Viscoelastic Hemostatic Assays for Orthopedic Trauma and Elective Procedures
Abstract
:1. Introduction
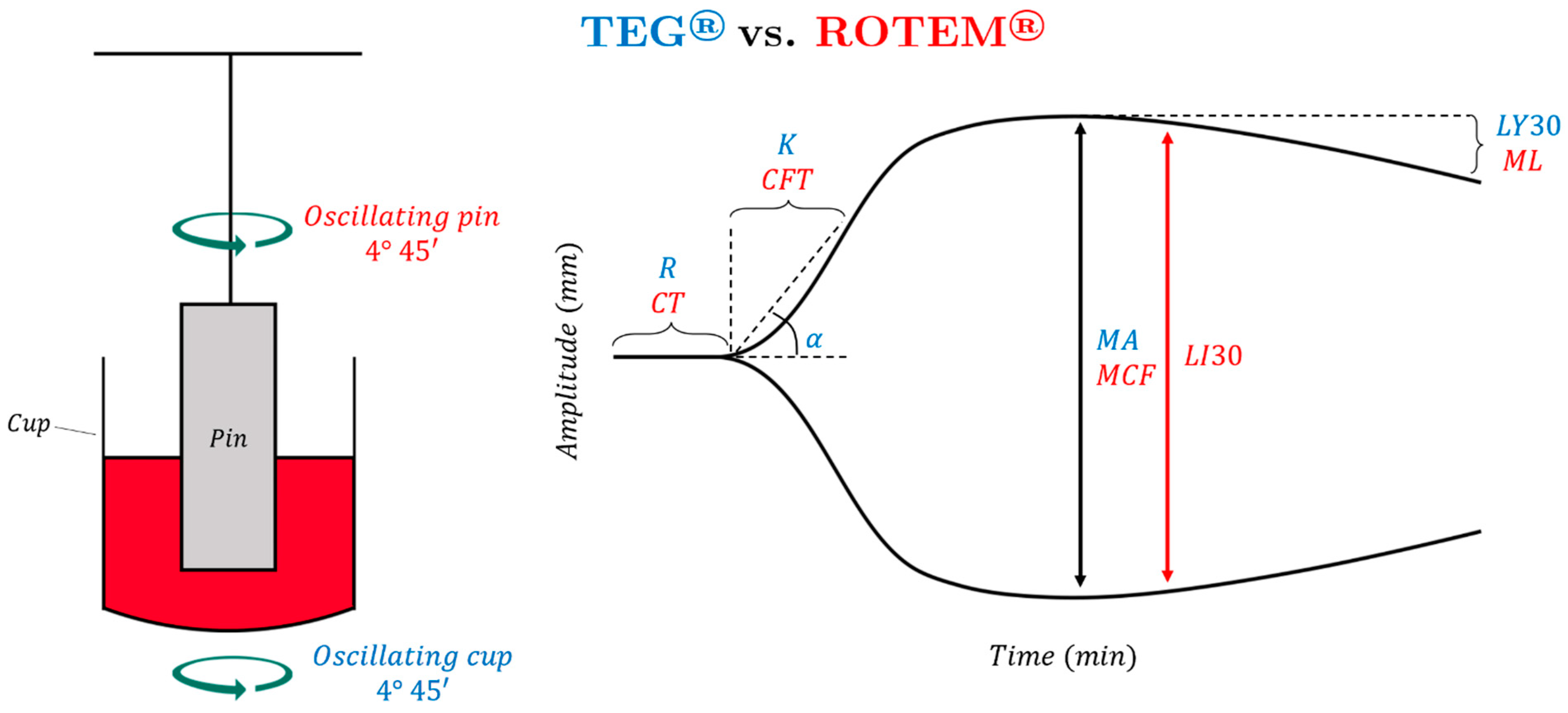
1.1. Thromboelastography (TEG)
1.2. Rotational Thromboelastometry (ROTEM)
1.3. Comparative Interpretation of Principle Viscoelastic Hemostatic Assays
1.4. Newer Viscoelastic Hemostatic Assays in Orthopedic Patients
2. Viscoelastic Hemostatic Assays in Orthopedic Surgery
2.1. Trauma
2.1.1. Pelvic Fractures
2.1.2. Long Bone Fractures
2.1.3. VHA-Directed Management of Orthopedic Trauma Patients
2.2. Viscoelastic Hemostatic Assays in the Orthopedic Subspecialties
2.2.1. Arthroplasty
2.2.2. Spine Surgery
2.2.3. VHA-Guided Operative Conduct in Elective Orthopedic Surgery
2.3. Prediction and Prevention of Venous Thromboembolism
2.4. Future Study
2.5. Summary of Principles for VHA Use in Orthopedic Patients
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A5 | amplitude at 5 min |
| A10 | amplitude at 10 min |
| A20 | amplitude at 20 min |
| ACT | activated clotting time |
| AIS | abbreviated injury severity score |
| BCT | blood component therapy |
| CA5 | clot amplitude at 5 min |
| CA10 | clot amplitude at 10 min |
| CCT | common coagulation test |
| CFT | clot formation time |
| CI | coagulation index |
| CL30 | clot lysis index at 30 min |
| CLI60 | clot lysis index at 60 min |
| CT | clotting time |
| DDAVP | desmopressin |
| DHS | dynamic hip screw |
| DVT | deep vein thrombosis |
| ECT | ecarin clotting time |
| ELISA | enzyme-linked immunosorbent assay |
| FCS | fibrinogen contribution to clot stiffness |
| FF | functional fibrinogen |
| FFP | fresh frozen plasma |
| GA | general anesthesia |
| HAT | hemostatic adjunct therapy |
| HES | hydroxyethyl starch |
| HF | hyperfibrinolysis |
| IL | interleukin |
| INR | international normalized ratio |
| ISS | injury severity score |
| ITACTIC | implementing treatment algorithms for the correction of trauma-induced coagulopathy |
| K | clot kinetics |
| k-TEG | kaolin thromboelastography |
| LMWH | low molecular weight heparin |
| LY30 | lysis at 30 min |
| MA | maximum amplitude |
| MAff | maximum amplitude functional fibrinogen |
| MCF | maximum clot firmness |
| MCP | monocyte chemotactic protein |
| ML | maximum lysis |
| MRTG | maximum rate of thrombus generation |
| NATEM | native thromboelastometry |
| NOAC | novel oral anticoagulant |
| PAI-1 | plasminogen activator inhibitor-1 |
| PBM | precision-based medicine |
| PCC | prothrombin complex concentrate |
| PCS | platelet contribution to clot stiffness |
| PE | pulmonary embolism |
| POD | postoperative day |
| pRBC | packed red blood cells |
| PT | prothrombin time |
| PTT | partial thromboplastin time |
| R | reaction time |
| RL | Ringer’s lactate |
| ROTEM | rotational thromboelastography |
| rTEG | rapid thromboelastography |
| TAT | thrombin–antithrombin complex |
| TDR | thrombodynamic ratio |
| TEG | thromboelastography |
| TEG/PM | thromboelastography with platelet mapping |
| THA | total hip arthroplasty |
| TIC | trauma-induced coagulopathy |
| TKA | total knee arthroplasty |
| TMRTG | time to maximum rate of thrombus generation |
| TNF | tumor necrosis factor |
| tPA | tissue plasminogen activator |
| TXA | tranexamic acid |
| VCM | viscocoagulation monitoring |
| VCT | viscocoagulation testing |
| VHA | viscoelastic hemostatic assay |
| VTE | venous thromboembolism |
References
- Brohi, K.; Singh, J.; Heron, M.; Coats, T. Acute traumatic coagulopathy. J. Trauma Acute Care Surg. 2003, 54, 1127–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacLeod, J.B.A.; Lynn, M.; McKenney, M.G.; Cohn, S.M.; Murtha, M. Early Coagulopathy Predicts Mortality in Trauma. J. Trauma Acute Care Surg. 2003, 55, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Brohi, K.; Cohen, M.J.; Davenport, R. Acute coagulopathy of trauma: Mechanism, identification and effect. Curr. Opin. Crit. Care 2007, 13, 680–685. [Google Scholar] [CrossRef]
- Niles, S.E.; McLaughlin, D.F.; Perkins, J.G.; Wade, C.E.; Li, Y.; Spinella, P.C.; Holcomb, J.B. Increased Mortality Associated with the Early Coagulopathy of Trauma in Combat Casualties. J. Trauma Acute Care Surg. 2008, 64, 1459–1465, discussion 63–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maegele, M.; Lefering, R.; Yucel, N.; Tjardes, T.; Rixen, D.; Paffrath, T. Early Coagulopathy in mulitple injury: An analysis from the German Trauma Registry on 8724 patients. Injury 2007, 38, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.E.; Moore, H.B.; Kornblith, L.Z.; Neal, M.D.; Hoffman, M.; Mutch, N.J.; Pierce, B. Trauma-induced coagulopathy. Nat. Rev. Dis. Primers 2021, 7, 30. [Google Scholar] [CrossRef]
- Wikkelsø, A.; Wetterslev, J.; Møller, A.M.; Afshari, A. Thromboelastography (TEG) or thromboelastometry (ROTEM) to monitor haemostatic treatment versus usual care in adults or children with bleeding. Cochrane Database Syst. Rev. 2016, 2016, CD007871. [Google Scholar] [CrossRef]
- Gonzalez, E.; Moore, E.E.; Moore, H.B.; Chapman, M.P.; Chin, T.L.; Ghasabyan, A.; Wohlauer, M.V.; Barnett, C.C.; Bensard, D.D.; Biffl, W.L.; et al. Goal-directed Hemostatic Resuscitation of Trauma-induced Coagulopathy: A Pragmatic Randomized Clinical Trial Comparing a Viscoelastic Assay to Conventional Coagulation Assays. Ann. Surg. 2016, 263, 1051–1059. [Google Scholar] [CrossRef]
