Dyslipidemia in Transplant Patients: Which Therapy?
Abstract
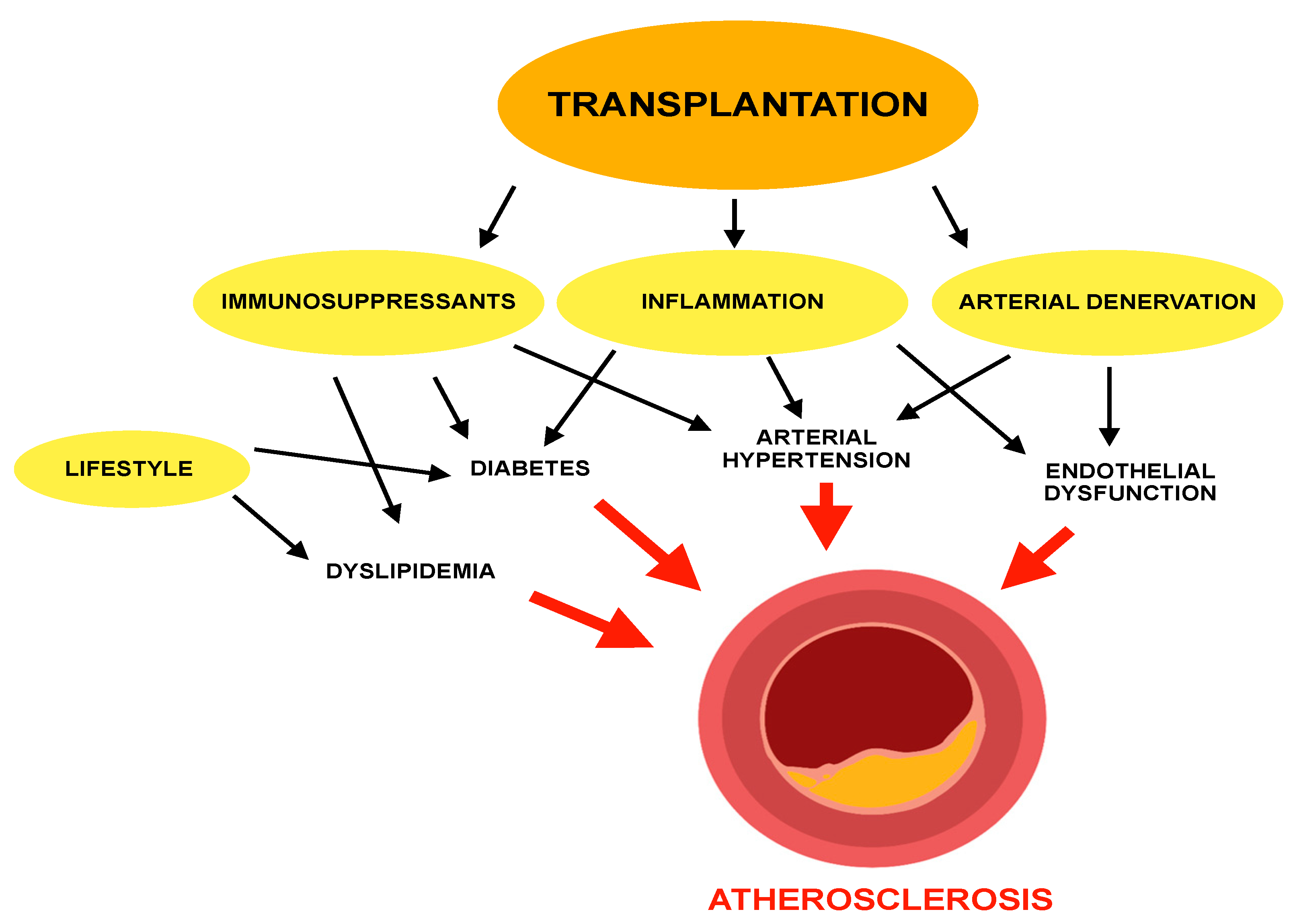
:1. Introduction
2. Role of Dyslipidemia in Atherosclerosis
3. Dyslipidemia and Atherosclerosis in Transplant Recipients
4. Immunosuppressants Effect on Dyslipidemia and Other CVD Risk Factors
5. Management of Dyslipidemia in Transplant Recipients
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Friera, L.; Peñalvo, J.L.; Fernández-Ortiz, A.; Ibañez, B.; López-Melgar, B.; Laclaustra, M.; Oliva, B.; Mocoroa, A.; Mendiguren, J.; Martínez de Vega, V.; et al. Prevalence, Vascular Distribution, and Multiterritorial Extent of Subclinical Atherosclerosis in a Middle-Aged Cohort: The PESA (Progression of Early Subclinical Atherosclerosis) Study. Circulation 2015, 131, 2104–2113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha, V.Z.; Libby, P. Obesity, inflammation, and atherosclerosis. Nat. Rev. Cardiol. 2009, 6, 399–409. [Google Scholar] [CrossRef]
- Després, J.P. Body fat distribution and risk of cardiovascular disease: An update. Circulation 2012, 126, 1301–1313. [Google Scholar] [CrossRef] [Green Version]
- Messner, B.; Bernhard, D. Smoking and cardiovascular disease: Mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 509–515. [Google Scholar] [CrossRef] [Green Version]
- Poznyak, A.; Grechko, A.V.; Poggio, P.; Myasoedova, V.A.; Alfieri, V.; Orekhov, A.N. The Diabetes Mellitus-Atherosclerosis Connection: The Role of Lipid and Glucose Metabolism and Chronic Inflammation. Int. J. Mol. Sci. 2020, 21, E1835. [Google Scholar] [CrossRef] [Green Version]
- Recio-Mayoral, A.; Banerjee, D.; Streather, C.; Kaski, J.C. Endothelial dysfunction, inflammation and atherosclerosis in chronic kidney disease—A cross-sectional study of predialysis, dialysis and kidney-transplantation patients. Atherosclerosis 2011, 216, 446–451. [Google Scholar] [CrossRef]
- Israni, A.K.; Snyder, J.J.; Skeans, M.A.; Peng, Y.; Maclean, J.R.; Weinhandl, E.D.; Kasiske, B.L.; PORT Investigators. Predicting coronary heart disease after kidney transplantation: Patient Outcomes in Renal Transplantation (PORT) Study. Am. J. Transplant. 2010, 10, 338–353. [Google Scholar] [CrossRef]
- Kobashigawa, J.A.; Starling, R.C.; Mehra, M.R.; Kormos, R.L.; Bhat, G.; Barr, M.L.; Sigouin, C.S.; Kolesar, J.; Fitzsimmons, W. Multicenter retrospective analysis of cardiovascular risk factors affecting long-term outcome of de novo cardiac transplant recipients. J. Heart Lung Transplant. 2006, 25, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Albeldawi, M.; Aggarwal, A.; Madhwal, S.; Cywinski, J.; Lopez, R.; Eghtesad, B.; Zein, N.N. Cumulative risk of cardiovascular events after orthotopic liver transplantation. Liver Transpl. 2012, 18, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Warden, B.A.; Duell, P.B. Management of dyslipidemia in adult solid organ transplant recipients. J. Clin. Lipidol. 2019, 13, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Patel, C.B.; Holley, C.L. Cardiac Allograft Vasculopathy: A Formidable Foe. J. Am. Coll. Cardiol. 2019, 74, 52–53. [Google Scholar] [CrossRef]
- Hüsing, A.; Kabar, I.; Schmidt, H.H. Lipids in liver transplant recipients. World J. Gastroenterol. 2016, 22, 3315–3324. [Google Scholar] [CrossRef]
- Kockx, M.; Kritharides, L. Hyperlipidaemia in immunosuppression. Curr. Opin. Lipidol. 2016, 27, 631–632. [Google Scholar] [CrossRef]
- Rodríguez, F.O.R.; Santiago, J.C.; Jiménez, G.M.; Carreño Rodríguez, Y.R.; Meléndez, A.R.; Medina Uicab, C.J.; Salas, L.N.; Quiñones Gamero, M.A.; Ramírez, C.D.R.G.; Covarrubias, L.G.; et al. Post-Transplant Cholesterol and Triglyceride Behavior: Effects of Sex, Age of the Recipient, and Type of Donor. Transplant. Proc. 2020, 52, 1157–1162. [Google Scholar] [CrossRef]
- Ference, B.A.; Kastelein, J.J.P.; Ray, K.K.; Ginsberg, H.N.; Chapman, M.J.; Packard, C.J.; Laufs, U.; Oliver-Williams, C.; Wood, A.M.; Butterworth, A.S.; et al. Association of Triglyceride-Lowering LPL Variants and LDL-C-Lowering LDLR Variants with Risk of Coronary Heart Disease. JAMA 2019, 321, 364–373. [Google Scholar] [CrossRef] [Green Version]
- Ajala, O.N.; Demler, O.V.; Liu, Y.; Farukhi, Z.; Adelman, S.J.; Collins, H.L.; Ridker, P.M.; Rader, D.J.; Glynn, R.J.; Mora, S. Anti-Inflammatory HDL Function, Incident Cardiovascular Events, and Mortality: A Secondary Analysis of the JUPITER Randomized Clinical Trial. J. Am. Heart Assoc. 2020, 9, e016507. [Google Scholar] [CrossRef]
