An Updated Review on the Psychoactive, Toxic and Anticancer Properties of Kava
Abstract
:1. Introduction
2. Materials and Methods
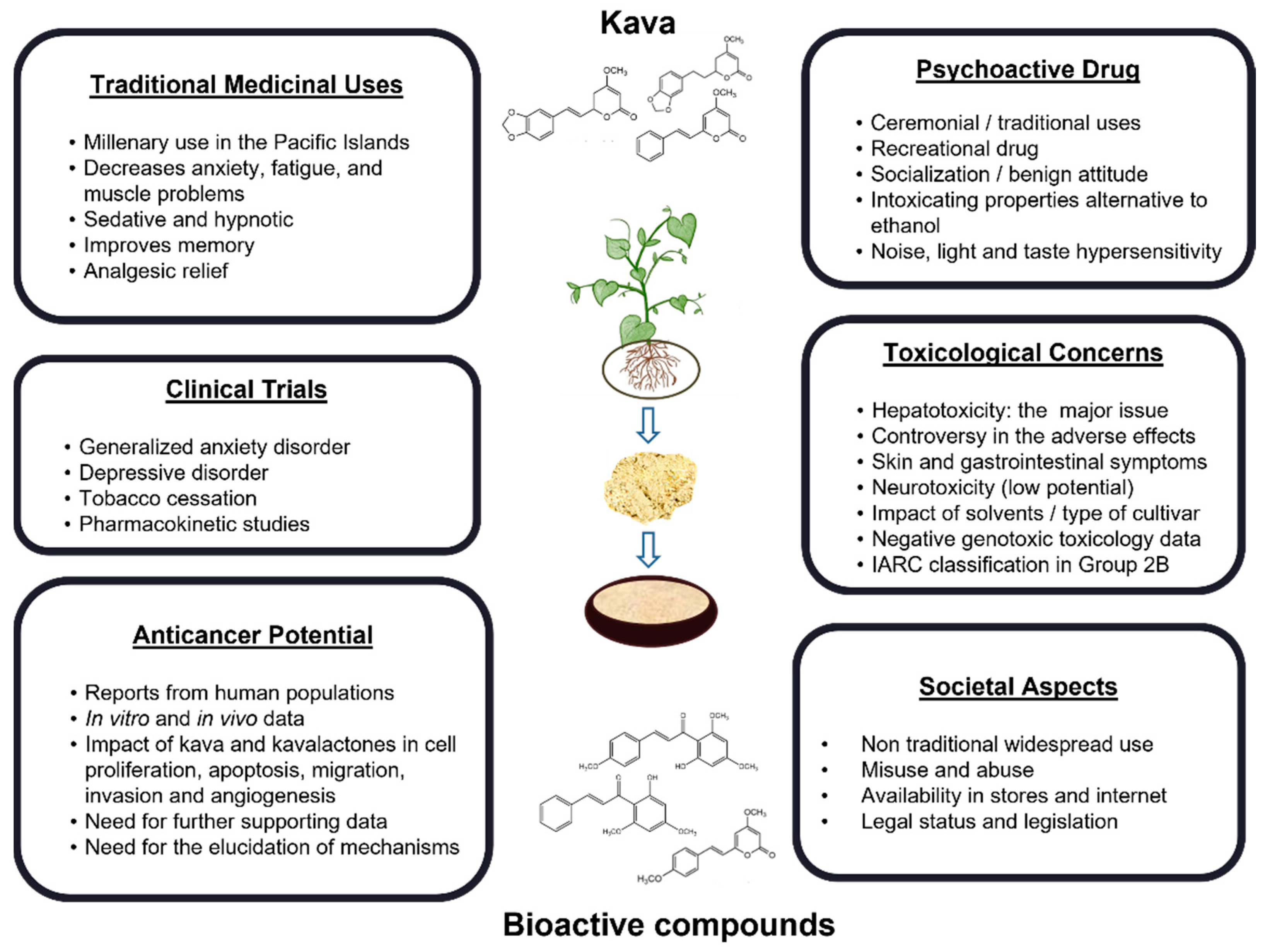
3. Historical and Social Perspectives on the Consumption of Kava
4. Chemical Aspects of Kava
4.1. Chemical Composition of Kava
4.2. Overview of the Methodologies Used for the Chemical Analysis of Kava
5. Toxicokinetics and Drug Interactions
5.1. ADME Aspects of Kava
5.2. Interactions and Metabolic Induction or Inhibition
6. Kava-Induced Toxicity and Safety Issues
6.1. Hepatotoxicity
6.2. Other Adverse Effects on Consumers
6.3. Genotoxicity and Carcinogenicity Potential
7. Clinical Potential Uses of Kava in Non-Cancer Diseases
8. Kava in Cancer Prevention and Treatment
8.1. Potential Roles of Kava in Cancer
8.2. Anticancer Properties of Kava Extracts
8.3. Anticancer Properties of Flavokavains
8.4. Anticancer Properties of Kavalactones
9. Final Considerations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Showman, A.F.; Baker, J.D.; Linares, C.; Naeole, C.K.; Borris, R.; Johnston, E.; Konanui, J.; Turner, H. Contemporary Pacific and Western perspectives on ’awa (Piper methysticum) toxicology. Fitoterapia 2015, 100, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Bian, T.; Corral, P.; Wang, Y.; Botello, J.; Kingston, R.; Daniels, T.; Salloum, R.; Johnston, E.; Huo, Z.; Lu, J.; et al. Kava as a Clinical Nutrient: Promises and Challenges. Nutrients 2020, 12, 3044. [Google Scholar] [CrossRef]
- Baker, J.D. Tradition and toxicity: Evidential cultures in the kava safety debate. Soc. Stud. Sci. 2011, 41, 361–384. [Google Scholar] [CrossRef] [PubMed]
- Humberston, C.L.; Akhtar, J.; Krenzelok, E.P. Acute Hepatitis Induced by Kava Kava. J. Toxicol. Clin. Toxicol. 2003, 41, 109–113. [Google Scholar] [CrossRef] [PubMed]
- National Toxicology Program. Toxicology and carcinogenesis studies of kava kava extract (CAS No. 9000-38-8) in F344/N rats and B6C3F1 mice (Gavage Studies). Natl. Toxicol. Program Tech. Rep. Ser. 2012, 571, 1–186. [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Some Drugs and Herbal Products. IARC Monogr. Eval. Carcinog. Risks Hum. 2016, 108, 7–419. [Google Scholar]
- Norton, S.A.; Ruze, P. Kava dermopathy. J. Am. Acad. Dermatol. 1994, 31, 89–97. [Google Scholar] [CrossRef]
- Stickel, F.; Shouval, D. Hepatotoxicity of herbal and dietary supplements: An update. Arch. Toxicol. 2015, 89, 851–865. [Google Scholar] [CrossRef]
- Narayanapillai, S.C.; Balbo, S.; Leitzman, P.; Grill, A.E.; Upadhyaya, P.; Shaik, A.A.; Zhou, B.; O’Sullivan, M.G.; Peterson, L.A.; Lu, J.; et al. Dihydromethysticin from kava blocks tobacco carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis and differentially reduces DNA damage in A/J mice. Carcinogenesis 2014, 35, 2365–2372. [Google Scholar] [CrossRef] [Green Version]
- Shaver, J.H.; Sosis, R. How Does Male Ritual Behavior Vary Across the Lifespan?: An Examination of Fijian Kava Cere-monies. Hum. Nat. 2014, 25, 136–160. [Google Scholar] [CrossRef]
- Singh, Y.N.; Singh, N.N. Therapeutic Potential of Kava in the Treatment of Anxiety Disorders. CNS Drugs 2002, 16, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Nosa, V.; Ofanoa, M. The social, cultural and medicinal use of kava for twelve Tongan born men living in Auckland, New Zealand. Pac. Health Dialog. 2009, 15, 96–102. [Google Scholar] [PubMed]
- Kuchta, K.; Schmidt, M.; Nahrstedt, A. German Kava Ban Lifted by Court: The Alleged Hepatotoxicity of Kava (Piper methysticum) as a Case of Ill-Defined Herbal Drug Identity, Lacking Quality Control, and Misguided Regulatory Politics. Planta Medica 2015, 81, 1647–1653. [Google Scholar] [CrossRef] [PubMed]
- Bilia, A.R.; Scalise, L.; Bergonzi, M.C.; Vincieri, F.F. Analysis of kavalactones from Piper methysticum (kava-kava). Anal. Technol. Biomed. Life Sci. 2004, 812, 203–214. [Google Scholar] [CrossRef]
- Celentano, A.; Tran, A.; Testa, C.; Thayanantha, K.; Tan-Orders, W.; Tan, S.; Syamal, M.; McCullough, M.J.; Yap, T. The protective effects of Kava (Piper methysticum) constituents in cancers: A systematic review. J. Oral Pathol. Med. 2019, 48, 510–529. [Google Scholar] [CrossRef] [PubMed]
- Sarris, J.; LaPorte, E.; Schweitzer, I. Kava: A Comprehensive Review of Efficacy, Safety, and Psychopharmacology. Aust. N. Z. J. Psychiatry 2011, 45, 27–35. [Google Scholar] [CrossRef]
- Côté, C.S.; Kor, C.; Cohen, J.; Auclair, K. Composition and biological activity of traditional and commercial kava extracts. Biochem. Biophys. Res. Commun. 2004, 322, 147–152. [Google Scholar] [CrossRef]
- White, C.M. The Pharmacology, Pharmacokinetics, Efficacy, and Adverse Events Associated with Kava. J. Clin. Pharmacol. 2018, 58, 1396–1405. [Google Scholar] [CrossRef]