- Johansson, P.I. Goal-directed hemostatic resuscitation for massively bleeding patients: The Copenhagen concept. Transfus. Apher. Sci. 2010, 43, 401–405. [Google Scholar] [CrossRef]
- Nardi, G.; Agostini, V.; Rondinelli, B.; Russo, E.; Bastianini, B.; Bini, G.; Bulgarelli, S.; Cingolani, E.; Donato, A.; Gambale, G.; et al. Trauma-induced coagulopathy: Impact of the early coagulation support protocol on blood product consumption, mortality and costs. Crit. Care 2015, 19, 83. [Google Scholar] [CrossRef] [Green Version]
- Holcomb, J.B.; Minei, K.M.; Scerbo, M.L.; Radwan, Z.A.; Wade, C.E.; Kozar, R.A.; Gill, B.S.; Albarado, R.; McNutt, M.K.; Khan, S.; et al. Admission rapid thrombelastography can replace conventional coagulation tests in the emergency department: Experience with 1974 consecutive trauma patients. Ann. Surg. 2012, 256, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Schöchl, H.; Nienaber, U.; Hofer, G.; Voelckel, W.; Jambor, C.; Scharbert, G.; Kozek-Langenecker, S.; Solomon, C. Goal-directed coagulation management of major trauma patients using thromboelastometry (ROTEM®)-guided administration of fibrinogen concentrate and prothrombin complex concentrate. Crit. Care 2010, 14, R55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spahn, D.R.; Bouillon, B.; Cerny, V.; Duranteau, J.; Filipescu, D.; Hunt, B.J.; Komadina, R.; Maegele, M.; Nardi, G.; Riddez, L.; et al. The European guideline on management of major bleeding and coagulopathy following trauma: Fifth edition. Crit. Care 2019, 23, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bugaev, N.; Como, J.J.; Golani, G.; Freeman, J.J.; Sawhney, J.S.; Vatsaas, C.J.; Yorkgitis, B.K.; Kreiner, L.A.; Garcia, N.M.; Aziz, H.A.; et al. Thromboelastography and rotational thromboelastometry in bleeding patients with coagulopathy: Practice management guideline from the Eastern Association for the Surgery of Trauma. J. Trauma Acute Care Surg. 2020, 89, 999–1017. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.; Thomas, S.; Kwaan, H.; Aversa, J.; Anderson, S.; Sundararajan, R.; Zimmer, D.; Bunch, C.; Stillson, J.; Draxler, D.; et al. Modern methods for monitoring hemorrhagic resuscitation in the United States: Why the delay? J. Trauma Acute Care Surg. 2020, 89, 1018–1022. [Google Scholar] [CrossRef] [PubMed]
- Schöchl, H.; Maegele, M.; Solomon, C.; Görlinger, K.; Voelckel, W. Early and individualized goal-directed therapy for trauma-induced coagulopathy. Scand. J. Trauma Resusc. Emerg. Med. 2012, 20, 15. [Google Scholar] [CrossRef] [Green Version]
- Görlinger, K.; Pérez-Ferrer, A.; Dirkmann, D.; Saner, F.; Maegele, M.; Calatayud, Á.A.P.; Kim, T.-Y. The role of evidence-based algorithms for rotational thromboelastometry-guided bleeding management. Korean J. Anesthesiol. 2019, 72, 297–322. [Google Scholar] [CrossRef] [Green Version]
- Vorweg, M.; Hanke, A.; Görlinger, K.; Lier, H. Thromboelastometry guided therapy of severe bleeding. Hämostaseologie 2013, 33, 51–61. [Google Scholar] [CrossRef]
- Baksaas-Aasen, K.; Gall, L.S.; Stensballe, J.; Juffermans, N.P.; Curry, N.; Maegele, M.; Brooks, A.; Rourke, C.; Gillespie, S.; Murphy, J.; et al. Viscoelastic Haemostatic Assay Augmented Protocols for Major Trauma Haemorrhage (ITACTIC): A Randomised, Controlled Trial. Intensive Care Med. 2020, 47, 49–59. [Google Scholar] [CrossRef]
- Kaufmann, C.R.; Dwyer, K.M.; Crews, J.D.; Dols, S.J.; Trask, A.L. Usefulness of Thrombelastography in Assessment of Trauma Patient Coagulation. J. Trauma Acute Care Surg. 1997, 42, 716–722. [Google Scholar] [CrossRef]
- Mauffrey, C.; Cuellar, D.; Pieracci, F.; Hak, D.; Hammerberg, E.; Stahel, P.; Burlew, C.C. Strategies for the management of haemorrhage following pelvic fractures and associated trauma-induced coagulopathy. Bone Jt. J. 2014, 96, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Mamczak, C.N.; Maloney, M.; Fritz, B.; Boyer, B.; Thomas, S.; Evans, E.; Ploplis, V.A.; Castellino, F.J.; McCollester, J.; Walsh, M. Thromboelastography in Orthopaedic Trauma Acute Pelvic Fracture Resuscitation: A Descriptive Pilot Study. J. Orthop. Trauma 2016, 30, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.T.; Coleman, J.R.; Carmichael, H.; Mauffrey, C.; Vintimilla, D.R.; Samuels, J.M.; Sauaia, A. High rate of fibrinolytic shutdown and venous thromboembolism in patients with severe pelvic fracture. J. Surg. Res. 2020, 246, 182–189. [Google Scholar] [CrossRef]
- Brill, J.B.; Badiee, J.; Zander, A.L.; Wallace, J.D.; Lewis, P.R.; Sise, M.J.; Bansal, V.; Shackford, S.R. The rate of deep vein thrombosis doubles in trauma patients with hypercoagulable thromboelastography. J. Trauma Acute Care Surg. 2017, 83, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Van, P.Y.; Cho, S.D.; Underwood, S.J.; Morris, M.; Watters, J.M.; Schreiber, M.A. Thrombelastography Versus AntiFactor Xa Levels in the Assessment of Prophylactic-Dose Enoxaparin in Critically Ill Patients. J. Trauma Acute Care Surg. 2009, 66, 1509–1517, discussion 15–17. [Google Scholar] [CrossRef]
- Hagedorn, J.C.; Bardes, J.M.; Paris, C.L.; Lindsey, R.W. Thromboelastography for the Orthopaedic Surgeon. J. Am. Acad. Orthop. Surg. 2019, 27, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Luddington, R.J. Thrombelastography/thromboelastometry. Clin. Lab. Haematol. 2005, 27, 81–90. [Google Scholar] [CrossRef]
- Thakur, M.; Ahmed, A.B. A review of thromboelastography. Int. J. Periop. Ultrasound Appl. Technol. 2012, 1, 25–29. [Google Scholar] [CrossRef]