- AIM-HIGH Investigators; Boden, W.E.; Probstfield, J.L.; Anderson, T.; Chaitman, B.R.; Desvignes-Nickens, P.; Koprowicz, K.; McBride, R.; Teo, K.; Weintraub, W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N. Engl. J. Med. 2011, 365, 2255–2267. [Google Scholar] [CrossRef] [Green Version]
- Van der Valk, F.M.; Bekkering, S.; Kroon, J.; Yeang, C.; Van den Bossche, J.; van Buul, J.D.; Ravandi, A.; Nederveen, A.J.; Verberne, H.J.; Scipione, C.; et al. Oxidized Phospholipids on Lipoprotein(a) Elicit Arterial Wall Inflammation and an Inflammatory Monocyte Response in Humans. Circulation 2016, 134, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Gentile, M.; Simeon, V.; Iannuzzo, G.; Mattiello, A.; Donata di Taranto, M.; Panico, S.; Rubba, P. Lipoprotein (a) is an independent predictor of cardiovascular events in Mediterranean women (Progetto Atena). Eur. J. Prev. Cardiol. 2020, 27, 2248–2250. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, B.G.; Chapman, M.J.; Ray, K.; Borén, J.; Andreotti, F.; Watts, G.F.; Ginsberg, H.; Amarenco, P.; Catapano, A.; Descamps, O.S.; et al. Lipoprotein(a) as a cardiovascular risk factor: Current status. Eur. Heart J. 2010, 31, 2844–2853. [Google Scholar] [CrossRef]
- O’Donoghue, M.L.; Fazio, S.; Giugliano, R.P.; Stroes, E.S.G.; Kanevsky, E.; Gouni-Berthold, I.; Im, K.; Lira Pineda, A.; Wasserman, S.M.; Češka, R.; et al. Lipoprotein(a), PCSK9 Inhibition, and Cardiovascular Risk. Circulation 2019, 139, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- Iannuzzo, G.; Gentile, M.; Bresciani, A.; Mallardo, V.; Di Lorenzo, A.; Merone, P.; Cuomo, G.; Pacileo, M.; Sarullo, F.M.; Venturini, E.; et al. Inhibitors of Protein Convertase Subtilisin/Kexin 9 (PCSK9) and Acute Coronary Syndrome (ACS): The State-of-the-Art. J. Clin. Med. 2021, 10, 1510. [Google Scholar] [CrossRef] [PubMed]
- Navab, M.; Ananthramaiah, G.M.; Reddy, S.T.; Van Lenten, B.J.; Ansell, B.J.; Fonarow, G.C.; Vahabzadeh, K.; Hama, S.; Hough, G.; Kamranpour, N.; et al. The oxidation hypothesis of atherogenesis: The role of oxidized phospholipids and HDL. J. Lipid Res. 2004, 45, 993–1007. [Google Scholar] [CrossRef] [Green Version]
- Ridker, P.M. A Test in Context: High-Sensitivity C-Reactive Protein. J. Am. Coll. Cardiol. 2016, 67, 712–723. [Google Scholar] [CrossRef]
- Libby, P.; Lichtman, A.H.; Hansson, G.K. Immune effector mechanisms implicated in atherosclerosis: From mice to humans. Immunity 2013, 38, 1092–1104. [Google Scholar] [CrossRef] [Green Version]
- Nus, M.; Mallat, Z. Immune-mediated mechanisms of atherosclerosis and implications for the clinic. Expert Rev. Clin. Immunol. 2016, 12, 1217–1237. [Google Scholar] [CrossRef]
- Gimbrone, M.A.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef] [Green Version]
- Di Minno, A.; Gentile, M.; Iannuzzo, G.; Calcaterra, I.; Tripaldella, M.; Porro, B.; Cavalca, V.; Di Taranto, M.D.; Tremoli, E.; Fortunato, G.; et al. Endothelial function improvement in patients with familial hypercholesterolemia receiving PCSK-9 inhibitors on top of maximally tolerated lipid lowering therapy. Thromb. Res. 2020, 194, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Chatzizisis, Y.S.; Coskun, A.U.; Jonas, M.; Edelman, E.R.; Feldman, C.L.; Stone, P.H. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: Molecular, cellular, and vascular behavior. J. Am. Coll. Cardiol. 2007, 49, 2379–2393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz, J.L.; Hutcheson, J.D.; Aikawa, E. Cardiovascular calcification: Current controversies and novel concepts. Cardiovasc. Pathol. 2015, 24, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Virmani, R.; Younis, H.; Burke, A.P.; Kamm, R.D.; Lee, R.T. The impact of calcification on the biomechanical stability of atherosclerotic plaques. Circulation 2001, 103, 1051–1056. [Google Scholar] [CrossRef] [Green Version]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef]
- Libby, P. Mechanisms of acute coronary syndromes and their implications for therapy. N. Engl. J. Med. 2013, 368, 2004–2013. [Google Scholar] [CrossRef] [Green Version]
- Kranzhöfer, R.; Browatzki, M.; Schmidt, J.; Kübler, W. Angiotensin II activates the proinflammatory transcription factor nuclear factor-kappaB in human monocytes. Biochem. Biophys. Res. Commun. 1999, 257, 826–828. [Google Scholar] [CrossRef]
- Tsai, H.I.; Liu, F.C.; Lee, C.W.; Kuo, C.F.; See, L.C.; Chung, T.T.; Yu, H.P. Cardiovascular disease risk in patients receiving organ transplantation: A national cohort study. Transpl. Int. 2017, 30, 1161–1171. [Google Scholar] [CrossRef] [Green Version]
