Performance of Artificial Intelligence-Based Algorithms to Predict Prolonged Length of Stay after Lumbar Decompression Surgery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Handling and Statistical Analysis
- CPU: AMD Ryzen 9 5950X 16-Core Processor (Santa Clara, CA, USA)
- RAM: 64 GB
- GPU: NVIDIA Geforce RTX 3090 (Santa Clara, CA, USA)
- Python version: 3.10.4 (64-bit) (Wilmington, DE, USA)
- OS: Windows 10 (Redmond, WA, USA)
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lurie, J.; Tomkins-Lane, C. Management of Lumbar Spinal Stenosis. BMJ 2016, 352, h6234. [Google Scholar] [CrossRef] [PubMed]
- Deer, T.; Sayed, D.; Michels, J.; Josephson, Y.; Li, S.; Calodney, A.K. A Review of Lumbar Spinal Stenosis with Intermittent Neurogenic Claudication: Disease and Diagnosis. Pain Med. 2019, 20, S32–S44. [Google Scholar] [CrossRef] [PubMed]
- Dagenais, S.; Caro, J.; Haldeman, S. A Systematic Review of Low Back Pain Cost of Illness Studies in the United States and Internationally. Spine J. 2008, 8, 8–20. [Google Scholar] [CrossRef]
- Deyo, R.A.; Mirza, S.K.; Martin, B.I.; Kreuter, W.; Goodman, D.C.; Jarvik, J.G. Trends, Major Medical Complications, and Charges Associated with Surgery for Lumbar Spinal Stenosis in Older Adults. JAMA 2010, 303, 1259–1265. [Google Scholar] [CrossRef] [Green Version]
- Raad, M.; Reidler, J.S.; El Dafrawy, M.H.; Amin, R.M.; Jain, A.; Neuman, B.J.; Riley, L.H.; Sciubba, D.M.; Kebaish, K.M.; Skolasky, R.L. US Regional Variations in Rates, Outcomes, and Costs of Spinal Arthrodesis for Lumbar Spinal Stenosis in Working Adults Aged 40–65 Years. J. Neurosurg. Spine 2018, 30, 83–90. [Google Scholar] [CrossRef]
- Bae, H.W.; Rajee, S.S.; Kanim, L.E. Nationwide Trends in the Surgical Management of Lumbar Spinal Stenosis. Spine 2013, 38, 916–926. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Pietrobon, R.; Sun, S.X.; Liu, G.G.; Hey, L. Estimates and Patterns of Direct Health Care Expenditures among Individuals with Back Pain in the United States. Spine 1976, 29, 86. [Google Scholar] [CrossRef] [PubMed]
- Emanuel, E.J.; Fuchs, V.R. The Perfect Storm of Overutilization. JAMA 2008, 299, 2789–2791. [Google Scholar] [CrossRef] [PubMed]
- Modhia, U.; Takemoto, S.; Braid-Forbes, M.J.; Weber, M.; Berven, S.H. Readmission Rates after Decompression Surgery in Patients with Lumbar Spinal Stenosis among Medicare Beneficiaries. Spine 2013, 38, 591–596. [Google Scholar] [CrossRef]