- Multipurpose System for In Vitro Coagulation Studies; US Food & Drug Administration: Silver Spring, MD, USA, 2019.
- Curry, N.S.; Davenport, R.; Pavord, S.; Mallett, S.V.; Kitchen, D.; Klein, A.A.; Maybury, H. The use of viscoelastic haemostatic assays in the management of major bleeding: A British Society for Haematology Guideline. Br. J. Haematol. 2018, 182, 789–806. [Google Scholar] [CrossRef] [Green Version]
- Gurbel, P.A.; Bliden, K.P.; Tantry, U.S.; Monroe, A.L.; Muresan, A.A.; Brunner, N.E.; Lopez-Espina, C.G.; Delmenico, P.R.; Cohen, E.; Raviv, G.; et al. First report of the point-of-care TEG: A technical validation study of the TEG-6S system. Platelets 2016, 27, 642–649. [Google Scholar] [CrossRef]
- Hartmann, J.; Murphy, M.; Dias, J.D. Viscoelastic Hemostatic Assays: Moving from the Laboratory to the Site of Care—A Review of Established and Emerging Technologies. Diagnostics 2020, 10, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shore-Lesserson, L.; Manspeizer, H.E.; DePerio, M.; Francis, S.; Vela-Cantos, F.; Ergin, M.A. Thromboelastography-guided transfusion algorithm reduces transfusions in complex cardiac surgery. Anesth. Analg. 1999, 88, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Spalding, G.J.; Hartrumpf, M.; Sierig, T.; Oesberg, N.; Kirschke, C.G.; Albes, J.M. Cost reduction of perioperative coagulation management in cardiac surgery: Value of ‘bedside’ thrombelastography (ROTEM). Eur. J. Cardiothorac. Surg. 2007, 31, 1052–1057. [Google Scholar] [CrossRef]
- Kang, Y.G.; Martin, D.J.; Marquez, J.; Lewis, J.H.; Bontempo, F.A.; Shaw, B.W.; Starzl, T.E.; Winter, P.M. Intraoperative changes in blood coagulation and thrombelastographic monitoring in liver transplantation. Anesth. Analg. 1985, 64, 888–896. [Google Scholar] [CrossRef] [Green Version]
- Trzebicki, J.; Flakiewicz, E.; Kosieradzki, M.; Blaszczyk, B.; Kołacz, M.; Jureczko, L.; Pacholczyk, M.; Chmura, A.; Lagiewska, B.; Lisik, W.; et al. The use of thromboelastometry in the assessment of hemostasis during orthotopic liver transplantation reduces the demand for blood products. Ann. Transplant. 2010, 15, 19–24. [Google Scholar] [PubMed]
- Snegovskikh, D.; Souza, D.; Walton, Z.; Dai, F.; Rachler, R.; Garay, A.; Snegovskikh, V.V.; Braveman, F.R.; Norwitz, E.R. Point-of-care viscoelastic testing improves the outcome of pregnancies complicated by severe postpartum hemorrhage. J. Clin. Anesthesia 2018, 44, 50–56. [Google Scholar] [CrossRef]
- Sankarankutty, A.; Nascimento, B.; da Luz, L.T.; Rizoli, S. TEG® and ROTEM® in trauma: Similar test but different results? World J. Emerg. Surg. 2012, 7, S3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whiting, D.; DiNardo, J.A. TEG and ROTEM: Technology and clinical applications. Am. J. Hematol. 2014, 89, 228–232. [Google Scholar] [CrossRef]
- Veigas, P.V.; Callum, J.; Rizoli, S.; Nascimento, B.; da Luz, L.T. A systematic review on the rotational thrombelastometry (ROTEM®) values for the diagnosis of coagulopathy, prediction and guidance of blood transfusion and prediction of mortality in trauma patients. Scand. J. Trauma Resusc. Emerg. Med. 2016, 24, 114. [Google Scholar] [CrossRef] [Green Version]
- Gillissen, A.; Akker, T.V.D.; Caram-Deelder, C.; Henriquez, D.D.C.A.; Bloemenkamp, K.W.M.; Eikenboom, J.; van der Bom, J.G.; de Maat, M.P.M. Comparison of thromboelastometry by ROTEM® Delta and ROTEM® Sigma in women with postpartum haemorrhage. Scand. J. Clin. Lab. Investig. 2019, 79, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Schenk, B.; Görlinger, K.; Treml, B.; Tauber, H.; Fries, D.; Niederwanger, C.; Oswald, E. A comparison of the new ROTEM® sigma with its predecessor, the ROTEMdelta. Anaesthesia 2019, 74, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Field, A.; Poole, T.; Bamber, J. ROTEM® sigma reference range validity. Anaesthesia 2019, 74, 1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volod, O.; Bunch, C.M.; Zackariya, N.; Moore, E.E.; Moore, H.B.; Kwaan, H.C.; Neal, M.D.; Al-Fadhl, M.D.; Patel, S.S.; Wiarda, G.; et al. Viscoelastic Hemostatic Assays: A Primer on Legacy and New Generation Devices. J. Clin. Med. 2022, 11, 860. [Google Scholar] [CrossRef] [PubMed]
- Hunt, H.; Stanworth, S.; Curry, N.; Woolley, T.; Cooper, C.; Ukoumunne, O.; Zhelev, Z.; Hyde, C. Thromboelastography (TEG) and rotational thromboelastometry (ROTEM) for trauma-induced coagulopathy in adult trauma patients with bleeding. Cochrane Database Syst. Rev. 2015, 2, CD010438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schöchl, H.; Voelckel, W.; Grassetto, A.; Schlimp, C.J. Practical application of point-of-care coagulation testing to guide treatment decisions in trauma. J. Trauma Acute Care Surg. 2013, 74, 1587–1598. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.S.P.; Meyer, M.A.S.; Sørensen, A.M.; Rasmussen, L.S.; Hansen, M.B.; Holcomb, J.B.; Cotton, B.A.; Wade, C.E.; Ostrowski, S.R.; Johansson, P.I. Thrombelastography and rotational thromboelastometry early amplitudes in 182 trauma patients with clinical suspicion of severe injury. J. Trauma Acute Care Surg. 2014, 76, 682–690. [Google Scholar] [CrossRef]
- Baumann, H.M.; Klinkspoor, J.H.; Goslings, J.C.; Juffermans, N.P.; Wirtz, M.R. Viscoelastic Testing in Trauma. Semin. Thromb. Hemost. 2017, 43, 375–385. [Google Scholar] [CrossRef]
- Solomon, C.; Schöchl, H.; Ranucci, M.; Schlimp, C. Can the Viscoelastic Parameter α-Angle Distinguish Fibrinogen from Platelet Deficiency and Guide Fibrinogen Supplementation? Anesth. Analg. 2015, 121, 289–301. [Google Scholar] [CrossRef]