- Bostom, A.D.; Brown, R.S.; Chavers, B.M.; Coffman, T.M.; Cosio, F.G.; Culver, K.; Curtis, J.J.; Danovitch, G.M.; Everson, G.T.; First, M.R.; et al. Prevention of post-transplant cardiovascular disease—Report and recommendations of an ad hoc group. Am. J. Transplant. 2002, 2, 491–500. [Google Scholar] [CrossRef]
- Gansevoort, R.T.; Correa-Rotter, R.; Hemmelgarn, B.R.; Jafar, T.H.; Heerspink, H.J.; Mann, J.F.; Matsushita, K.; Wen, C.P. Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet 2013, 382, 339–352. [Google Scholar] [CrossRef]
- Weiner, D.E.; Tighiouart, H.; Elsayed, E.F.; Griffith, J.L.; Salem, D.N.; Levey, A.S.; Sarnak, M.J. The Framingham predictive instrument in chronic kidney disease. J. Am. Coll. Cardiol. 2007, 50, 217–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansell, H.; Stewart, S.A.; Shoker, A. Validity of cardiovascular risk prediction models in kidney transplant recipients. Sci. World J. 2014, 2014, 750579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Markell, M.S.; Armenti, V.; Danovitch, G.; Sumrani, N. Hyperlipidemia and glucose intolerance in the post-renal transplant patient. J. Am. Soc. Nephrol. 1994, 4, S37–S47. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.M.; Markakis, M.; Sension, M.; Vitalis, S.; Baughman, K.; Swank, R.; Kwiterovich, P.O.; Pearson, T.A.; Achuff, S.; Baumgartner, W.A.; et al. Prevalence of hyperlipidemia in heart transplant recipients. Transplantation 1987, 44, 323–325. [Google Scholar] [CrossRef]
- Pan, L.; Yang, Z.; Wu, Y.; Yin, R.X.; Liao, Y.; Wang, J.; Gao, B.; Zhang, L.; China National Survey of Chronic Kidney Disease Working Group. The prevalence, awareness, treatment and control of dyslipidemia among adults in China. Atherosclerosis 2016, 248, 2–9. [Google Scholar] [CrossRef]
- Tóth, P.P.; Potter, D.; Ming, E.E. Prevalence of lipid abnormalities in the United States: The National Health and Nutrition Examination Survey 2003–2006. J. Clin. Lipidol. 2012, 6, 325–330. [Google Scholar] [CrossRef]
- González-Amieva, A.; López-Miranda, J.; Marín, C.; Pérez-Martinez, P.; Gómez, P.; Paz-Rojas, E.; Arizón, J.M.; Jiménez-Perepérez, J.A.; Concha, M.; Pérez-Jiménez, F. The apo A-I gene promoter region polymorphism determines the severity of hyperlipidemia after heart transplantation. Clin. Transplant. 2003, 17, 56–62. [Google Scholar] [CrossRef]
- Taegtmeyer, A.B.; Breen, J.B.; Smith, J.; Rogers, P.; Kullak-Ublick, G.A.; Yacoub, M.H.; Banner, N.R.; Barton, P.J. Effect of ABCB1 genotype on pre- and post-cardiac transplantation plasma lipid concentrations. J. Cardiovasc. Transl. Res. 2011, 4, 304–312. [Google Scholar] [CrossRef]
- Numakura, K.; Kagaya, H.; Yamamoto, R.; Komine, N.; Saito, M.; Hiroshi, T.; Akihama, S.; Inoue, T.; Narita, S.; Tsuchiya, N.; et al. Characterization of clinical and genetic risk factors associated with dyslipidemia after kidney transplantation. Dis. Markers 2015, 2015, 179434. [Google Scholar] [CrossRef]
- Pinto, A.S.; Chedid, M.F.; Guerra, L.T.; Cabeleira, D.D.; Kruel, C.D.P. Dietary management for dyslipidemia in liver transplant recipients. Arq. Bras. Cir. Dig. 2016, 29, 246–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, L.W. Cardiovascular toxicities of immunosuppressive agents. Am. J. Transplant. 2002, 2, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Claes, K.; Meier-Kriesche, H.U.; Schold, J.D.; Vanrenterghem, Y.; Halloran, P.F.; Ekberg, H. Effect of different immunosuppressive regimens on the evolution of distinct metabolic parameters: Evidence from the Symphony study. Nephrol. Dial. Transplant. 2012, 27, 850–857. [Google Scholar] [CrossRef] [Green Version]
- Miller, L.W.; Schlant, R.C.; Kobashigawa, J.; Kubo, S.; Renlund, D.G. 24th Bethesda conference: Cardiac transplantation. Task Force 5: Complications. J. Am. Coll. Cardiol. 1993, 22, 41–54. [Google Scholar] [CrossRef] [Green Version]
- Sander, M.; Lyson, T.; Thomas, G.D.; Victor, R.G. Sympathetic neural mechanisms of cyclosporine-induced hypertension. Am. J. Hypertens. 1996, 9, 121S–138S. [Google Scholar] [CrossRef]
- Wang, J.; Guo, R.; Liu, S.; Chen, Q.; Zuo, S.; Yang, M.; Zuo, X. Molecular mechanisms of FK506-induced hypertension in solid organ transplantation patients. Chin. Med. J. 2014, 127, 3645–3650. [Google Scholar]
- Singer, D.R.; Jenkins, G.H. Hypertension in transplant recipients. J. Hum. Hypertens. 1996, 10, 395–402. [Google Scholar]
- Arner, P.; Gunnarsson, R.; Blomdahl, S.; Groth, C.G. Some characteristics of steroid diabetes: A study in renal-transplant recipients receiving high-dose corticosteroid therapy. Diabetes Care 1983, 6, 23–25. [Google Scholar] [CrossRef]