- Martin, B.I.; Mirza, S.K.; Flum, D.R.; Wickizer, T.M.; Heagerty, P.J.; Lenkoski, A.F.; Deyo, R.A. Repeat Surgery after Lumbar Decompression for Herniated Disc: The Quality Implications of Hospital and Surgeon Variation. Spine J. 2012, 12, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Yeom, J.S.; Buchowski, J.M.; Shen, H.X.; Liu, G.; Bunmaprasert, T.; Riew, K.D. Effect of Fibrin Sealant on Drain Output and Duration of Hospitalization after Multilevel Anterior Cervical Fusion: A Retrospective Matched Pair Analysis. Spine 2008, 33, E543–E547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epstein, N.E.; Schwall, G.; Reillly, T.; Insinna, T.; Bahnken, A.; Hood, D.C. Surgeon Choices, and the Choice of Surgeons, Affect Total Hospital Charges for Single-Level Anterior Cervical Surgery. Spine 2011, 36, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, J.N.; Lurie, J.D.; Olson, P.; Bronner, K.K.; Fisher, E.S.; Morgan, M.T.S. United States Trends and Regional Variations in Lumbar Spine Surgery: 1992–2003. Spine 2006, 31, 2707. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.N.; Lipson, S.J.; Lew, R.A.; Grobler, L.J.; Weinstein, J.N.; Brick, G.W.; Fossel, A.H.; Liang, M.H. Lumbar Laminectomy Alone or with Instrumented or Noninstrumented Arthrodesis in Degenerative Lumbar Spinal Stenosis: Patient Selection, Costs, and Surgical Outcomes. Spine 1997, 22, 1123–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phan, K.; Kim, J.; Di Capua, J.; Lee, N.J.; Kothari, P.; Dowdell, J.; Overley, S.C.; Guzman, J.Z.; Cho, S.K. Impact of Operation Time on 30-Day Complications after Adult Spinal Deformity Surgery. Glob. Spine J. 2017, 7, 664–671. [Google Scholar] [CrossRef]
- Klineberg, E.O.; Passias, P.G.; Jalai, C.M.; Worley, N.; Sciubba, D.M.; Burton, D.C.; Gupta, M.C.; Soroceanu, A.; Zebala, L.P.; Mundis, G.M. Predicting Extended Length of Hospital Stay in an Adult Spinal Deformity Surgical Population. Spine 2016, 41, 798–805. [Google Scholar] [CrossRef] [Green Version]
- Saravi, B.; Lang, G.; Ülkümen, S.; Südkamp, N.; Hassel, F. Case-Matched Radiological and Clinical Outcome Evaluation of Interlaminar versus Microsurgical Decompression of Lumbar Spinal Stenosis. In Proceedings of the German Congress of Orthopaedics and Traumatology, Berlin, Germany, 26–29 October 2021. [Google Scholar] [CrossRef]
- Saravi, B.; Hassel, F.; Ülkümen, S.; Zink, A.; Shavlokhova, V.; Couillard-Despres, S.; Boeker, M.; Obid, P.; Lang, G.M. Artificial Intelligence-Driven Prediction Modeling and Decision Making in Spine Surgery Using Hybrid Machine Learning Models. J. Pers. Med. 2022, 12, 509. [Google Scholar] [CrossRef]
- Gellman, D.D. Cost-Benefit in Health Care: We Need to Know Much More. Can. Med. Assoc. J. 1974, 111, 988–989. [Google Scholar]
- Dagenais, S.; Roffey, D.M.; Wai, E.K.; Haldeman, S.; Caro, J. Can Cost Utility Evaluations Inform Decision Making about Interventions for Low Back Pain? Spine J. 2009, 9, 944–957. [Google Scholar] [CrossRef]
- Krell, R.W.; Girotti, M.; Dimick, J.B. Extended Hospital Stay after Surgery: A Marker of Hospital Quality or Efficiency? J. Surg. Res. 2013, 179, 219. [Google Scholar] [CrossRef]