- Groves, D.S.; Welsby, I.J.; Naik, B.I.; Tanaka, K.; Hauck, J.N.; Greenberg, C.S.; Winegar, D.A.; Viola, F. Multicenter Evaluation of the Quantra QPlus System in Adult Patients Undergoing Major Surgical Procedures. Anesth. Analg. 2020, 130, 899–909. [Google Scholar] [CrossRef]
- Naik, B.I.; Tanaka, K.; Sudhagoni, R.G.; Viola, F. Prediction of hypofibrinogenemia and thrombocytopenia at the point of care with the Quantra® QPlus® System. Thromb. Res. 2020, 197, 88–93. [Google Scholar] [CrossRef]
- Brearton, C.; Rushton, A.; Parker, J.; Martin, H.; Hodgson, J. Performance Evaluation of a New Point of Care Viscoelastic Coagulation Monitoring System in Major Abdominal, Orthopaedic and Vascular Surgery. Platelets 2020, 31, 1052–1059. [Google Scholar] [CrossRef] [PubMed]
- Bostian, P.A.; Ray, J.J.; Karolcik, B.A.; Bramer, M.A.; Wilson, A.; Dietz, M.J. Thromboelastography is predictive of mortality, blood transfusions, and blood loss in patients with traumatic pelvic fractures: A retrospective cohort study. Eur. J. Trauma Emerg. Surg. 2020, 48, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Kane, I.; Ong, A.; Orozco, F.R.; Post, Z.D.; Austin, L.S.; Radcliff, K.E. Thromboelastography Predictive of Death in Trauma Patients. Orthop. Surg. 2015, 7, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Moore, H.B.; Moore, E.E.; Neal, M.D.; Sheppard, F.R.; Kornblith, L.Z.; Draxler, D.F.; Walsh, M.; Medcalf, R.L.; Cohen, M.J.; Cotton, B.A.; et al. Fibrinolysis Shutdown in Trauma: Historical Review and Clinical Implications. Anesth. Analg. 2019, 129, 762–773. [Google Scholar] [CrossRef]
- Moore, H.; Moore, E.; Gonzalez, E.; Chapman, M.; Chin, T.; Silliman, C.; Banerjee, A. Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: The spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. J. Trauma Acute Care Surg. 2014, 77, 811–817, discussion 7. [Google Scholar] [CrossRef] [Green Version]
- Holcomb, J.B.; Tilley, B.C.; Baraniuk, S.; Fox, E.E.; Wade, C.E.; Podbielski, J.M.; del Junco, D.J.; Brasel, K.J.; Bulger, E.M.; Callcut, R.A.; et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs. a 1:1:2 ratio and mortality in patients with severe trauma: The PROPPR randomized clinical trial. JAMA 2015, 313, 471–482. [Google Scholar] [CrossRef]
- McCurdy, M.; Spilger-Liew Walsh, M. Mortality and ratio of blood products used in patients with severe trauma. JAMA 2015, 313, 2077. [Google Scholar] [CrossRef]
- Spinella, P.C.; Wade, C.E.; Blackbourne, L.H.; Borgman, M.A.; Zarzabal, L.A.; Du, F.; Perkins, J.G.; Maegele, M.; Schreiber, M.; Hess, J.R.; et al. The Association of Blood Component Use Ratios with the Survival of Massively Transfused Trauma Patients With and Without Severe Brain Injury. J. Trauma Acute Care Surg. 2011, 71, S343–S352. [Google Scholar] [CrossRef] [Green Version]
- Coleman, J.J.; Zarzaur, B.L.; Katona, C.W.; Plummer, Z.J.; Johnson, L.S.; Fecher, A.; O’Rear, J.M.; Feliciano, D.V.; Rozycki, G.S. Factors Associated with Pulmonary Embolism Within 72 Hours of Admission after Trauma: A Multicenter Study. J. Am. Coll. Surg. 2015, 220, 731–736. [Google Scholar] [CrossRef]
- Wilson, D.; Cooke, E.; McNally, M.; Wilson, H.; Yeates, A.; Mollan, R. Changes in coagulability as measured by thrombelastography following surgery for proximal femoral fracture. Injury 2001, 32, 765–770. [Google Scholar] [CrossRef]
- Liu, C.; Guan, Z.; Xu, Q.; Zhao, L.; Song, Y.; Wang, H. Relation of thromboelastography parameters to conventional coagulation tests used to evaluate the hypercoagulable state of aged fracture patients. Medicine 2016, 95, e3934. [Google Scholar] [CrossRef] [PubMed]
- Laursen, T.H.; Meyer, M.A.S.; Meyer, A.S.P.; Gaarder, T.; Naess, P.A.; Stensballe, J.; Ostrowski, S.R.; Johansson, P.I. Thrombelastography early amplitudes in bleeding and coagulopathic trauma patients: Results from a multicenter study. J. Trauma Acute Care Surg. 2018, 84, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.-J.; Park, S.-W.; Bae, B.-K.; Lee, S.-H.; Choi, H.J.; Ahn, T.Y.; Goh, T.S.; Lee, M.J.; Yeom, S.R.; Park, S.J. FIBTEM Improves the Sensitivity of Hyperfibrinolysis Detection in Severe Trauma Patients: A Retrospective Study Using Thromboelastometry. Sci. Rep. 2020, 10, 6980. [Google Scholar] [CrossRef]
- Tsantes, A.G.; Trikoupis, I.G.; Papadopoulos, D.V.; Tsante, K.A.; Mavrogenis, A.F.; Koulouvaris, P.; Savvidou, O.D.; Kontogeorgakos, V.A.; Piovani, D.; Kriebardis, A.G.; et al. Higher coagulation activity in hip fracture patients: A case-control study using rotational thromboelastometry. Int. J. Lab. Hematol. 2020, 43, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.F.J. Changes in Thrombelastograph Variables Associated with Aging. Anesth. Analg. 2004, 99, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, H.-L.; Shi, Z.-J.; Zhang, Y. The Application of Thromboelastography in Understanding and Management of Ecchymosis after Total Knee Arthroplasty. J. Arthroplast. 2018, 33, 3754–3758. [Google Scholar] [CrossRef]
- Lloyd-Donald, P.; Lee, W.-S.; Liu, G.-M.; Bellomo, R.; McNicol, L.; Weinberg, L. Thromboelastography in elective total hip arthroplasty. World J. Orthop. 2021, 12, 555–564. [Google Scholar] [CrossRef]