- Backhauß, C.; Krieglstein, J. Extract of kava (Piper methysticum) and its methysticin constituents protect brain tissue against ischemic damage in rodents. Eur. J. Pharmacol. 1992, 215, 265–269. [Google Scholar] [CrossRef]
- Hegazy, N.H.; Breitinger, H.-G.; Breitinger, U. Kavalactones from Kava (Piper methysticum) root extract as modulators of recombinant human glycine receptors. Biol. Chem. 2019, 400, 1205–1215. [Google Scholar] [CrossRef]
- Kong, Y.; Gao, X.; Wang, C.; Ning, C.; Liu, K.; Liu, Z.; Sun, H.; Ma, X.; Sun, P.; Meng, Q. Protective effects of yangonin from an edible botanical Kava against lithocholic acid-induced cholestasis and hepatotoxicity. Eur. J. Pharmacol. 2018, 824, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-F.; Su, Y.-Z.; Tseng, Y.-H.; Wang, S.-Y.; Wang, H.-M.; Chueh, P.J. Flavokawain B, a novel chalcone from Alpinia pricei Hayata with potent apoptotic activity: Involvement of ROS and GADD153 upstream of mitochondria-dependent apoptosis in HCT116 cells. Free Radic. Biol. Med. 2010, 49, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Fields, C. A UHPLC-UV Method Development and Validation for Determining Kavalactones and Flavokavains in Piper methysticum (Kava). Molecules 2019, 24, 1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Lund, J.A.; Murch, S.J.; Brown, P.N. Single-Lab Validation for Determination of Kavalactones and Flavokavains in Piper methysticum (Kava). Planta Medica 2018, 84, 1213–1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teschke, R.; Sarris, J.; Lebot, V. Kava hepatotoxicity solution: A six-point plan for new kava standardization. Phytomedicine 2011, 18, 96–103. [Google Scholar] [CrossRef]
- Dragull, K.; Yoshida, W.Y.; Tang, C.-S. Piperidine alkaloids from Piper methysticum. Phytochemistry 2003, 63, 193–198. [Google Scholar] [CrossRef]
- Dinh, L.D.; Simmen, U.; Bueter, K.B.; Bueter, B.; Lundstrom, K.; Schaffner, W. Interaction of Various Piper methysticum Cultivars with CNS Receptors in vitro. Planta Medica 2001, 67, 306–311. [Google Scholar] [CrossRef] [Green Version]
- Lebot, V.; Do, T.; Legendre, L. Detection of flavokavins (A, B, C) in cultivars of kava (Piper methysticum) using high performance thin layer chromatography (HPTLC). Food Chem. 2014, 151, 554–560. [Google Scholar] [CrossRef]
- Kuchta, K.; de Nicola, P.; Schmidt, M. Randomized, dose-controlled double-blind trial: Efficacy of an ethanolic kava (Piper methysticum rhizome) extract for the treatment of anxiety in elderly patients. Tradit. Kamp Med. 2017, 5, 3–10. [Google Scholar] [CrossRef]
- Sarris, J.; Kavanagh, D.J.; Byrne, G.; Bone, K.M.; Adams, J.; Deed, G. The Kava Anxiety Depression Spectrum Study (KADSS): A randomized, placebo-controlled crossover trial using an aqueous extract of Piper methysticum. Psychopharmacology 2009, 205, 399–407. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Monographs on Selected Medicinal Plants; World Health Organization (WHO): Geneva, Switzerland, 2002; Volume 2. [Google Scholar]
- Zhao, X.; Su, C.; Ren, R.; Zhang, B.; Wang, Y.; Su, X.; Lu, F.; Zong, R.; Yang, L.; Zhang, W.; et al. Simultaneous determination of both kavalactone and flavokawain constituents by different single-marker methods in kava. J. Sep. Sci. 2021, 44, 2705–2716. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Eans, S.O.; Stacy, H.M.; Narayanapillai, S.C.; Sharma, A.; Fujioka, N.; Haddad, L.; McLaughlin, J.; Avery, B.A.; Xing, C. A stable isotope dilution tandem mass spectrometry method of major kavalactones and its applications. PLoS ONE 2018, 13, e0197940. [Google Scholar] [CrossRef]
- Ferreira, J.V.; Pierotte, I.C.; Pianetti, G.A.; César, I.C. Simultaneous quantitation of kavalactones in kava dry extracts: Comparison of multi-standards and single standard validation approaches. Phytochem. Anal. 2021, 32, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Mathews, J.M.; Etheridge, A.S.; Valentine, J.L.; Black, S.R.; Coleman, D.P.; Patel, P.; So, J.; Burka, L.T. Pharmacokinetics and Disposition of the Kavalactone Kawain: Interaction with Kava Extract and Kavalactones in Vivo and in Vitro. Drug Metab. Dispos. 2005, 33, 1555–1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, L.; Li, Q.; Xia, Q.; Dial, S.; Chan, P.-C.; Fu, P. Analysis of gene expression changes of drug metabolizing enzymes in the livers of F344 rats following oral treatment with kava extract. Food Chem. Toxicol. 2009, 47, 433–442. [Google Scholar] [CrossRef] [Green Version]
- Fu, P.P.; Xia, Q.; Guo, L.; Yu, H.; Chan, P.-C. Toxicity of Kava Kava. J. Environ. Sci. Health Part C 2008, 26, 89–112. [Google Scholar] [CrossRef] [Green Version]
- Mathews, J.M.; Etheridge, A.S.; Black, S.R. Inhibition of human cytochrome P450 activities by kava extract and kavalactones. Drug Metab. Dispos. 2002, 30, 1153–1157. [Google Scholar] [CrossRef]