- Bottle, A.; Middleton, S.; Kalkman, C.J.; Livingston, E.H.; Aylin, P. Global Comparators Project: International Comparison of Hospital Outcomes Using Administrative Data. Health Serv. Res. 2013, 48, 2081–2100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoy, D.; March, L.; Brooks, P.; Blyth, F.; Woolf, A.; Bain, C.; Williams, G.; Smith, E.; Vos, T.; Barendregt, J.; et al. The Global Burden of Low Back Pain: Estimates from the Global Burden of Disease 2010 Study. Ann. Rheum. Dis. 2014, 73, 968–974. [Google Scholar] [CrossRef] [Green Version]
- Deyo, R.A.; Mirza, S.K.; Turner, J.A.; Martin, B.A. Overtreating Chronic Back Pain: Time to Back Off? J. Am. Board Fam. Med. 2009, 22, 62–68. [Google Scholar] [CrossRef] [Green Version]
- Friedly, J.; Standaert, C.; Chan, L. Epidemiology of Spine Care: The Back Pain Dilemma. Phys. Med. Rehabil. Clin. N. Am. 2010, 21, 659–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, B.I.; Turner, J.A.; Mirza, S.K.; Lee, M.J.; Comstock, B.A.; Deyo, R.A. Trends in Health Care Expenditures, Utilization, Ad Health Status among US Adults with Spine Problems, 1997–2006. Spine 2009, 34, 2077–2084. [Google Scholar] [CrossRef] [PubMed]
- Freburger, J.K.; Holmes, G.M.; Agans, R. The Rising Prevalence of Chronic Low Back Pain. Arch. Intern. Med. 2009, 169, 251–258. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, I.M.; Hostin, R.A.; O’Brien, M.F.; Fleming, N.S.; Ogola, G.; Kudyakov, R.; Richter, K.M.; Saigal, R.; Berven, S.H.; Ames, C.P. Analysis of the Direct Cost of Surgery for Four Diagnostic Categories of Adult Spinal Deformity. Spine J. 2013, 13, 1843–1848. [Google Scholar] [CrossRef]
- Shields, L.B.; Clark, L.; Glassman, S.D.; Shields, C.B. Decreasing Hospital Length of Stay Following Lumbar Fusion Utilizing Multidisciplinary Committee Meetings Involving Surgeons and Other Caretakers. Surg. Neurol. Int. 2017, 8, 5. [Google Scholar] [CrossRef]
- Ansari, S.F.; Yan, H.; Zou, J.; Worth, R.M.; Barbaro, N.M. Hospital Length of Stay and Readmission Rate for Neurosurgical Patients. Neurosurgery 2018, 82, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Gruskay, J.A.; Fu, M.; Bohl, D.D.; Webb, M.L.; Grauer, J.N. Factors Affecting Length of Stay after Elective Posterior Lumbar Spine Surgery: A Multivariate Analysis. Spine J. 2015, 15, 1188–1195. [Google Scholar] [CrossRef]
- Linzey, J.R.; Kahn, E.N.; Shlykov, M.A.; Johnson, K.T.; Sullivan, K.; Pandey, A.S. Length of Stay Beyond Medical Readiness in Neurosurgical Patients: A Prospective Analysis. Neurosurgery 2019, 85, 60–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siccoli, A.; de Wispelaere, M.P.; Schröder, M.L. Machine Learning– Based Preoperative Predictive Analytics for Lumbar Spinal Stenosis. Neurosurg. Focus 2019, 46, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biron, D.R.; Sinha, I.; Kleiner, J.E. A Novel Machine Learning Model Developed to Assist in Patient Selection for Outpatient Total Shoulder Arthroplasty. J. Am. Acad. Orthop. Surg. 2019, 28, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Navarro, S.M.; Wang, E.Y.; Haeberle, H.S. Machine Learning and Primary Total Knee Arthroplasty: Patient Forecasting for a Patient-Specific Payment Model. J. Arthroplasty 2018, 33, 3617–3623. [Google Scholar] [CrossRef] [PubMed]