- Yang, Y.; Yao, Z.; Dai, W.; Shi, P.; Luo, L.; Zhang, C. Changes of thrombelastography in patients undergoing elective primary total knee and total hip replacement with low molecular heparin prophylaxis. J. Orthop. Surg. Res. 2014, 9, 52. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.-D.; Chen, Y.; Tian, M.; He, Y.; Tao, Y.-Z.; Xu, W.; Cheng, Q.; Chen, C.; Liu, W.; Huang, W. Application of thrombelastography (TEG) for safety evaluation of tranexamic acid in primary total joint arthroplasty. J. Orthop. Surg. Res. 2019, 14, 214. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.-C.; Sun, M.-J.; Pan, S.; Rui, M.; Zhao, F.-C.; Zha, G.-C.; Pang, Y.; Zheng, X.; Guo, K.-J. Intravenous administration of tranexamic acid in total hip arthroplasty does not change the blood coagulopathy: A prospective thrombelastography analysis. J. Orthop. Surg. 2020, 28, 2309499020959516. [Google Scholar] [CrossRef]
- Grant, A.L.; Letson, H.L.; Morris, J.L.; McEwen, P.; Hazratwala, K.; Wilkinson, M.; Dobson, G.P. Tranexamic acid is associated with selective increase in inflammatory markers following total knee arthroplasty (TKA): A pilot study. J. Orthop. Surg. Res. 2018, 13, 149. [Google Scholar] [CrossRef] [PubMed]
- Kohro, S.; Yamakage, M.; Arakawa, J.; Kotaki, M.; Omote, T.; Namiki, A. Surgical/tourniquet pain accelerates blood coagulability but not fibrinolysis. Br. J. Anaesth. 1998, 80, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Topçu, I.; Çivi, M.; Öztürk, T.; Keleş, G.; Çoban, S.; Yentür, E.; Okçu, G. Evaluation of hemostatic changes using thromboelastography after crystalloid or colloid fluid administration during major orthopedic surgery. Braz. J. Med Biol. Res. 2012, 45, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Oswald, E.; Finsterwalder, T.; Innerhofer, N.; Haas, T.; Mittermayr, M.; Strohmaier, S.; Innerhofer, P. Comparison of arterial versus venous parameters of Rotational thromboelastometry and multiple platelet function analyzer: Results of a pilot study. Scand. J. Clin. Lab. Investig. 2013, 73, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, J.; Zhang, S.; Liu, S.; Yuan, J.; Wang, C.; Miao, X.; Lin, X.; Li, J.; Shi, Z. Perioperative Coagulation Profile with Thromboelastography in Aspirin-Treated Patients Undergoing Posterior Lumbar Fusion. Pain Physician 2020, 23, E619–E628. [Google Scholar]
- Mittermayr, M.; Streif, W.; Haas, T.; Fries, D.; Velik-Salchner, C.; Klingler, A.; Innerhofer, P. Effects of colloid and crystalloid solutions on endogenous activation of fibrinolysis and resistance of polymerized fibrin to recombinant tissue plasminogen activator added ex vivo. Br. J. Anaesth. 2008, 100, 307–314. [Google Scholar] [CrossRef] [Green Version]
- Mittermayr, M.; Streif, W.; Haas, T.; Fries, D.; Velik-Salchner, C.; Klingler, A.; Oswald, E.; Bach, C.; Schnapka-Koepf, M.; Innerhofer, P. Hemostatic Changes After Crystalloid or Colloid Fluid Administration During Major Orthopedic Surgery: The Role of Fibrinogen Administration. Anesth. Analg. 2007, 105, 905–917. [Google Scholar] [CrossRef]
- Theusinger, O.M.; Spahn, D.R. Perioperative blood conservation strategies for major spine surgery. Best Pract. Res. Clin. Anaesthesiol. 2015, 30, 41–52. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, Y.; Zhang, Y.; Yu, L.; Zhang, K. Thromboelastogram-Guided Transfusion Therapy Reduces Blood-Component Transfusion and Improves Coagulation Function during Orthopedic Surgery. J. Nanomater. 2021, 2021, 1–6. [Google Scholar] [CrossRef]
- Spiezia, L.; Vasques, F.; Behr, A.U.; Campello, E.; Maggiolo, S.; Berizzi, A.; Gavasso, S.; Woodhams, B.; Biancari, F.; Simioni, P. Perioperative coagulation assessment of patients undergoing major elective orthopedic surgery. Intern. Emerg. Med. 2016, 11, 793–801. [Google Scholar] [CrossRef]
- Hanke, A.A.; Bartlau, J.; Flöricke, F.; Przemeck, M.; Horstmann, H.; Weber-Spickschen, T.S.; Sieg, L.; Schumacher, C. Coagulation monitoring and transfusion in major non-emergency orthopaedic surgery—An observational study. J. Orthop. 2020, 22, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Froessler, B.; Weber, I.; Hodyl, N.A.; Saadat-Gilani, K. Dynamic changes in clot formation determined using thromboelastometry after reinfusion of unwashed anticoagulated cell-salvaged whole blood in total hip arthroplasty. Blood Transfus. 2015, 13, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.O.; Kim, Y.S.; Chang, H.W.; Kim, H.; Lim, B.G.; Lee, M. In vitro investigation of the effects of exogenous sugammadex on coagulation in orthopedic surgical patients. BMC Anesthesiol. 2018, 18, 56. [Google Scholar] [CrossRef] [PubMed]
- Geerts, W.H.; Code, K.I.; Jay, R.M.; Chen, E.; Szalai, J.P. A Prospective Study of Venous Thromboembolism after Major Trauma. N. Engl. J. Med. 1994, 331, 1601–1606. [Google Scholar] [CrossRef]
- Cotton, B.A.; Minei, K.M.; Radwan, Z.A.; Matijevic, N.; Pivalizza, E.; Podbielski, J.; Wade, C.E.; Kozar, R.A.; Holcomb, J.B. Admission rapid thrombelastography predicts development of pulmonary embolism in trauma patients. J. Trauma Acute Care Surg. 2012, 72, 1470–1477. [Google Scholar] [CrossRef]
- Stawicki, S.P.; Grossman, M.D.; Cipolla, J.; Hoff, W.S.; Hoey, B.A.; Wainwright, G.; Reed III, J.F. Deep venous thrombosis and pulmonary embolism in trauma patients: An overstatement of the problem? Am. Surg. 2005, 71, 387–391. [Google Scholar] [CrossRef]
- Nathens, A.B.; McMurray, M.K.; Cuschieri, J.; Durr, E.A.; Moore, E.E.; Bankey, P.E.; Freeman, B.; Harbrecht, B.G.; Johnson, J.L.; Minei, J.P.; et al. The Practice of Venous Thromboembolism Prophylaxis in the Major Trauma Patient. J. Trauma Acute Care Surg. 2007, 62, 557–563. [Google Scholar] [CrossRef]