- Drachenberg, C.B.; Klassen, D.K.; Weir, M.R.; Wiland, A.; Fink, J.C.; Bartlett, S.T.; Cangro, C.B.; Blahut, S.; Papadimitriou, J.C. Islet cell damage associated with tacrolimus and cyclosporine: Morphological features in pancreas allograft biopsies and clinical correlation. Transplantation 1999, 68, 396–402. [Google Scholar] [CrossRef]
- Ghanem, H.; van den Dorpel, M.A.; Weimar, W.; Man in ’T Veld, A.J.; El-Kannishy, M.H.; Jansen, H. Increased low density lipoprotein oxidation in stable kidney transplant recipients. Kidney Int. 1996, 49, 488–493. [Google Scholar] [CrossRef] [Green Version]
- Taylor, J.E.; Scott, N.; Hill, A.; Bridges, A.; Henderson, I.S.; Stewart, W.K.; Belch, J.J. Oxygen free radicals and platelet and granulocyte aggregability in renal transplant patients. Transplantation 1993, 55, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, W.H.; Walker, R.J.; Ball, M.J.; Stapley, S.A.; Robertson, M.C. Oxidation of low density lipoproteins from patients with renal failure or renal transplants. Kidney Int. 1995, 48, 227–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosmans, J.L.; Holvoet, P.; Dauwe, S.E.; Ysebaert, D.K.; Chapelle, T.; Jürgens, A.; Kovacic, V.; Van Marck, E.A.; De Broe, M.E.; Verpooten, G.A. Oxidative modification of low-density lipoproteins and the outcome of renal allografts at 1 1/2 years. Kidney Int. 2001, 59, 2346–2356. [Google Scholar] [CrossRef] [PubMed]
- Yates, P.J.; Nicholson, M.L. The aetiology and pathogenesis of chronic allograft nephropathy. Transpl. Immunol. 2006, 16, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Najafian, B.; Kasiske, B.L. Chronic allograft nephropathy. Curr. Opin. Nephrol. Hypertens. 2008, 17, 149–155. [Google Scholar] [CrossRef]
- Ruiz, M.C.; Medina, A.; Moreno, J.M.; Gómez, I.; Ruiz, N.; Bueno, P.; Asensio, C.; Osuna, A. Relationship between oxidative stress parameters and atherosclerotic signs in the carotid artery of stable renal transplant patients. Transplant. Proc. 2005, 37, 3796–3798. [Google Scholar] [CrossRef]
- Urbanowicz, T.K.; Michalak, M.; Gąsecka, A.; Olasińska-Wiśniewska, A.; Perek, B.; Rodzki, M.; Bociański, M.; Jemielity, M. A Risk Score for Predicting Long-Term Mortality Following Off-Pump Coronary Artery Bypass Grafting. J. Clin. Med. 2021, 10, 3032. [Google Scholar] [CrossRef]
- Khush, K.K.; Cherikh, W.S.; Chambers, D.C.; Goldfarb, S.; Hayes, D., Jr.; Kucheryavaya, A.Y.; Levvey, B.J.; Meiser, B.; Rossano, J.W.; Stehlik, J.; et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-fifth Adult Heart Transplantation Report-2018, Focus Theme: Multiorgan Transplantation. J. Heart Lung Transplant. 2018, 37, 1155–1168. [Google Scholar] [CrossRef]
- Lu, W.H.; Palatnik, K.; Fishbein, G.A.; Lai, C.; Levi, D.S.; Perens, G.; Alejos, J.; Kobashigawa, J.; Fishbein, M.C. Diverse morphologic manifestations of cardiac allograft vasculopathy: A pathologic study of 64 allograft hearts. J. Heart Lung Transplant. 2011, 30, 1044–1050. [Google Scholar] [CrossRef]
- Schmauss, D.; Weis, M. Cardiac allograft vasculopathy: Recent developments. Circulation 2008, 117, 2131–2141. [Google Scholar] [CrossRef]
- Lee, F.; Nair, V.; Chih, S. Cardiac allograft vasculopathy: Insights on pathogenesis and therapy. Clin. Transplant. 2020, 34, e13794. [Google Scholar] [CrossRef] [PubMed]
- Nishi, H.; Higashihara, T.; Inagi, R. Lipotoxicity in Kidney, Heart, and Skeletal Muscle Dysfunction. Nutrients 2019, 11, 1664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancuso, P. The role of adipokines in chronic inflammation. Immunotargets Ther. 2016, 5, 47–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamb, K.E.; Lodhi, S.; Meier-Kriesche, H.U. Long-term renal allograft survival in the United States: A critical reappraisal. Am. J. Transplant. 2011, 11, 450–462. [Google Scholar] [CrossRef]
- Cain, D.W.; Cidlowski, J.A. Immune regulation by glucocorticoids. Nat. Rev. Immunol. 2017, 17, 233–247. [Google Scholar] [CrossRef]
- Coutinho, A.E.; Chapman, K.E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell. Endocrinol. 2011, 335, 2–13. [Google Scholar] [CrossRef]
- Miyata, M.; Lee, J.Y.; Susuki-Miyata, S.; Wang, W.Y.; Xu, H.; Kai, H.; Kobayashi, K.S.; Flavell, R.A.; Li, J.D. Glucocorticoids suppress inflammation via the upregulation of negative regulator IRAK-M. Nat. Commun. 2015, 6, 6062. [Google Scholar] [CrossRef] [Green Version]
- Montero, N.; Pascual, J. Immunosuppression and Post-transplant Hyperglycemia. Curr. Diabetes Rev. 2015, 11, 144–154. [Google Scholar] [CrossRef]