- Durand, W.M.; DePasse, J.M.; Daniels, A.H. Predictive Modeling for Blood Transfusion after Adult Spinal Deformity Surgery: A Tree-Based Machine Learning Approach. Spine 2018, 43, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Fontana, M.A.; Lyman, S.; Sarker, G.K.; Padgett, D.E.; MacLean, C.H. Can Machine Learning Algorithms Predict Which Patients Will Achieve Minimally Clinically Important Differences from Total Joint Arthroplasty? Clin. Orthop. Relat. Res. Lippincott Williams Wilkins 2019, 477, 1267–1279. [Google Scholar] [CrossRef] [Green Version]
- Malik, A.T.; Khan, S.N. Predictive Modeling in Spine Surgery. Ann. Transl. Med. 2019, 7, 173. [Google Scholar] [CrossRef]
- Kobayashi, K.; Ando, K.; Kato, F.; Kanemura, T.; Sato, K.; Hachiya, Y.; Matsubara, Y.; Kamiya, M.; Sakai, Y.; Yagi, H. Predictors of Prolonged Length of Stay after Lumbar Interbody Fusion: A Multicenter Study. Glob. Spine J. 2019, 9, 466–472. [Google Scholar] [CrossRef] [Green Version]
- Adogwa, O.; Lilly, D.T.; Khalid, S.; Desai, S.A.; Vuong, V.D.; Davison, M.A.; Ouyang, B.; Bagley, C.A.; Cheng, J. Extended Length of Stay after Lumbar Spine Surgery: Sick Patients, Postoperative Complications, or Practice Style Differences among Hospitals and Physicians? World Neurosurg. 2019, 123, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.D.; Hsu, W.K.; De Oliveira, G.S.; Saha, S.; Kim, J.Y.S. Operative Duration as an Independent Risk Factor for Postoperative Complications in Single-Level Lumbar Fusion: An Analysis of 4588 Surgical Cases. Spine 2014, 39, 510–520. [Google Scholar] [CrossRef]
- Dibra, F.F.; Silverberg, A.J.; Vasilopoulos, T.; Gray, C.F.; Parvataneni, H.K.; Prieto, H.A. Arthroplasty Care Redesign Impacts the Predictive Accuracy of the Risk Assessment and Prediction Tool. J. Arthroplast. 2019, 34, 2549–2554. [Google Scholar] [CrossRef] [PubMed]
- Vigushin, D.M.; Pepys, M.B.; Hawkins, P.N. Metabolic and Scintigraphic Studies of Radioiodinated Human C-Reactive Protein in Health and Disease. J. Clin. Investig. 1993, 91, 1351–1357. [Google Scholar] [CrossRef]
- Pepys, M.B.; Hirschfield, G.M. C-Reactive Protein: A Critical Update. J. Clin. Investig. 2003, 111, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M. Clinical Application of C-Reactive Protein for Cardiovascular Disease Detection and Prevention. Circulation 2003, 107, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Colley, C.M.; Fleck, A.; Goode, A.W.; Muller, B.R.; Myers, M.A. Early Time Course of the Acute Phase Protein Response in Man. J. Clin. Pathol. 1983, 36, 203–207. [Google Scholar] [CrossRef] [Green Version]