- Brown, W.; Lunati, M.; Maceroli, M.; Ernst, A.; Staley, C.; Johnson, R.; Schenker, M. Ability of Thromboelastography to Detect Hypercoagulability: A Systematic Review and Meta-Analysis. J. Orthop. Trauma 2020, 34, 278–286. [Google Scholar] [CrossRef]
- Gary, J.L.; Schneider, P.S.; Galpin, M.; Radwan, Z.; Munz, J.W.; Achor, T.S.; Cotton, B.A. Can thrombelastography predict venous thromboembolic events in patients with severe extremity trauma? J. Orthop. Trauma 2016, 30, 294–298. [Google Scholar]
- Tsantes, A.G.; Papadopoulos, D.V.; Trikoupis, I.G.; Tsante, K.A.; Mavrogenis, A.F.; Koulouvaris, P.; Piovani, D.; Kriebardis, A.G.; Gialeraki, A.; Nikolopoulos, G.K.; et al. Rotational Thromboelastometry Findings Are Associated with Symptomatic Venous Thromboembolic Complications after Hip Fracture Surgery. Clin. Orthop. Relat. Res. 2021. ahead of print. [Google Scholar] [CrossRef]
- Parameswaran, A.; Krishnamoorthy, V.P.; Oommen, A.T.; Jasper, A.; Korula, R.J.; Nair, S.C.; Poonnoose, P.M. Is pre-operative assessment of coagulation profile with Thrombelastography (TEG) useful in predicting venous thromboembolism (VTE) following orthopaedic surgery? J. Clin. Orthop. Trauma 2016, 7, 225–229. [Google Scholar] [CrossRef] [Green Version]
- Hepner, D.L.; Concepcion, M.; Bhavani-Shankar, K. Coagulation Status Using Thromboelastography in Patients Receiving Warfarin Prophylaxis and Epidural Analgesia. J. Clin. Anesth. 2002, 14, 405–410. [Google Scholar] [CrossRef]
- Klein, S.M.; Slaughter, T.F.; Vail, P.T.; Ginsberg, B.; El-Moalem, H.E.; Alexander, R.; D’Ercole, F.; Greengrass, R.A.; Perumal, T.T.; Welsby, I.; et al. Thromboelastography as a perioperative measure of anticoagulation resulting from low molecular weight heparin: A comparison with anti-Xa concentrations. Anesth. Analg. 2000, 91, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chang, D.; Chui, J.; Cao, J.; Negus, J. The efficacy and cost-effectiveness of enoxaparin versus rivaroxaban in the prevention of venous thromboembolism following total hip or knee arthroplasty: A meta-analysis. J. Orthop. 2022, 30, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Solbeck, S.; Ostrowski, S.R.; Stensballe, J.; Johansson, P.I. Thrombelastography detects dabigatran at therapeutic concentrations in vitro to the same extent as gold-standard tests. Int. J. Cardiol. 2016, 208, 14–18. [Google Scholar] [CrossRef]
- Dias, J.D.; Norem, K.; Doorneweerd, D.D.; Thurer, R.L.; Popovsky, M.A.; Omert, L.A. Use of Thromboelastography (TEG) for Detection of New Oral Anticoagulants. Arch. Pathol. Lab. Med. 2015, 139, 665–673. [Google Scholar] [CrossRef] [Green Version]
- Oswald, E.; Velik-Salchner, C.; Innerhofer, P.; Tauber, H.; Auckenthaler, T.; Ulmer, H.; Streif, W. Results of rotational thromboelastometry, coagulation activation markers and thrombin generation assays in orthopedic patients during thromboprophylaxis with rivaroxaban and enoxaparin: A prospective cohort study. Blood Coagul. Fibrinolysis 2015, 26, 136–144. [Google Scholar] [CrossRef]
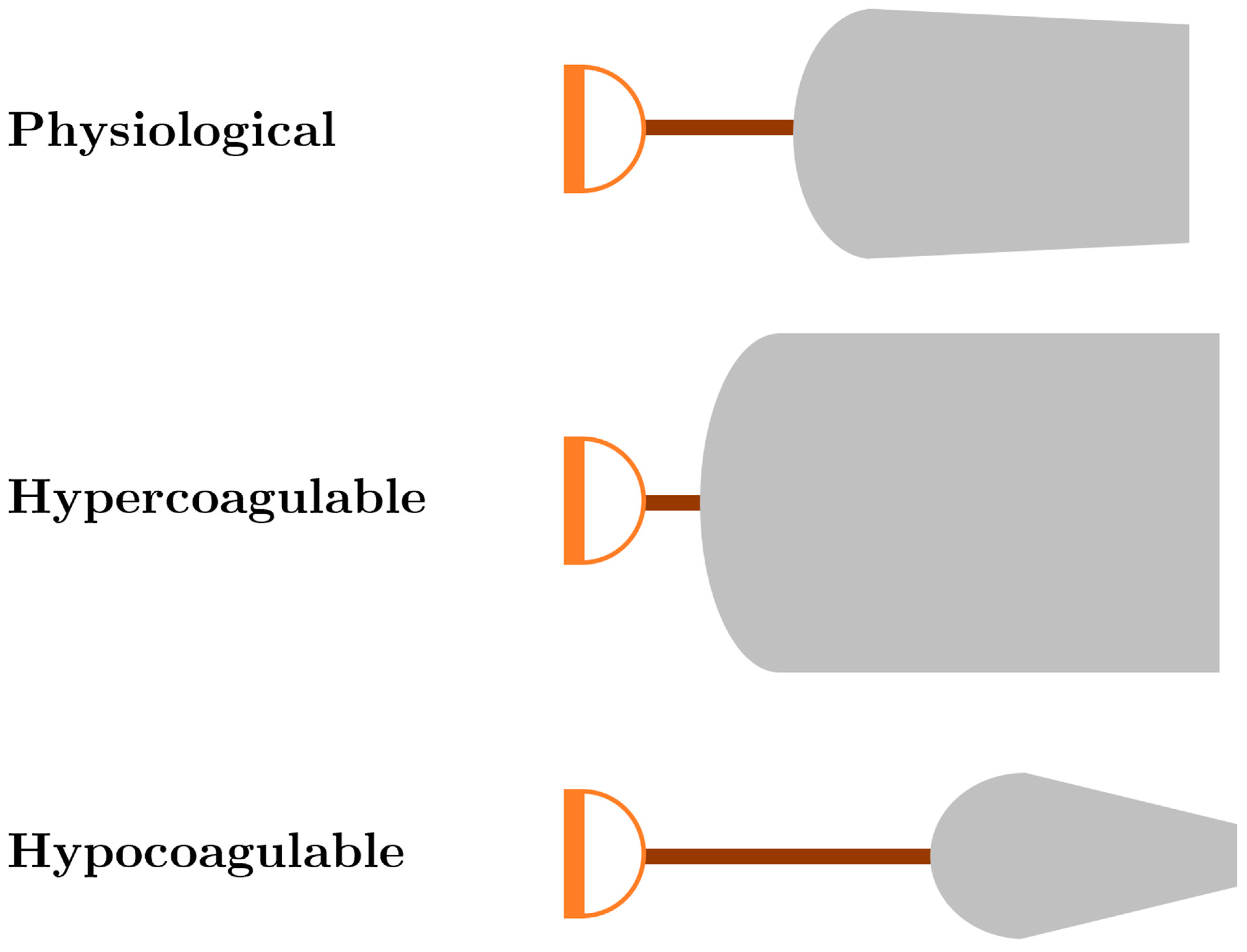
| ROTEM/TEG Parameter Abnormality | Intervention |
|---|---|
| Prolonged CT/R, “Long handle” | Fresh frozen plasma and/or multifactor concentrates (e.g., prothrombin complex concentrate) |
| Decreased CT/R “Short handle” | Prophylactic anticoagulation |
| Decreased alpha angle, “Decreased slope of blade” | Cryoprecipitate/fibrinogen concentrate |
| Decreased MCF/MA, “Decreased width of blade” | Platelets/cryoprecipitate/fibrinogen concentrate |
| Increased MCF/MA “Increased width of blade” | Prophylactic anticoagulation and/or antiplatelet agents |
| Increased CLI60/LY30, “Sharp point of blade” | Antifibrinolytic (e.g., tranexamic acid) |
| First Author | Study Design | No. of Patients | Conclusions |
|---|---|---|---|
| Bostian et al., 2020 [53] | Retrospective Cohort | 141 pelvic and/or acetabular fractures | Increased LY30 on index TEG significantly correlated with mortality, blood loss, and pRBCs transfused. |
| Kane et al., 2015 [54] | Retrospective Cohort | 131 pelvic and/or acetabular fractures | Index TEG R >6 was an independent predictor of mortality (13/25, 52% death rate). Forty-one patients had an abnormal R value at index presentation. |
| Mamczak et al., 2016 [22] | Retrospective Cohort | 40 pelvic and/or acetabular fractures | TEG/PM-guided resuscitation yielded an average transfusion ratio of 2.5:1:2.8 pRBC:FFP:platelets. TEG may optimize resuscitation over standard 1:1:1 fixed ratio guidelines. |
| Nelson et al., 2020 [23] | Retrospective Cohort | 210 severe pelvic fractures | At index presentation, 59% of patients demonstrated fibrinolytic shutdown on rTEG. VTE incidence was 11%, and fibrinolysis shutdown on rTEG at index did not predict VTE. |
| First Author | Study Design | No. of Patients | Conclusions |
|---|---|---|---|
| Wilson et al., 2001 [61] | Prospective Cohort | 250 femoral neck fractures | Patients who developed postoperative DVT demonstrated significantly greater hypercoagulability by TEG on PODs 1–7 compared to those who did not develop DVT. |
| Liu et al., 2016 [62] | Retrospective Cohort | 40 fractures (13 humerus and 27 femur) in adults >60 years old, compared to 40 age-matched controls | Prior to surgery and 4 h post injury, aged fracture patients showed significant hypercoagulability on TEG by decreased K and R and increased alpha angle, MA, and CI compared to controls. TEG parameters correlated well with CCTs. |