- Keledjian, J.; Duffield, P.; Jamieson, D.; Lidgard, R.; Duffield, A. Uptake into Mouse Brain of Four Compounds Present in the Psychoactive Beverage Kava. J. Pharm. Sci. 1988, 77, 1003–1006. [Google Scholar] [CrossRef]
- Rasmussen, A.K.; Scheline, R.R.; Solheim, E.; Hänsel, R. Metabolism of Some Kava Pyrones in the Rat. Xenobiotica 1979, 9, 1–16. [Google Scholar] [CrossRef]
- Clouatre, D.L. Kava kava: Examining new reports of toxicity. Toxicol. Lett. 2004, 150, 85–96. [Google Scholar] [CrossRef]
- Duffield, A.; Jamieson, D.; Lidgard, R.; Duffield, P.; Bourne, D. Identification of some human urinary metabolites of the intoxicating beverage kava. J. Chromatogr. A 1989, 475, 273–281. [Google Scholar] [CrossRef]
- Zou, L.; Harkey, M.R.; Henderson, G.L. Synthesis, in vitro Reactivity, and Identification of 6-Phenyl-3-hexen-2-one in Human Urine after Kava-Kava (Piper methysticum) Ingestion. Planta Medica 2005, 71, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.M.; Qiu, S.-X.; Zhang, S.; Zhang, F.; Burdette, J.E.; Yu, L.; Bolton, J.L.; van Breemen, R. Identification of Novel Electrophilic Metabolites of Piper methysticum Forst. (Kava). Chem. Res. Toxicol. 2003, 16, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Pantano, F.; Tittarelli, R.; Mannocchi, G.; Zaami, S.; Ricci, S.; Giorgetti, R.; Terranova, D.; Busardò, F.P.; Marinelli, E. Hepatotoxicity Induced by “the 3Ks”: Kava, Kratom and Khat. Int. J. Mol. Sci. 2016, 17, 580. [Google Scholar] [CrossRef]
- Clayton, N.P.; Yoshizawa, K.; Kissling, G.E.; Burka, L.T.; Chan, P.-C.; Nyska, A. Immunohistochemical analysis of expressions of hepatic cytochrome P450 in F344 rats following oral treatment with kava extract. Exp. Toxicol. Pathol. 2007, 58, 223–236. [Google Scholar] [CrossRef] [Green Version]
- Zou, L.; Harkey, M.; Henderson, G. Effects of herbal components on cDNA-expressed cytochrome P450 enzyme catalytic activity. Life Sci. 2002, 71, 1579–1589. [Google Scholar] [CrossRef]
- Russmann, S.; Lauterburg, B.H.; Barguil, Y.; Choblet, E.; Cabalion, P.; Rentsch, K.; Wenk, M. Traditional aqueous kava extracts inhibit cytochrome P450 1A2 in humans: Protective effect against environmental carcinogens? Clin. Pharmacol. Ther. 2005, 77, 453–454. [Google Scholar] [CrossRef]
- Steiner, G.G. The correlation between cancer incidence and kava consumption. Hawaii Med. J. 2000, 59, 420–422. [Google Scholar]
- Li, Y.; Mei, H.; Wu, Q.; Zhang, S.; Fang, J.-L.; Shi, L.; Guo, L. Methysticin and 7,8-Dihydromethysticin are Two Major Kavalactones in Kava Extract to Induce CYP1A1. Toxicol. Sci. 2011, 124, 388–399. [Google Scholar] [CrossRef] [Green Version]
- Gurley, B.J.; Gardner, S.F.; Hubbard, M.A.; Williams, D.K.; Gentry, W.B.; Khan, I.A.; Shah, A. In vivo effects of goldenseal, kava kava, black cohosh, and valerian on human cytochrome P450 1A2, 2D6, 2E1, and 3A4/5 phenotypes. Clin. Pharmacol. Ther. 2005, 77, 415–426. [Google Scholar] [CrossRef]
- Krum, B.N.; Molz de Freitas, C.; Chiapinotto Ceretta, A.P.; Barbosa, C.P.; de Moraes Reis, E.; Scussel, R.; da Silva Córneo, E.; Machado-de-Ávila, R.A.; Boligon, A.A.; Fachinetto, R. Kava decreases the stereotyped behavior induced by amphetamine in mice. J. Ethnopharmacol. 2021, 265, 113293. [Google Scholar] [CrossRef] [PubMed]
- Krum, B.N.; de Freitas, C.M.; Busanello, A.; Schaffer, L.F.; Fachinetto, R. Ex vivo and in vitro inhibitory potential of Kava extract on monoamine oxidase B activity in mice. J. Tradit. Complement. Med. 2022, 12, 115–122. [Google Scholar] [CrossRef]
- Melchert, P.W.; Qian, Y.; Zhang, Q.; Klee, B.O.; Xing, C.; Markowitz, J.S. In vitro inhibition of carboxylesterase 1 by Kava (Piper methysticum) Kavalactones. Chem. Interact. 2022, 357, 109883. [Google Scholar] [CrossRef] [PubMed]
- Foo, H.; Lemon, J. Acute effects of kava, alone or in combination with alcohol, on subjective measures of impairment and intoxication and on cognitive performance. Drug Alcohol Rev. 1997, 16, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, D.D.; Duffield, P.H. Positive Interaction of Ethanol and Kava Resin in Mice. Clin. Exp. Pharmacol. Physiol. 1990, 17, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Herberg, K.W. Effect of Kava-Special Extract WS 1490 combined with ethyl alcohol on safety-relevant performance parameters. Blutalkohol 1993, 30, 96–105. [Google Scholar]
- Abdulabba Hasan, M.; Mohan, S.; Rahman, H.S.; Hamasalih Othman, H.; Omer, S.H.; Farasani, A. The sub-acute toxicity of kavalactone in rats: A study of the effect of oral doses and the mechanism of toxicity in combination with ethanol. Drug Chem. Toxicol. 2022, in press. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Assessments of the Risk of Hepatotoxicity with Kava Products; WHO Document Production Services: Geneva, Switzerland, 2007. [Google Scholar]