- White, J.; Kelly, M.; Dunsmuir, R. C-Reactive Protein Level after Total Hip and Total Knee Replacement. J. Bone Jt. Surg. Br. 1998, 80, 909–911. [Google Scholar] [CrossRef]
- Perry, T.E.; Muehlschlegel, J.D.; Liu, K.-Y.; Fox, A.A.; Collard, C.D.; Body, S.C.; Shernan, S.K. CABG Genomics Investigators Preoperative C-Reactive Protein Predicts Long-Term Mortality and Hospital Length of Stay after Primary, Nonemergent Coronary Artery Bypass Grafting. Anesthesiology 2010, 112, 607–613. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, H.J.; Christensen, I.J.; Sørensen, S.; Moesgaard, F.; Brünner, N. Preoperative Plasma Plasminogen Activator Inhibitor Type-1 and Serum C-Reactive Protein Levels in Patients with Colorectal Cancer. The RANX05 Colorectal Cancer Study Group. Ann. Surg. Oncol. 2000, 7, 617–623. [Google Scholar] [CrossRef]
- Nozoe, T.; Matsumata, T.; Kitamura, M.; Sugimachi, K. Significance of Preoperative Elevation of Serum C-Reactive Protein as an Indicator for Prognosis in Colorectal Cancer. Am. J. Surg. 1998, 176, 335–338. [Google Scholar] [CrossRef]
- Fransen, E.J.; Maessen, J.G.; Elenbaas, T.W.; van Aarnhem, E.E.; van Dieijen-Visser, M.P. Enhanced Preoperative C-Reactive Protein Plasma Levels as a Risk Factor for Postoperative Infections after Cardiac Surgery. Ann. Thorac. Surg. 1999, 67, 134–138. [Google Scholar] [CrossRef]
- Brewster, N.; Guthrie, C.; McBirnie, J. CRP Levels as a Measure of Surgical Trauma: A Comparison of Different General Surgical Procedures. J. R. Coll. Surg. Edinb. 1994, 39, 86–88. [Google Scholar] [PubMed]
- Grande, M.; Tucci, G.F.; Adorisio, O.; Barini, A.; Rulli, F.; Neri, A.; Franchi, F.; Farinon, A.M. Systemic Acute-Phase Response after Laparoscopic and Open Cholecystectomy. Surg. Endosc. 2002, 16, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, U.; Kessler, K.; Plusczyk, T.; Pistorius, G.; Vollmar, B.; Menger, M.D. Comparison of Surgical Stress between Laparoscopic and Open Colonic Resections. Surg. Endosc. 2003, 17, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Saravi, B.; Ülkümen, S.; Couillard-Despres, S.; Lang, G.; Hassel, F. One-Year Clinical Outcomes of Minimal-Invasive Dorsal Percutaneous Fixation of Thoracolumbar Spine Fractures. Medicina 2022, 58, 606. [Google Scholar] [CrossRef]
- Cappabianca, G.; Paparella, D.; Visicchio, G.; Capone, G.; Lionetti, G.; Numis, F.; Ferrara, P.; D’Agostino, C.; de Luca Tupputi Schinosa, L. Preoperative C-Reactive Protein Predicts Mid-Term Outcome after Cardiac Surgery. Ann. Thorac. Surg. 2006, 82, 2170–2178. [Google Scholar] [CrossRef] [PubMed]
- Cole, D.S.; Watts, A.; Scott-Coombes, D.; Avades, T. Clinical Utility of Peri-Operative C-Reactive Protein Testing in General Surgery. Ann. R. Coll. Surg. Engl. 2008, 90, 317–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