| First Author | Study Design | No. of Patients | Conclusions |
|---|---|---|---|
| Meyer et al., 2014 [47] | Prospective Cohort | 182 trauma patients | A5 and A10 may be better predictors of TIC and need for goal-directed transfusion compared to MA/MCF in severely injured trauma patients. |
| Laursen et al., 2018 [63] | Prospective Cohort | 404 trauma patients | The following admission TEG parameters were found to predict mortality: kTEG A10 and MA; FF A5, A10, and MA. For transfused patients, all TEG parameters were predictive of mortality except for rTEG MA and kTEG MA. |
| Wang et al., 2020 [64] | Retrospective Cohort | 402 trauma patients with ISS > 15 | Hyperfibrinolysis on FIBTEM at index presentation was independently associated with a significantly higher mortality (22.3%) compared to those without hyperfibrinolysis (10.3%). |
| Tsantes et al., 2021 [65] | Case-Control Study | 198 hip fractures undergoing arthroplasty or intramedullary nailing, age-matched to 52 controls | Post injury, hip fracture patients demonstrated hypercoagulability by abnormal EXTEM MCF and alpha angle, and INTEM MCF, A10, and alpha angle. Postoperative ROTEM analysis trended towards increased hypercoagulability |
| Ng 2004 [66] | Retrospective Cohort | 132 fracture repair patients | Prior to anesthesia induction, age weakly correlated to increasing hypercoagulability on TEG. |
| First Author | Study Design | No. of Patients | Conclusions |
|---|---|---|---|
| Wang et al., 2018 [67] | Prospective Cohort | 75 unilateral TKA patients. All patients received 10 mg rivaroxaban postoperatively once daily. | Ecchymosis postoperatively was significantly associated with a postoperatively increased R and decreased alpha angle and CI compared to preoperative TEG analysis. |
| Lloyd-Donald et al., 2021 [68] | Retrospective Cohort | 52 elective THA patients (20 general anesthesia, 32 spinal anesthesia). All patients received postoperative DVT chemoprophylaxis. | Regardless of anesthesia technique, postoperative TEG demonstrated hypercoagulability by gradually increased MA through POD5. CCTs only demonstrated significantly elevated fibrinogen levels postop for both anesthesia techniques. TEG/CCT correlation to VTE was not reported. |
| Yang et al., 2014 [69] | Prospective Cohort | 90 TKA or THA patients. All received 10 days thromboprophylaxis with enoxaparin | On POD9, 37.8% of patients were hypercoagulable on TEG despite thromboprophylaxis. There was a significant trend towards hypercoagulability on PODs 1–4 for parameters K, MA, alpha angle, and CI. MA and alpha angle remained increased on PODs 4–9. |
| Wu et al., 2019 [70] | Retrospective Cohort | 359 THA or TKA patients who received at least 1 dose of TXA immediately prior to surgery. | Patients who received multiple doses of TXA preoperatively demonstrated significantly greater hypercoagulability by TEG for 7 days postoperation compared to those who only received a single dose of TXA. However, using multiple doses of TXA was not correlated to VTE. |
| Grant et al., 2018 [72] | Prospective Cohort | 23 TKA (12 received TXA preoperatively and immediately after surgery) | No significant difference in ROTEM parameters was detected for those who received TXA compared to those who did not receive TXA. |
| Zhang et al., 2020 [71] | Prospective Cohort | 174 THA (86 received TXA, 88 no TXA) | There was no significant difference in coagulation status by TEG or CCT analysis on the day before operation, POD1, or POD7 for those patients who received TXA. There was no difference in VTE rate. The TXA group had significantly lower blood loss and transfusion requirement. |
| Kohro et al., 1998 [73] | Prospective Cohort | 22 TKA patients (11 extradural anesthesia (EA) and 11 general anesthesia (GA)) | Tourniquet inflation was associated with an increase in MA greater in the GA group. Fibrinolysis significantly increased in both anesthesia groups 5 min after tourniquet deflation. |
| Topcu et al., 2012 [74] | Prospective Cohort | 75 orthopedic patients undergoing TKA or THA | Maintenance fluids of Ringer’s lactate, 6% hydroxyethyl starch, and 4% gelofusine solution each had mild changes on TEG parameters immediately and 24 h postoperation, but all changes were within normal limits. |
| First Author | Study Design | No. of Patients | Conclusions |
|---|---|---|---|
| Li et al., 2020 [76] | Retrospective Cohort | 80 lumbar fusion patients (39 aspirin-treated and 41 aspirin-naïve) | There was no significant difference in perioperative TEG values between the two treatment groups. |
| Mittermayr et al., 2007 [78] | Prospective Cohort | 66 spinal fusion patients | Magnitude of MCF reduction is affected by the type of intraoperative maintenance fluid used. Colloids showed a greater change in MCF compared to crystalloid. FIBTEM MCF <7 mm predicted clinical bleeding and the need for fibrinogen replacement. Hydroxyethyl starch demonstrates the most significant decrease in fibrin polymerization. |
| Mittermayr et al., 2008 [77] | Prospective Cohort | 66 spinal fusion patients | As determined by an in vitro tPA challenge test (invoked hyperfibrinolysis), patients administered intraoperative maintenance fluids with colloids demonstrated more rapid clot dissolution compared to those provided crystalloid. |