- Hays, A.P.; Hill, R.B. Enzymes of lipid synthesis in the liver of the cortisone-treated rat. Biochim. Biophys. Acta 1965, 98, 646–648. [Google Scholar] [CrossRef]
- Bagdade, J.D.; Yee, E.; Albers, J.; Pykalisto, O.J. Glucocorticoids and triglyceride transport: Effects on triglyceride secretion rates, lipoprotein lipase, and plasma lipoproteins in the rat. Metabolism 1976, 25, 533–542. [Google Scholar] [CrossRef]
- Becker, D.M.; Chamberlain, B.; Swank, R.; Hegewald, M.G.; Girardet, R.; Baughman, K.L.; Kwiterovich, P.O.; Pearson, T.A.; Ettinger, W.H.; Renlund, D. Relationship between corticosteroid exposure and plasma lipid levels in heart transplant recipients. Am. J. Med. 1988, 85, 632–638. [Google Scholar] [CrossRef]
- Vathsala, A.; Weinberg, R.B.; Schoenberg, L.; Grevel, J.; Goldstein, R.A.; Van Buren, C.T.; Lewis, R.M.; Kahan, B.D. Lipid abnormalities in cyclosporine-prednisone-treated renal transplant recipients. Transplantation 1989, 48, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Keogh, A.; Macdonald, P.; Harvison, A.; Richens, D.; Mundy, J.; Spratt, P. Initial steroid-free versus steroid-based maintenance therapy and steroid withdrawal after heart transplantation: Two views of the steroid question. J. Heart Lung Transplant. 1992, 11, 421–427. [Google Scholar] [PubMed]
- Pascual, J.; van Hooff, J.P.; Salmela, K.; Lang, P.; Rigotti, P.; Budde, K. Three-year observational follow-up of a multicenter, randomized trial on tacrolimus-based therapy with withdrawal of steroids or mycophenolate mofetil after renal transplant. Transplantation 2006, 82, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Macian, F. NFAT proteins: Key regulators of T-cell development and function. Nat. Rev. Immunol. 2005, 5, 472–484. [Google Scholar] [CrossRef]
- Derfler, K.; Hayde, M.; Heinz, G.; Hirschl, M.M.; Steger, G.; Hauser, A.C.; Balcke, P.; Widhalm, K. Decreased postheparin lipolytic activity in renal transplant recipients with cyclosporin A. Kidney Int. 1991, 40, 720–727. [Google Scholar] [CrossRef] [Green Version]
- Princen, H.M.; Meijer, P.; Wolthers, B.G.; Vonk, R.J.; Kuipers, F. Cyclosporin A blocks bile acid synthesis in cultured hepatocytes by specific inhibition of chenodeoxycholic acid synthesis. Biochem. J. 1991, 275, 501–505. [Google Scholar] [CrossRef] [Green Version]
- De Groen, P.C. Cyclosporine, low-density lipoprotein, and cholesterol. Mayo Clin. Proc. 1988, 63, 1012–1021. [Google Scholar] [CrossRef]
- Apanay, D.C.; Neylan, J.F.; Ragab, M.S.; Sgoutas, D.S. Cyclosporine increases the oxidizability of low-density lipoproteins in renal transplant recipients. Transplantation 1994, 58, 663–669. [Google Scholar] [CrossRef]
- Ballantyne, C.M.; Podet, E.J.; Patsch, W.P.; Harati, Y.; Appel, V.; Gotto, A.M., Jr.; Young, J.B. Effects of cyclosporine therapy on plasma lipoprotein levels. JAMA 1989, 262, 53–56. [Google Scholar] [CrossRef]
- Hricik, D.E.; Mayes, J.T.; Schulak, J.A. Independent effects of cyclosporine and prednisone on posttransplant hypercholesterolemia. Am. J. Kidney Dis. 1991, 18, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Vincenti, F.; Jensik, S.C.; Filo, R.S.; Miller, J.; Pirsch, J. A long-term comparison of tacrolimus (FK506) and cyclosporine in kidney transplantation: Evidence for improved allograft survival at five years. Transplantation 2002, 73, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Vincenti, F.; Friman, S.; Scheuermann, E.; Rostaing, L.; Jenssen, T.; Campistol, J.M.; Uchida, K.; Pescovitz, M.D.; Marchetti, P.; Tuncer, M.; et al. Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus. Am. J. Transplant. 2007, 7, 1506–1514. [Google Scholar] [CrossRef]
- Kotha, S.; Lawendy, B.; Asim, S.; Serón, D.; Burgos, M.D.; Pallardó, L.M.; Kanter, J.; Díaz Corte, C.; Rodríguez, M.; Diaz, J.M.; et al. Impact of immunosuppression on incidence of post-transplant diabetes mellitus in solid organ transplant recipients: Systematic review and meta-analysis. World J. Transplant. 2021, 11, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Hernández, D.; Moreso, F.; Serón, D.; Burgos, M.D.; Pallardó, L.M.; Kanter, J.; Corte, C.D.; Rodríguez, M.; Diaz, J.M.; et al. Randomized Controlled Trial Assessing the Impact of Tacrolimus Versus Cyclosporine on the Incidence of Posttransplant Diabetes Mellitus. Kidney Int. Rep. 2018, 3, 1304–1315. [Google Scholar] [CrossRef] [Green Version]
- Sander, M.; Victor, R.G. Hypertension after cardiac transplantation: Pathophysiology and management. Curr. Opin. Nephrol. Hypertens. 1995, 4, 443–451. [Google Scholar] [CrossRef]