- Ulbricht, C.; Basch, E.; Boon, H.; Ernst, E.; Hammerness, P.; Sollars, D.; Tsourounis, C.; Woods, J.; Bent, S. Safety review of kava (Piper methysticum) by the Natural Standard Research Collaboration. Expert Opin. Drug Saf. 2005, 4, 779–794. [Google Scholar] [CrossRef]
- Teschke, R.; Qiu, S.X.; Lebot, V. Herbal hepatotoxicity by kava: Update on pipermethystine, flavokavain B, and mould hepatotoxins as primarily assumed culprits. Dig. Liver Dis. 2011, 43, 676–681. [Google Scholar] [CrossRef]
- Nerurkar, P.V.; Dragull, K.; Tang, C.-S. In Vitro Toxicity of Kava Alkaloid, Pipermethystine, in HepG2 Cells Compared to Kavalactones. Toxicol. Sci. 2004, 79, 106–111. [Google Scholar] [CrossRef] [Green Version]
- Tugcu, G.; Kırmızıbekmez, H.; Aydın, A. The integrated use of in silico methods for the hepatotoxicity potential of Piper methysticum. Food Chem. Toxicol. 2020, 145, 111663. [Google Scholar] [CrossRef] [PubMed]
- Lechtenberg, M.; Quandt, B.; Schmidt, M.; Nahrstedt, A. Is the alkaloid pipermethystine connected with the claimed liver toxicity of Kava products? Die Pharm.-Int. J. Pharm. Sci. 2008, 63, 71–74. [Google Scholar] [CrossRef]
- Zhou, P.; Gross, S.; Liu, J.-H.; Yu, B.-Y.; Feng, L.-L.; Nolta, J.; Sharma, V.; Piwnica-Worms, D.; Qiu, S.X. Flavokawain B, the hepatotoxic constituent from kava root, induces GSH-sensitive oxidative stress through modulation of IKK/NF-κB and MAPK signaling pathways. FASEB J. 2010, 24, 4722–4732. [Google Scholar] [CrossRef] [PubMed]
- Narayanapillai, S.C.; Leitzman, P.; O’Sullivan, M.G.; Xing, C. Flavokawains A and B in Kava, Not Dihydromethysticin, Potentiate Acetaminophen-Induced Hepatotoxicity in C57BL/6 Mice. Chem. Res. Toxicol. 2014, 27, 1871–1876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowe, A.; Ramzan, I. Are Mould Hepatotoxins Responsible for Kava Hepatotoxicity? Phytotherapy Res. 2012, 26, 1768–1770. [Google Scholar] [CrossRef]
- Whitton, P.A.; Lau, A.; Salisbury, A.; Whitehouse, J.; Evans, C.S. Kava lactones and the kava-kava controversy. Phytochemistry 2003, 64, 673–679. [Google Scholar] [CrossRef]
- Russmann, S.; Lauterburg, B.H.; Helbling, A. Kava Hepatotoxicity. Ann. Intern. Med. 2001, 135, 68. [Google Scholar] [CrossRef] [Green Version]
- Peter, S. The Use of Herbal OTC Products in South Africa: Main Topic. C. CME Your SA J. CPD 2003, 21, 89–95. [Google Scholar]
- Sarris, J.; Laporte, E.; Scholey, A.; King, R.; Pipingas, A.; Schweitzer, I.; Stough, C. Does a Medicinal Dose of Kava Impair Driving? A Randomized, Placebo-Controlled, Double-Blind Study. Traffic Inj. Prev. 2013, 14, 13–17. [Google Scholar] [CrossRef]
- Sarris, J.; Stough, C.; Teschke, R.; Wahid, Z.T.; Bousman, C.A.; Murray, G.; Savage, K.M.; Mouatt, P.; Ng, C.; Schweitzer, I. Kava for the Treatment of Generalized Anxiety Disorder RCT: Analysis of Adverse Reactions, Liver Function, Addiction, and Sexual Effects. Phytotherapy Res. 2013, 27, 1723–1728. [Google Scholar] [CrossRef]
- Meseguer, E.; Taboada, R.; Sánchez, V.; Mena, M.A.; Campos, V.; De Yébenes, J.G. Life-threatening parkinsonism induced by kava-kava. Mov. Disord. 2002, 17, 195–196. [Google Scholar] [CrossRef] [PubMed]
- Vignier, N.; Lert, F.; Salomon, C.; Hamelin, C. Kava drinking associated with suicidal behaviour among young Kanaks using kava in New Caledonia. Aust. N. Z. J. Public Health 2011, 35, 427–433. [Google Scholar] [CrossRef]
- Wainiqolo, I.; Kafoa, B.; Kool, B.; Robinson, E.; Herman, J.; McCaig, E.; Ameratunga, S. Driving following Kava Use and Road Traffic Injuries: A Population-Based Case-Control Study in Fiji (TRIP 14). PLoS ONE 2016, 11, e0149719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whittaker, P.; Clarke, J.J.; San, R.H.; Betz, J.M.; Seifried, H.E.; Dejager, L.; Dunkel, V.C. Evaluation of commercial kava extracts and kavalactone standards for mutagenicity and toxicity using the mammalian cell gene mutation assay in L5178Y mouse lymphoma cells. Food Chem. Toxicol. 2008, 46, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Jhoo, J.-W.; Ang, C.Y.W.; Heinze, T.M.; Deck, J.; Schnackenberg, L.K.; Beger, R.D.; Dragull, K.; Tang, C.-S. Identification of C-glycoside Flavonoids as Potential Mutagenic Compounds in Kava. J. Food Sci. 2007, 72, C120–C125. [Google Scholar] [CrossRef]
- Teixeira da Silva, T.; Braga Martins, J.; Do Socorro de Brito Lopes, M.; de Almeida, P.M.; Silva Sá, J.L.; Alline Martins, F. Modulating effect of DL-kavain on the mutagenicity and carcinogenicity induced by doxorubicin in Drosophila melanogaster. J. Toxicol. Environ. Health Part A 2021, 84, 769–782. [Google Scholar] [CrossRef]
