] (2017) | Australia | 43 healthy eyes | Corynebacterium (11.1%) Acinetobacteria (11.0%) Pseudomonas (10.4%) Sphingomonas (10.2%) Streptococcus (4.8%) Massilia (3.2%) Rothia (1.9%) | |
| Kara M et al. [22] (2017) | America | 52 healthy eyes in children | Staphylococcus (56.5%) Streptococcus (16.9%) Corynebacterium (6.2%) Moraxella (8%) Oceanospirillaceae (7.32%) Listeriaceae (4.42 %) Psychomonadaceae (2.57%) Leuconostocaceae (2.07%) | |
| Jiang et al. [21] (2018) | China | 58 healthy eyes and 82MGD eyes | Staphylococcus (G+) (13.6%) S. epidermidis (10.7%) S. aureus (1.4%) S. hominis (1.4%) S. capitis (0.7%) Corynebacterium (G+) (2.9%) C. macginleyi (2.9%) Microbacteriaceae (G+) (2.9%) Microbacterium (0.7%) Micrococcaceae (2.1%) Moraxella osloensis (G−) (2.1%) | Staphylococcus (G+) (47.9%) S. epidermidis (46.4%) S. aureus (2.9%) S. hominis (0.7%) S. capitis (1.4%) S. warneri (0.7%) Corynebacterium (G+) (4.3%) C. macginleyi (3.6%) C. pseudodiphtheriticum (0.7%) Microbacteriaceae (G+) (10.0%) Microbacterium (2.1%) Micrococcaceae (5.0%) |
| Li et al. [24] (2019) | China | 54 healthy eyes and 35 dry eyes | Proteobacteria (51.70%) Firmicutes (16.86%) Bacteroidetes (13.60%) Actinobacteria (6.12%) Cyanobacteria (1.72%) Acidobacteria (1.66%) Chloroflexi (1.54%) Planctomycetes (1.43%) Epsilonbacteraeota (1.25%) Verrucomicrobia (1.06%) | Proteobacteria (47.62%) Firmicutes (17.20%) Bacteroidetes (16.54%) Actinobacteria (6.24%) Cyanobacteria (2.01%) Acidobacteria (1.69%) Chloroflexi (1.58%) Planctomycetes (1.40%) Epsilonbacteraeota (1.00%) Verrucomicrobia (0.95%) |
| Dong et al. [23] (2019) | China | 42 healthy eyes and 47 MGD eyes | Corynebacterium (46.43%) Staphylococcus (7.88%) Sphingomonas (0.79%) Snodgrassella (3.60%) Propionibacterium (5.44%) Streptococcus (3.89%) | Staphylococcus (20.71%) Corynebacterium (20.22%) Propionibacterium (9.29%) Sphingomonas (5.73%) Snodgrassella (4.17%) Streptococcus (2.80%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Ding, Y.; Jiang, X.; Yang, J.; Li, X. Bacteria and Dry Eye: A Narrative Review. J. Clin. Med. 2022, 11, 4019. https://doi.org/10.3390/jcm11144019
Wang Y, Ding Y, Jiang X, Yang J, Li X. Bacteria and Dry Eye: A Narrative Review. Journal of Clinical Medicine. 2022; 11(14):4019. https://doi.org/10.3390/jcm11144019
Chicago/Turabian StyleWang, Yuchen, Yi Ding, Xiaodan Jiang, Jiarui Yang, and Xuemin Li. 2022. "Bacteria and Dry Eye: A Narrative Review" Journal of Clinical Medicine 11, no. 14: 4019. https://doi.org/10.3390/jcm11144019
APA StyleWang, Y., Ding, Y., Jiang, X., Yang, J., & Li, X. (2022). Bacteria and Dry Eye: A Narrative Review. Journal of Clinical Medicine, 11(14), 4019. https://doi.org/10.3390/jcm11144019