- Taylor, D.O.; Barr, M.L.; Radovancevic, B.; Renlund, D.G.; Mentzer, R.M., Jr.; Smart, F.W.; Tolman, D.E.; Frazier, O.H.; Young, J.B.; VanVeldhuisen, P. A randomized, multicenter comparison of tacrolimus and cyclosporine immunosuppressive regimens in cardiac transplantation: Decreased hyperlipidemia and hypertension with tacrolimus. J. Heart Lung Transplant. 1999, 18, 336–345. [Google Scholar] [CrossRef]
- Bracht, C.; Yan, X.W.; LaRocca, H.P.; Sütsch, G.; Kiowski, W. Cyclosporine A and control of vascular tone in the human forearm: Influence of post-transplant hypertension. J. Hypertens. 1999, 17, 357–363. [Google Scholar] [CrossRef]
- Ricoult, S.J.H.; Manning, B.D. The multifaceted role of mTORC1 in the control of lipid metabolism. EMBO Rep. 2013, 14, 242–251. [Google Scholar] [CrossRef] [Green Version]
- Kurdi, A.; Martinet, W.; De Meyer, G.R.Y. mTOR Inhibition and Cardiovascular Diseases: Dyslipidemia and Atherosclerosis. Transplantation 2018, 102 (Supp. S1), S44–S46. [Google Scholar] [CrossRef]
- Murakami, N.; Riella, L.V.; Funakoshi, T. Risk of metabolic complications in kidney transplantation after conversion to mTOR inhibitor: A systematic review and meta-analysis. Am. J. Transplant. 2014, 14, 2317–2327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karpe, K.M.; Talaulikar, G.S.; Walters, G.D. Calcineurin inhibitor withdrawal or tapering for kidney transplant recipients. Cochrane Database Syst. Rev. 2017, 7, CD006750. [Google Scholar] [CrossRef] [PubMed]
- De Sévaux, R.G.; Hilbrands, L.B.; Tiggeler, R.G.; Koene, R.A.; Hoitsma, A.J. A randomised, prospective study on the conversion from cyclosporine-prednisone to cyclosporine-azathioprine at 6 months after renal transplantation. Transpl. Int. 1998, 11, S322–S324. [Google Scholar] [CrossRef] [PubMed]
- D’Avola, D.; Cuervas-Mons, V.; Martí, J.; Ortiz de Urbina, J.; Lladó, L.; Jimenez, C.; Otero, E.; Suarez, F.; Rodrigo, J.M.; Gómez, M.A.; et al. Cardiovascular morbidity and mortality after liver transplantation: The protective role of mycophenolate mofetil. Liver Transpl. 2017, 23, 498–509. [Google Scholar] [CrossRef]
- Yeung, M.Y.; Gabardi, S.; Sayegh, M.H. Use of polyclonal/monoclonal antibody therapies in transplantation. Expert Opin. Biol. Ther. 2017, 17, 339–352. [Google Scholar] [CrossRef]
- Noble, J.; Jouve, T.; Janbon, B.; Rostaing, L.; Malvezzi, P. Belatacept in kidney transplantation and its limitations. Expert Rev. Clin. Immunol. 2019, 15, 359–367. [Google Scholar] [CrossRef]
- El Hennawy, H.; Safar, O.; Al Faifi, A.S.; El Nazer, W.; Kamal, A.; Mahedy, A.; Zaitoun, M.; Fahmy, A.E. Belatacept rescue therapy of CNI-induced nephrotoxicity, meta-analysis. Transplant. Rev. 2021, 35, 100653. [Google Scholar] [CrossRef]
- Böhmig, G.A.; Eskandary, F.; Doberer, K.; Halloran, P.F. The therapeutic challenge of late antibody-mediated kidney allograft rejection. Transpl. Int. 2019, 32, 775–788. [Google Scholar] [CrossRef] [Green Version]
- Authors/Task Force Members; ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis 2019, 290, 140–205. [Google Scholar] [CrossRef] [Green Version]
- Chronic Kidney Disease Prognosis Consortium; Matsushita, K.; van der Velde, M.; Astor, B.C.; Woodward, M.; Levey, A.S.; de Jong, P.E.; Coresh, J.; Gansevoort, R.T. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 2010, 375, 2073–2081. [Google Scholar] [CrossRef] [Green Version]
- Harris, J.; Teuteberg, J.; Shullo, M. Optimal low-density lipoprotein concentration for cardiac allograft vasculopathy prevention. Clin. Transplant. 2018, 32, e13248. [Google Scholar] [CrossRef]
- Barbagallo, C.M.; Cefalù, A.B.; Gallo, S.; Rizzo, M.; Noto, D.; Cavera, G.; Rao Camemi, A.; Marino, G.; Caldarella, R.; Notarbartolo, A.; et al. Effects of Mediterranean diet on lipid levels and cardiovascular risk in renal transplant recipients. Nephron 1999, 82, 199–204. [Google Scholar] [CrossRef]
- Page, R.L.; Miller, G.G.; Lindenfeld, J. Drug therapy in the heart transplant recipient: Part IV: Drug-drug interactions. Circulation 2005, 111, 230–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hricik, D.E.; Bartucci, M.R.; Mayes, J.T.; Schulak, J.A. The effects of steroid withdrawal on the lipoprotein profiles of cyclosporine-treated kidney and kidney-pancreas transplant recipients. Transplantation 1992, 54, 868–871. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, J.R.; Sorroche, P.; Legal, S.; Oyhamburu, J.; Brandi, P.; Pasqualini, T. Effect of therapy with deflazacort on dyslipoproteinemia after pediatric renal transplantation. J. Pediatr. 1998, 133, 533–536. [Google Scholar] [CrossRef]