- Sarris, J. Herbal medicines in the treatment of psychiatric disorders: 10-year updated review. Phytother. Res. 2018, 32, 1147–1162. [Google Scholar] [CrossRef]
- Ooi, S.L.; Henderson, P.; Pak, S.C. Kava for Generalized Anxiety Disorder: A Review of Current Evidence. J. Altern. Complement. Med. 2018, 24, 770–780. [Google Scholar] [CrossRef]
- Volgin, A.; Yang, L.; Amstislavskaya, T.G.; Demin, K.; Wang, D.; Yan, D.; Wang, J.; Wang, M.; Alpyshov, E.; Hu, G.; et al. DARK Classics in Chemical Neuroscience: Kava. ACS Chem. Neurosci. 2020, 11, 3893–3904. [Google Scholar] [CrossRef]
- Smith, K.; Leiras, C. The effectiveness and safety of Kava Kava for treating anxiety symptoms: A systematic review and analysis of randomized clinical trials. Complement. Ther. Clin. Pract. 2018, 33, 107–117. [Google Scholar] [CrossRef]
- Sarris, J.; Stough, C.; Bousman, C.; Wahid, Z.T.; Murray, G.; Teschke, R.; Savage, K.M.; Dowell, A.; Ng, C.; Schweitzer, I. Kava in the Treatment of Generalized Anxiety Disorder: A Double-Blind, Randomized, Placebo-Controlled Study. J. Clin. Psychopharmacol. 2013, 33, 643–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarris, J.; Scholey, A.; Schweitzer, I.; Bousman, C.; Laporte, E.; Ng, C.; Murray, G.; Stough, C. The acute effects of kava and oxazepam on anxiety, mood, neurocognition; and genetic correlates: A randomized, placebo-controlled, double-blind study. Hum. Psychopharmacol. Clin. Exp. 2012, 27, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Volz, H.-P.; Kieser, M. Kava-kava Extract WS 1490 versus Placebo in Anxiety Disorders—A Randomized Placebo-controlled 25-week Outpatient Trial. Pharmacopsychiatry 1997, 30, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Huntley, A.L.; Ernst, E. A systematic review of herbal medicinal products for the treatment of menopausal symptoms. Menopause 2003, 10, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Geller, S.E.; Studee, L. Botanical and dietary supplements for mood and anxiety in menopausal women. Menopause 2007, 14, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, B.P.; Bent, S.; Tice, J.A.; Blackwell, T.; Cummings, S.R. An Internet-Based Randomized, Placebo-Controlled Trial of Kava and Valerian for Anxiety and Insomnia. Medicine 2005, 84, 197–207. [Google Scholar] [CrossRef]
- Wheatley, D. Stress-induced insomnia treated with kava and valerian: Singly and in combination. Hum. Psychopharmacol. Clin. Exp. 2001, 16, 353–356. [Google Scholar] [CrossRef]
- Cairney, S.; Clough, A.R.; Maruff, P.; Collie, A.; Currie, B.J.; Currie, J. Saccade and Cognitive Function in Chronic Kava Users. Neuropsychopharmacology 2003, 28, 389–396. [Google Scholar] [CrossRef]
- Tawfiq, R.A.; Nassar, N.N.; Eleraky, W.; El-Denshary, E.S. Enhanced efficacy and reduced side effects of diazepam by kava combination. J. Adv. Res. 2014, 5, 587–594. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, Y.; Shaikh, M.; Wang, I.; Yong, Y.; Lee, V.L.L.; Williams, L. Evaluation of Anti-Convulsive Properties of Aqueous Kava Extract on Zebrafish Using the PTZ-Induced Seizure Model. Brain Sci. 2020, 10, 541. [Google Scholar] [CrossRef]
- Le Marchand, L.; Hankin, J.H.; Bach, F.; Kolonel, L.N.; Wilkens, L.R.; Stacewicz-Sapuntzakis, M.; Bowen, P.E.; Beecher, G.R.; Laudon, F.; Baqué, P.; et al. An ecological study of diet and lung cancer in the South Pacific. Int. J. Cancer 1995, 63, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Paksoy, N.; Bouchardy, C.; Parkin, D.M. Cancer Incidence in Western Samoa. Int. J. Epidemiol. 1991, 20, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.E.; Kolonel, L.N.; Dworsky, R.; Kerford, D.; Mori, E.; Singh, K.; Thevenot, H. Cancer incidence in the islands of the Pacific. Natl. Cancer Inst. Monogr. 1985, 69, 73–81. [Google Scholar] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Last 25 Years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umezawa, S.; Higurashi, T.; Komiya, Y.; Arimoto, J.; Horita, N.; Kaneko, T.; Iwasaki, M.; Nakagama, H.; Nakajima, A. Chemoprevention of colorectal cancer: Past, present, and future. Cancer Sci. 2019, 110, 3018–3026. [Google Scholar] [CrossRef]
- Martin, A.C.; Johnston, E.; Xing, C.; Hegeman, A.D. Measuring the Chemical and Cytotoxic Variability of Commercially Available Kava (Piper methysticum G. Forster). PLoS ONE 2014, 9, e111572. [Google Scholar] [CrossRef]
- Einbond, L.; Negrin, A.; Kulakowski, D.; Wu, H.-A.; Antonetti, V.; Jalees, F.; Law, W.; Roller, M.; Redenti, S.; Kennelly, E.; et al. Traditional preparations of kava (Piper methysticum) inhibit the growth of human colon cancer cells in vitro. Phytomedicine 2017, 24, 1–13. [Google Scholar] [CrossRef]