| First Author | Study Design | No. of Patients | Conclusions |
|---|---|---|---|
| Zhang et al., 2021 [80] | Retrospective Cohort | 480 orthopedic surgery patients (266 TEG-guided, 214 CCT-guided; 202 spinal surgeries, 180 fracture surgeries, and 98 TKA) | TEG guided intraoperative transfusion required lower volumes of pRBCs, FFP, cryoprecipitate, and platelets. |
| Spiezia et al., 2016 [81] | Retrospective Cohort | 40 elective orthopedic patients (THA, femur fracture fixation, and spinal surgery) who had ≥250 mL/h blood loss intraoperatively | Intraoperative blood loss significantly correlated with preoperative low platelet count and low FIBTEM MCF, and postoperative low fibrinogen and platelet count, prolonged CFT, and decreased alpha angle and MCF |
| Hanke et al., 2020 [82] | Retrospective Cohort | 70 THA, TKA, or spinal fusion patients (23 received transfusions) | CT was significantly prolonged and FIBTEM decreased preoperatively in the group that required postoperative transfusions. |
| Froessler et al., 2015 [83] | Prospective Cohort | 25 orthopedic patients undergoing THA | ROTEM did not indicate coagulopathy after reinfusion of unwashed salvaged whole blood. |
| Lee et al., 2018 [84] | Prospective Cohort | 14 healthy adults undergoing elective orthopedic surgery compared to controls | Increasing supratherapeutic concentrations of sugammadex were significantly associated with decreases in coagulation as manifested by prolongation in TEG R time, time to maximum rate of thrombus generation (TMRTG), and decreases in the alpha angle, MA, and maximum rate of thrombus generation (MRTG). |
| First Author | Study Design | No. of Patients | Conclusions |
|---|---|---|---|
| Cotton et al., 2012 [86] | Retrospective Cohort | 2070 trauma activations (53 developed PE) | Elevated MA at admission was an independent predictor of PE. MA >65 had an odds ratio of 3.5, and MA >72 had an odds ratio of 5.8. |
| Gary et al., 2016 [90] | Retrospective Cohort | 1818 (310 extremity AIS ≥ 2, 1508 extremity AIS < 2) | Admission rTEG MA predicted VTE in patients with severe extremity trauma with an OR = 3.66 for MA ≥65 and OR = 6.7 for MA ≥72. |
| Brill et al., 2017 [24] | Prospective Cohort | 684 trauma patients who received surveillance u/s and admission TEG | Admission TEG demonstrated hypercoagulability in 582 (85.1%) patients. LE DVT was diagnosed in 99 (14.5%) patients. Despite prophylaxis, hypercoagulable TEG carried a two-fold risk of DVT (OR 2.41, 95% CI 1.11–5.24). |
| Tsantes et al., 2021 [91] | Retrospective Cohort | 354 femoral neck and peritrochanteric fracture patients | Several abnormal ROTEM values were found predictive of VTE: Increased preop MCF (median 70 mm), decreased preop CFT (median 61 s), and decreased postop CFT (median 52 s). Preop CFT demonstrated the greatest prediction of VTE with sensitivity of 81% and specificity of 86%. |
| Parameswaran et al., 2016 [92] | Prospective Cohort | 101 hip fracture or elective THA/TKA patients who did not receive postoperative chemoprophylaxis for DVT | DVT incidence was 7%. Only 1 patient with DVT demonstrated hypercoagulability on preoperative TEG. For those without DVT, 37/94 demonstrated hypercoagulability on preoperative TEG. No TEG parameter was predictive of DVT. |
| Brown et al., 2020 [89] | Systematic review (35 studies) & meta-analysis (5 studies) | 8939 postoperative patients | MA >65 was not predictive of VTE (OR 1.31, 95% CI 0.74–2.34). There was wide variability across studies for the threshold MA value of hypercoagulability and the pooled mean threshold value was 66.7 mm. TEG consistently showed hypercoagulability on POD1. |
| Hepner et al., 2002 [93] | Prospective cohort | 52 TKA patients receiving DVT prophylaxis with warfarin | On the day of epidural catheter removal, R was increased compared to preoperative values but still within normal range. CI was normal. Only INR was elevated to an average of 1.48 at the time of catheter removal. |
| Klein et al., 2000 [94] | Prospective Cohort | 24 unilateral TKA/THA patients | TEG parameters R and K correlated well with anticipated peak and trough of postoperative LMWH and anti-Xa levels. |
| Oswald et al., 2015 [98] | Prospective Cohort | 188 orthopaedic surgery patients (receiving 40 mg enoxaparin or 10 mg rivaroxaban) | Increase in EXTEM CT was greater with rivaroxaban than enoxaparin. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamczak, C.N.; Speybroeck, J.; Stillson, J.E.; Dynako, J.; Piscoya, A.; Peck, E.E.; Aboukhaled, M.; Cancel, E.; McDonald, M.; Garcia, D.; et al. Viscoelastic Hemostatic Assays for Orthopedic Trauma and Elective Procedures. J. Clin. Med. 2022, 11, 4029. https://doi.org/10.3390/jcm11144029
Mamczak CN, Speybroeck J, Stillson JE, Dynako J, Piscoya A, Peck EE, Aboukhaled M, Cancel E, McDonald M, Garcia D, et al. Viscoelastic Hemostatic Assays for Orthopedic Trauma and Elective Procedures. Journal of Clinical Medicine. 2022; 11(14):4029. https://doi.org/10.3390/jcm11144029
Chicago/Turabian StyleMamczak, Christiaan N., Jacob Speybroeck, John E. Stillson, Joseph Dynako, Andres Piscoya, Ethan E. Peck, Michael Aboukhaled, Emily Cancel, Michael McDonald, Diego Garcia, and et al. 2022. "Viscoelastic Hemostatic Assays for Orthopedic Trauma and Elective Procedures" Journal of Clinical Medicine 11, no. 14: 4029. https://doi.org/10.3390/jcm11144029