- Friemann, S.; Feuring, E.; Padberg, W.; Ernst, W. Improvement of nephrotoxicity, hypertension, and lipid metabolism after conversion of kidney transplant recipients from cyclosporine to tacrolimus. Transplant. Proc. 1998, 30, 1240–1242. [Google Scholar] [CrossRef]
- Manzarbeitia, C.; Reich, D.J.; Rothstein, K.D.; Braitman, L.E.; Levin, S.; Munoz, S.J. Tacrolimus conversion improves hyperlipidemic states in stable liver transplant recipients. Liver Transpl. 2001, 7, 93–99. [Google Scholar] [CrossRef]
- Marcén, R.; Chahin, J.; Alarcón, A.; Bravo, J. Conversion from cyclosporine microemulsion to tacrolimus in stable kidney transplant patients with hypercholesterolemia is related to an improvement in cardiovascular risk profile: A prospective study. Transplant. Proc. 2006, 38, 2427–2430. [Google Scholar] [CrossRef] [PubMed]
- White, M.; Haddad, H.; Leblanc, M.H.; Giannetti, N.; Pflugfelder, P.; Davies, R.; Isaac, D.; Burton, J.; Chan, M.; Azevedo, E.; et al. Conversion from cyclosporine microemulsion to tacrolimus-based immunoprophylaxis improves cholesterol profile in heart transplant recipients with treated but persistent dyslipidemia: The Canadian multicentre randomized trial of tacrolimus vs. cyclosporine microemulsion. J. Heart Lung Transplant. 2005, 24, 798–809. [Google Scholar] [CrossRef]
- Palmer, S.C.; Navaneethan, S.D.; Craig, J.C.; Perkovic, V.; Johnson, D.W.; Nigwekar, S.U.; Hegbrant, J.; Strippoli, G.F. HMG CoA reductase inhibitors (statins) for kidney transplant recipients. Cochrane Database Syst. Rev. 2014, 2014, CD005019. [Google Scholar] [CrossRef]
- Blum, A.; Shamburek, R. The pleiotropic effects of statins on endothelial function, vascular inflammation, immunomodulation and thrombogenesis. Atherosclerosis 2009, 203, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Zeiser, R. Immune modulatory effects of statins. Immunology 2018, 154, 69–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hölschermann, H.; Hilgendorff, A.; Kemkes-Matthes, B.; Schönburg, M.; Bauer, E.P.; Tillmanns, H.; Haberbosch, W. Simvastatin attenuates vascular hypercoagulability in cardiac transplant recipients. Transplantation 2000, 69, 1830–1836. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, I.; Sata, M.; Saiura, A.; Kaneda, Y.; Yashiro, H.; Hirata, Y.; Makuuchi, M.; Nagai, R. Atorvastatin attenuates transplant-associated coronary arteriosclerosis in a murine model of cardiac transplantation. Biomed. Pharmacother. 2007, 61, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Campana, C.; Regazzi, M.B.; Buggia, I.; Molinaro, M. Clinically significant drug interactions with cyclosporin. An update. Clin. Pharmacokinet. 1996, 30, 141–179. [Google Scholar] [CrossRef]
- Ballantyne, C.M.; Corsini, A.; Davidson, M.H.; Holdaas, H.; Jacobson, T.A.; Leitersdorf, E.; März, W.; Reckless, J.P.; Stein, E.A. Risk for myopathy with statin therapy in high-risk patients. Arch. Intern. Med. 2003, 163, 553–564. [Google Scholar] [CrossRef] [Green Version]
- Cheng-Lai, A. Rosuvastatin: A new HMG-CoA reductase inhibitor for the treatment of hypercholesterolemia. Heart Dis. 2003, 5, 72–78. [Google Scholar] [CrossRef]
- Wiggins, B.S.; Saseen, J.J.; Page, R.L.; Reed, B.N.; Sneed, K.; Kostis, J.B.; Lanfear, D.; Virani, S.; Morris, P.B.; American Heart Association Clinical Pharmacology Committee of the Council on Clinical Cardiology; et al. Recommendations for Management of Clinically Significant Drug-Drug Interactions with Statins and Select Agents Used in Patients with Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2016, 134, e468–e495. [Google Scholar] [CrossRef] [Green Version]
- Shaw, S.M.; Chaggar, P.; Ritchie, J.; Shah, M.K.H.; Baynes, A.C.; O’Neill, N.; Fildes, J.E.; Yonan, N.; Williams, S.G. The efficacy and tolerability of ezetimibe in cardiac transplant recipients taking cyclosporin. Transplantation 2009, 87, 771–775. [Google Scholar] [CrossRef]
- Almutairi, F.; Peterson, T.C.; Molinari, M.; Walsh, M.J.; Alwayn, I.; Peltekian, K.M. Safety and effectiveness of ezetimibe in liver transplant recipients with hypercholesterolemia. Liver Transpl. 2009, 15, 504–508. [Google Scholar] [CrossRef]