- Leitzman, P.; Narayanapillai, S.C.; Balbo, S.; Zhou, B.; Upadhyaya, P.; Shaik, A.A.; O’Sullivan, M.G.; Hecht, S.S.; Lu, J.; Xing, C. Kava Blocks 4-(Methylnitrosamino)-1-(3-pyridyl)-1-Butanone–Induced Lung Tumorigenesis in Association with Reducing O6-methylguanine DNA Adduct in A/J Mice. Cancer Prev. Res. 2014, 7, 86–96. [Google Scholar] [CrossRef] [Green Version]
- Hu, Q.; Corral, P.; Narayanapillai, S.C.; Leitzman, P.; Upadhyaya, P.; O’Sullivan, M.G.; Hecht, S.S.; Lu, J.; Xing, C. Oral Dosing of Dihydromethysticin Ahead of Tobacco Carcinogen NNK Effectively Prevents Lung Tumorigenesis in A/J Mice. Chem. Res. Toxicol. 2020, 33, 1980–1988. [Google Scholar] [CrossRef]
- Triolet, J.; Shaik, A.A.; Gallaher, D.; O’Sullivan, M.G.; Xing, C. Reduction in Colon Cancer Risk by Consumption of Kava or Kava Fractions in Carcinogen-Treated Rats. Nutr. Cancer 2012, 64, 838–846. [Google Scholar] [CrossRef]
- Johnson, T.E.; Hermanson, D.; Wang, L.; Kassie, F.; Upadhyaya, P.; O’Sullivan, M.G.; Hecht, S.S.; Lu, J.; Xing, C. Lung Tumorigenesis Suppressing Effects of a Commercial Kava Extract and Its Selected Compounds in A/J Mice. Am. J. Chin. Med. 2011, 39, 727–742. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S.S. Biochemistry, Biology, and Carcinogenicity of Tobacco-Specific N-Nitrosamines. Chem. Res. Toxicol. 1998, 11, 559–603. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Narayanapillai, S.C.; Tessier, K.M.; Strayer, L.G.; Upadhyaya, P.; Hu, Q.; Kingston, R.; Salloum, R.G.; Lu, J.; Hecht, S.S.; et al. The Impact of One-week Dietary Supplementation with Kava on Biomarkers of Tobacco Use and Nitrosamine-based Carcinogenesis Risk among Active Smokers. Cancer Prev. Res. 2020, 13, 483–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zi, X.; Simoneau, A.R. Flavokawain A, a Novel Chalcone from Kava Extract, Induces Apoptosis in Bladder Cancer Cells by Involvement of Bax Protein-Dependent and Mitochondria-Dependent Apoptotic Pathway and Suppresses Tumor Growth in Mice. Cancer Res. 2005, 65, 3479–3486. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A Privileged Structure in Medicinal Chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef]
- Jandial, D.D.; Krill, L.S.; Chen, L.; Wu, C.; Ke, Y.; Xie, J.; Hoang, B.H.; Zi, X. Induction of G2M Arrest by Flavokawain A, a Kava Chalcone, Increases the Responsiveness of HER2-Overexpressing Breast Cancer Cells to Herceptin. Molecules 2017, 22, 462. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, W.; Wang, Z.; Gao, M.; Wang, X.; Han, W.; Zhang, N.; Xu, X. Flavokawain A inhibits prostate cancer cells by inducing cell cycle arrest and cell apoptosis and regulating the glutamine metabolism pathway. J. Pharm. Biomed. Anal. 2020, 186, 113288. [Google Scholar] [CrossRef]
- Abu, N.; Akhtar, M.N.; Yeap, S.K.; Lim, K.L.; Ho, W.Y.; Zulfadli, A.J.; Omar, A.R.; Sulaiman, M.R.; Abdullah, M.P.; Alitheen, N.B. Flavokawain A Induces Apoptosis in MCF-7 and MDA-MB231 and Inhibits the Metastatic Process In Vitro. PLoS ONE 2014, 9, e105244. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, X.; Li, X.; Liu, S.; Simoneau, A.R.; He, F.; Wu, X.-R.; Zi, X. KAVA Chalcone, Flavokawain A, Inhibits Urothelial Tumorigenesis in the UPII-SV40T Transgenic Mouse Model. Cancer Prev. Res. 2013, 6, 1365–1375. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zheng, L.; Yan, M.; Wu, J.; Liu, Y.; Tian, X.; Jiang, W.; Zhang, L.; Wang, R. Activity and mechanism of flavokawain A in inhibiting permeability-glycoprotein expression in paclitaxel resistance of lung cancer. Oncol. Lett. 2020, 19, 379–387. [Google Scholar] [CrossRef]
- Abu, N.; Mohamed, N.E.; Yeap, S.K.; Lim, K.L.; Akhtar, M.N.; Zulfadli, A.J.; Kee, B.B.; Abdullah, M.P.; Omar, A.R.; Alitheen, N.B. In vivo antitumor and antimetastatic effects of flavokawain B in 4T1 breast cancer cell-challenged mice. Drug Des. Dev. Ther. 2015, 9, 1401–1417. [Google Scholar] [CrossRef] [Green Version]
- Abu, N.; Akhtar, M.N.; Yeap, S.K.; Lim, K.L.; Ho, W.Y.; Abdullah, M.P.; Ho, C.L.; Omar, A.R.; Ismail, J.; Alitheen, N.B. Flavokawain B induced cytotoxicity in two breast cancer cell lines, MCF-7 and MDA-MB231 and inhibited the metastatic potential of MDA-MB231 via the regulation of several tyrosine kinases In vitro. BMC Complement. Altern. Med. 2016, 16, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palko-Łabuz, A.; Kostrzewa-Susłow, E.; Janeczko, T.; Środa-Pomianek, K.; Poła, A.; Uryga, A.; Michalak, K. Cyclization of flavokawain B reduces its activity against human colon cancer cells. Hum. Exp. Toxicol. 2020, 39, 262–275. [Google Scholar] [CrossRef]
- Hseu, Y.-C.; Lin, R.-W.; Shen, Y.-C.; Lin, K.-Y.; Liao, J.-W.; Thiyagarajan, V.; Yang, H.-L. Flavokawain B and Doxorubicin Work Synergistically to Impede the Propagation of Gastric Cancer Cells via ROS-Mediated Apoptosis and Autophagy Pathways. Cancers 2020, 12, 2475. [Google Scholar] [CrossRef]