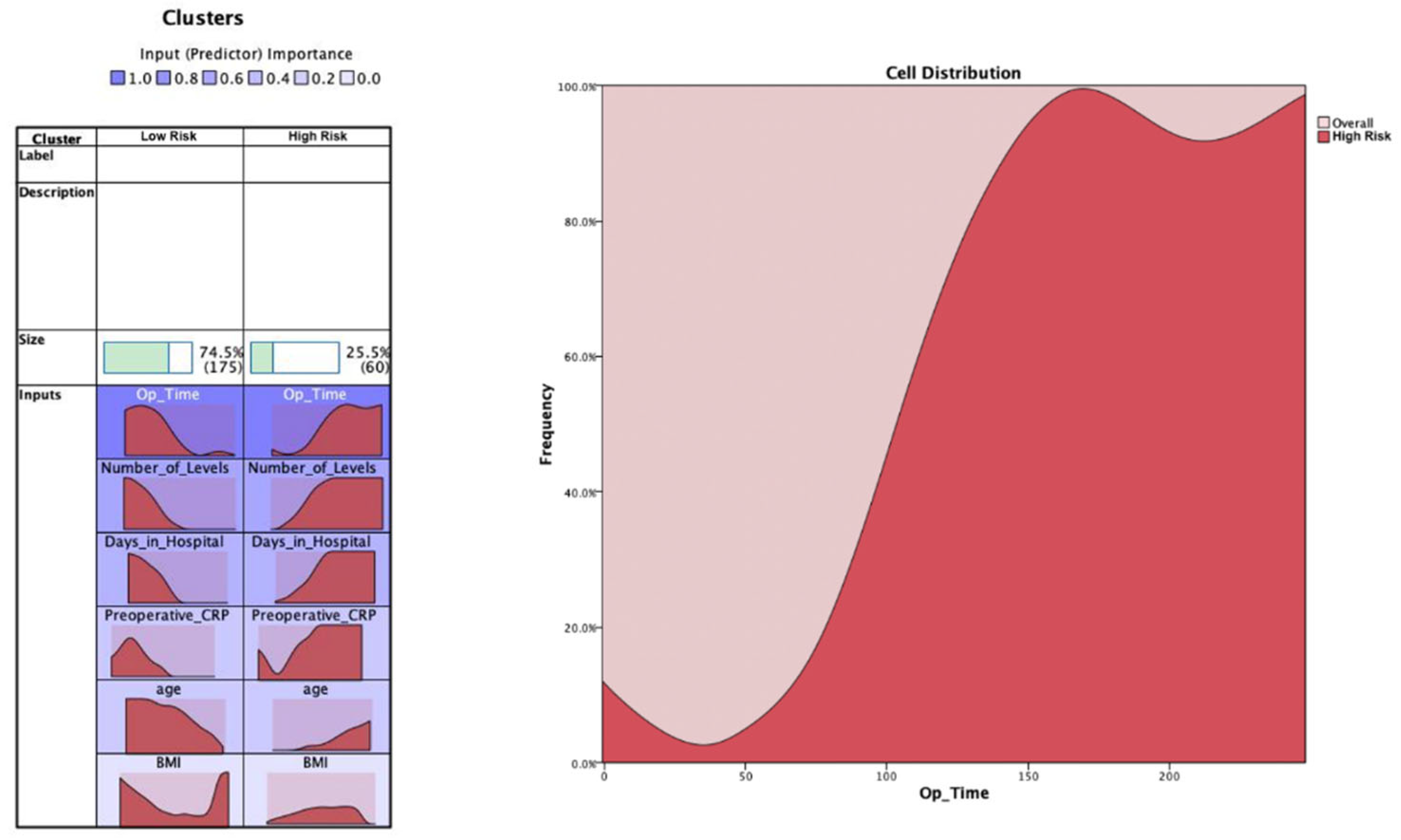






| BMI | Number_of_Levels | Op_Time | Preoperative_CRP | Age | Days_in_Hospital | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Std. Deviation | Mean | Std. Deviation | Mean | Std. Deviation | Mean | Std. Deviation | Mean | Std. Deviation | Mean | Std. Deviation | ||
| Cluster | Low risk | 27.39 | 5.670 | 1.04 | 0.242 | 55.04 | 29.086 | 4.45 | 13.325 | 57.2366 | 16.37208 | 11.70 | 5.482 |
| High risk | 28.75 | 5.145 | 1.92 | 0.973 | 137.63 | 51.662 | 40.90 | 66.902 | 72.2849 | 11.97116 | 24.27 | 14.429 | |
| Combined | 27.74 | 5.562 | 1.27 | 0.656 | 76.13 | 51.034 | 13.76 | 38.912 | 61.0787 | 16.69396 | 14.91 | 10.247 | |
| p-value | **** | **** | **** | **** | **** | **** | **** | **** | **** | **** | **** | **** | |
| BMI | Number_of_Levels | Op_Time | Preoperative_CRP | Age | Frequencies | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Std. Deviation | Mean | Std. Deviation | Mean | Std. Deviation | Mean | Std. Deviation | Mean | Std. Deviation | Normal | Prolonged | ||
| Cluster | High risk | 28.31 | 5.649 | 1.62 | 1.022 | 104.92 | 58.253 | 23.72 | 51.250 | 73.2094 | 12.37884 | 0 | 63 |
| Low risk | 27.53 | 5.531 | 1.14 | 0.382 | 65.58 | 43.776 | 10.11 | 32.706 | 56.6354 | 15.86603 | 172 | 0 | |
| Combined | 27.74 | 5.562 | 1.27 | 0.656 | 76.13 | 51.034 | 13.76 | 38.912 | 61.0787 | 16.69396 | 172 | 63 | |
| p-value | **** | **** | **** | **** | **** | **** | **** | **** | **** | **** | **** | ||
| Algorithm | Accuracy | AUC |
|---|---|---|
| LogisticRegression | 0.814 | 0.814 |
| Random Forest classifier | 0.808 | 0.826 |
| SGD classifier | 0.771 | 0.804 |
| K-nearest neighbors | 0.755 | 0.769 |
| Decision Trees classifier | 0.739 | 0.675 |
| GaussianNB | 0.755 | 0.799 |
| SVM | 0.771 | 0.794 |
| Custom CNN | 0.771 | 0.862 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saravi, B.; Zink, A.; Ülkümen, S.; Couillard-Despres, S.; Hassel, F.; Lang, G. Performance of Artificial Intelligence-Based Algorithms to Predict Prolonged Length of Stay after Lumbar Decompression Surgery. J. Clin. Med. 2022, 11, 4050. https://doi.org/10.3390/jcm11144050
Saravi B, Zink A, Ülkümen S, Couillard-Despres S, Hassel F, Lang G. Performance of Artificial Intelligence-Based Algorithms to Predict Prolonged Length of Stay after Lumbar Decompression Surgery. Journal of Clinical Medicine. 2022; 11(14):4050. https://doi.org/10.3390/jcm11144050
Chicago/Turabian StyleSaravi, Babak, Alisia Zink, Sara Ülkümen, Sebastien Couillard-Despres, Frank Hassel, and Gernot Lang. 2022. "Performance of Artificial Intelligence-Based Algorithms to Predict Prolonged Length of Stay after Lumbar Decompression Surgery" Journal of Clinical Medicine 11, no. 14: 4050. https://doi.org/10.3390/jcm11144050