- Wang, J.; Qi, Q.; Zhou, W.; Feng, Z.; Huang, B.; Chen, A.; Zhang, D.; Li, W.; Zhang, Q.; Jiang, Z.; et al. Inhibition of glioma growth by flavokawain B is mediated through endoplasmic reticulum stress induced autophagy. Autophagy 2018, 14, 2007–2022. [Google Scholar] [CrossRef] [Green Version]
- Hua, R.; Pei, Y.; Gu, H.; Sun, Y.; He, Y. Antitumor effects of flavokawain-B flavonoid in gemcitabine-resistant lung cancer cells are mediated via mitochondrial-mediated apoptosis, ROS production, cell migration and cell invasion inhibition and blocking of PI3K/AKT Signaling pathway. J. Buon 2020, 25, 262–267. [Google Scholar] [PubMed]
- Sakai, T.; Eskander, R.N.; Guo, Y.; Kim, K.J.; Mefford, J.; Hopkins, J.; Bhatia, N.N.; Zi, X.; Hoang, B.H. Flavokawain B, a kava chalcone, induces apoptosis in synovial sarcoma cell lines. J. Orthop. Res. 2012, 30, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Eskander, R.N.; Randall, L.M.; Sakai, T.; Guo, Y.; Hoang, B.; Zi, X. Flavokawain B, a novel, naturally occurring chalcone, exhibits robust apoptotic effects and induces G2/M arrest of a uterine leiomyosarcoma cell line. J. Obstet. Gynaecol. Res. 2012, 38, 1086–1094. [Google Scholar] [CrossRef] [Green Version]
- Phang, C.-W.; Karsani, S.A.; Sethi, G.; Malek, S.N.A. Flavokawain C Inhibits Cell Cycle and Promotes Apoptosis, Associated with Endoplasmic Reticulum Stress and Regulation of MAPKs and Akt Signaling Pathways in HCT 116 Human Colon Carcinoma Cells. PLoS ONE 2016, 11, e0148775. [Google Scholar] [CrossRef] [Green Version]
- Phang, C.-W.; Karsani, S.A.; Malek, S.N.A. Induction of apoptosis and cell cycle arrest by flavokawain C on HT-29 human colon adenocarcinoma via enhancement of reactive oxygen species generation, upregulation of p21, p27, and Gadd153, and inactivation of inhibitor of apoptosis proteins. Pharmacogn. Mag. 2017, 13, S321–S328. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Ha, U.S.; Yu, K.; Wu, C.; Yokoyama, N.; Zi, X. Kavalactone yangonin induces autophagy and sensitizes bladder cancer cells to flavokawain A and docetaxel via inhibition of the mTOR pathway. J. Biomed. Res. 2017, 31, 408–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, H.; Liu, F.; Wang, J.; Zhao, M.; Wang, D.; Jia, C.; Wang, T.; Chen, Z.; Fan, Y.; Liang, D.; et al. Dihydromethysticin, a natural molecule from Kava, suppresses the growth of colorectal cancer via the NLRC3/PI3K pathway. Mol. Carcinog. 2020, 59, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.-Q.; Huang, Y.-G.; He, A.-N. Dihydromethysticin kavalactone induces apoptosis in osteosarcoma cells through modulation of PI3K/Akt pathway, disruption of mitochondrial membrane potential and inducing cell cycle arrest. Int. J. Clin. Exp. Pathol. 2015, 8, 4356–4366. [Google Scholar] [PubMed]
- Hecht, S.S.; Kassie, F.; Hatsukami, D.K. Chemoprevention of lung carcinogenesis in addicted smokers and ex-smokers. Nat. Cancer 2009, 9, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Puppala, M.; Narayanapillai, S.C.; Leitzman, P.; Sun, H.; Upadhyaya, P.; O’Sullivan, M.G.; Hecht, S.S.; Xing, C. Pilot in Vivo Structure–Activity Relationship of Dihydromethysticin in Blocking 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone-Induced O6-Methylguanine and Lung Tumor in A/J Mice. J. Med. Chem. 2017, 60, 7935–7940. [Google Scholar] [CrossRef] [Green Version]

| Flavokavain | Cancer | Study Type | Possible Mechanisms Involved | Key Findings | References |
|---|---|---|---|---|---|
| Flavokavain A | Bladder cancer | In vivo | Upregulation of p27 and DR5 and downregulation of Ki67, survivin and XIAP | Inhibition of occurrence of high-grade papillary UCC by 42.1% and promotion of apoptosis in UPII-SV40T transgenic mice | [111] |
| Bladder cancer | In vitro /In vivo | Bax protein-dependent and mitochondria-dependent apoptotic pathways | Inhibition of growth tumor cells by apoptosis (57% decrease) in xenograft mouse model | [106] | |
| Breast cancer | In vitro /Ex vivo | Intrinsic mitochondrial pathway with potential dependency on the p53 status | Cell cycle arrest G2/M in MDA-MB-231 and G1 in MCF-7. Induction of apoptosis in both cell lines | [110] | |
| Breast cancer | In vitro | Inhibition of Cdc2 and Cdc25C phosphorylation and upregulation of Bim and BAX | Cell cycle arrest G2/M. Flavokavain A in the presence of herceptin enhanced treatment. Induction of apoptosis in SKBR3. | [108] | |
| Lung cancer | In vitro | Downregulation of P-gp by inhibition of PI3K/Akt pathway | Inhibition of cell proliferation and induction of apoptosis of PTX-resistant A549/T cells in a concentration-dependent manner | [112] | |
| Prostate cancer | In vitro | Glutamine metabolism pathway upregulated, reducing the levels of glutamine, glutamic acid, and proline in PC3 cells | Reduced glutamine decreased GSH levels, which increased ROS levels and consequently cell apoptosis. Cell cycle arrest in G2/M | [109] | |
| Flavokavain B | Breast cancer | In vitro | - | Inhibition of proliferation, migration, and invasiveness in 4T1 cells. Reduced weight and size tumors after 28-days of treatment in cell-challenge mice | [113] |
| Breast cancer | In vitro /Ex vivo | Tyrosine kinase pathways | Induction of apoptosis and cell cycle arrest in G2/M in MDA-MB 231 and MCF-7 cells. Inhibition of migration and invasion in MDA-MB 231 cells and angiogenesis in HUVEC cells and in the rat aortic ring assay | [114] | |
| Colon cancer | In vitro | Cyclization of flavokavain B to 5,7-dimethoxyflavone | Inhibition of cell proliferation and cell cycle arrest in G2/M in LoVo and LoVo/Dx cell lines | [115] | |
| Gastric cancer | In vitro /In vivo | Extrinsic and intrinsic apoptotic pathways | Flavokavain B in the presence of doxorubicin suppresses cell growth and induces apoptosis and autophagy in BALB/c mice and in AGS cells | [116] | |
| Glioblastoma multiforme | In vitro /In vivo | Induction of autophagy | Inhibition of cell growth through autophagy in U251, U87 and T98 cell lines and combined with autophagy inhibitors led to apoptosis in mice | [117] | |
| Lung cancer | In vitro | Intrinsic apoptosis pathway and blockage of PI3K/Akt signaling pathway | Flavokavain B-induced apoptosis, ROS production and inhibits migration and invasion in A549 cell line | [118] | |
| Synovial Sarcoma | In vitro | Extrinsic and intrinsic apoptotic pathways | Inhibition of cell growth in SYO-I and HS-SY-II cell lines in a concentration-dependent manner | [119] | |
| Uterine Leiomyosarcoma | In vitro | Upregulation of DR5, Puma and Bin and downregulation of survivin | Cell cycle arrest in the G2/M and induction of apoptosis in SK_LMS-1 and ECC-1 cell lines | [120] | |
| Flavokavain C | Colorectal cancer | In vitro | Induction of intrinsic and extrinsic apoptosis pathways by an inactivation of Akt pathway and modulation of MAPK pathway | High cytotoxicity in HCT 116 cells in a time- and concentration-dependent manner. Disruption of the mitochondrial membrane potential and cell cycle arrest in the S phase | [121] |
| Colorectal cancer | In vitro | Inactivation of inhibitor of apoptotic proteins and endoplasmic reticulum stress pathways | Decreased cell viability and SOD activity and increased of ROS in HT-29 cells | [122] |
| Cancer | Study Type | Compound | Possible Mechanisms Involved | Key Findings | References |
|---|---|---|---|---|---|
| Bladder cancer | In vitro | Yangonin | Inhibition of mTOR pathway | Induction of autophagic cell death in UMUC-3 and T24 cells and growth inhibition in RT4, T24, UMUC3, HT1376 and HT1197 cell lines. | [123] |
| Colorectal cancer | In vitro/ In vivo | Dihydromethysticin | NLRC3/PI3K pathway | Inhibition of proliferation, migration, invasion and promotion of cell apoptosis and cell cycle arrest in HCT116, HT29 and LoVo. Inhibition of tumor growth in male BALB/C nude mice. | [124] |
| Lung cancer | In vivo | Dihydromethysticin | Inhibition of NNAL activation/increased NNAL detoxification | Reduction in adenocarcinoma multiplicity (97% decrease) and DNA adducts in A/J mice. | [9] |
| Lung cancer | In vivo | Dihydromethysticin | Inhibition of NNK-induced O6-mG | Temporally complete inhibition of lung adenoma in A/J mice. Pre-NNK administration of dihydromethysticin highly effective. | [101] |
| Osteosarcoma | In vitro | Dihydromethysticin | Decreased activity of PI3K/Akt pathway/disruption of MMP | Cell apoptosis and cell cycle arrest in G0/G1 in MG-63 cells. Inhibition of proliferation. | [125] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, R.B.; Dinis-Oliveira, R.J.; Oliveira, N.G. An Updated Review on the Psychoactive, Toxic and Anticancer Properties of Kava. J. Clin. Med. 2022, 11, 4039. https://doi.org/10.3390/jcm11144039
Soares RB, Dinis-Oliveira RJ, Oliveira NG. An Updated Review on the Psychoactive, Toxic and Anticancer Properties of Kava. Journal of Clinical Medicine. 2022; 11(14):4039. https://doi.org/10.3390/jcm11144039
Chicago/Turabian StyleSoares, Rita B., Ricardo Jorge Dinis-Oliveira, and Nuno G. Oliveira. 2022. "An Updated Review on the Psychoactive, Toxic and Anticancer Properties of Kava" Journal of Clinical Medicine 11, no. 14: 4039. https://doi.org/10.3390/jcm11144039
APA StyleSoares, R. B., Dinis-Oliveira, R. J., & Oliveira, N. G. (2022). An Updated Review on the Psychoactive, Toxic and Anticancer Properties of Kava. Journal of Clinical Medicine, 11(14), 4039. https://doi.org/10.3390/jcm11144039