- Sapan, M.; Ozben, B.; Yakupoglu, G.; Suleymanlar, G.; Ozben, T. Effects of diet and gemfibrozil on posttransplant hyperlipidemia in renal transplant recipients. J. Investig. Med. 2009, 57, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Prasad, G.V.R. Post-transplant dyslipidemia: Mechanisms, diagnosis and management. World J. Transplant. 2016, 6, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Kasiske, B.; Cosio, F.G.; Beto, J.; Bolton, K.; Chavers, B.M.; Grimm Jr, R.; Levin, A.; Masri, B.; Parekh, R.; Wanner, C.; et al. Clinical practice guidelines for managing dyslipidemias in kidney transplant patients: A report from the Managing Dyslipidemias in Chronic Kidney Disease Work Group of the National Kidney Foundation Kidney Disease Outcomes Quality Initiative. Am. J. Transplant. 2004, 4, 13–53. [Google Scholar] [CrossRef] [PubMed]
- Mir, O.; Poinsignon, V.; Arnedos, M.; Delaloge, S.; Paci, A. Pharmacokinetic interaction involving fenofibrate and everolimus. Ann. Oncol. 2015, 26, 248–249. [Google Scholar] [CrossRef]
- Fehrman-Ekholm, I.; Jogestrand, T.; Angelin, B. Decreased cyclosporine levels during gemfibrozil treatment of hyperlipidemia after kidney transplantation. Nephron 1996, 72, 483. [Google Scholar] [CrossRef]
- Canner, P.L.; Berge, K.G.; Wenger, N.K.; Stamler, J.; Friedman, L.; Prineas, R.J.; Friedewald, W. Fifteen year mortality in Coronary Drug Project patients: Long-term benefit with niacin. J. Am. Coll. Cardiol. 1986, 8, 1245–1255. [Google Scholar] [CrossRef] [Green Version]
- Brown, B.G.; Zhao, X.Q.; Chait, A.; Fisher, L.D.; Cheung, M.C.; Morse, J.S.; Dowdy, A.A.; Marino, E.K.; Bolson, E.L.; Alaupovic, P.; et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N. Engl. J. Med. 2001, 345, 1583–1592. [Google Scholar] [CrossRef]
- Lal, S.M.; Hewett, J.E.; Petroski, G.F.; Van Stone, J.C.; Ross, G. Effects of nicotinic acid and lovastatin in renal transplant patients: A prospective, randomized, open-labeled crossover trial. Am. J. Kidney Dis. 1995, 25, 616–622. [Google Scholar] [CrossRef]
- Ponticelli, C.; Arnaboldi, L.; Moroni, G.; Corsini, A. Treatment of dyslipidemia in kidney transplantation. Expert Opin. Drug Saf. 2020, 19, 257–267. [Google Scholar] [CrossRef]
- Jensen, R.A.; Lal, S.M.; Diaz-Arias, A.; James-Kracke, M.; Van Stone, J.C.; Ross, G. Does cholestyramine interfere with cyclosporine absorption? A prospective study in renal transplant patients. ASAIO J. 1995, 41, M704–M706. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T., Jr.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Effects of Icosapent Ethyl on Total Ischemic Events: From REDUCE-IT. J. Am. Coll. Cardiol. 2019, 73, 2791–2802. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Asano, T.; Suzuki, S.; Tokonabe, S.; Hayakawa, M. Evaluation of ethyl icosapentate in the treatment of hypercholesterolemia in kidney transplant recipients. Transplant. Proc. 1998, 30, 3047–3048. [Google Scholar] [CrossRef]
- Yessoufou, A.; Plé, A.; Moutairou, K.; Hichami, A.; Khan, N.A. Docosahexaenoic acid reduces suppressive and migratory functions of CD4+CD25+ regulatory T-cells. J. Lipid Res. 2009, 50, 2377–2388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreassen, A.K.; Hartmann, A.; Offstad, J.; Geiran, O.; Kvernebo, K.; Simonsen, S. Hypertension prophylaxis with omega-3 fatty acids in heart transplant recipients. J. Am. Coll. Cardiol. 1997, 29, 1324–1331. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, G.G.; Steg, P.G.; Szarek, M.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Edelberg, J.M.; Goodman, S.G.; Hanotin, C.; Harrington, R.A.; et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N. Engl. J. Med. 2018, 379, 2097–2107. [Google Scholar] [CrossRef] [PubMed]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, G.; Cioffi, G.; Di Lorenzo, A.; Iannone, F.P.; Cudemo, G.; Iannicelli, A.M.; Pacileo, M.; D’Andrea, A.; Vigorito, C.; Iannuzzo, G.; et al. Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitors Use for Atherogenic Dyslipidemia in Solid Organ Transplant Patients. J. Clin. Med. 2022, 11, 3247. [Google Scholar] [CrossRef]
- Warden, B.A.; Kaufman, T.; Minnier, J.; Duell, P.B.; Fazio, S.; Shapiro, M.D. Use of PCSK9 Inhibitors in Solid Organ Transplantation Recipients. JACC Case Rep. 2020, 2, 396–399. [Google Scholar] [CrossRef]
- Agarwala, A.; Jones, P.; Nambi, V. The role of antisense oligonucleotide therapy in patients with familial hypercholesterolemia: Risks, benefits, and management recommendations. Curr. Atheroscler. Rep. 2015, 17, 467. [Google Scholar] [CrossRef]
- Creta, M.; Calogero, A.; Sagnelli, C.; Peluso, G.; Incollingo, P.; Candida, M.; Minieri, G.; Longo, N.; Fusco, F.; Tammaro, V.; et al. Donor and Recipient Outcomes following Robotic-Assisted Laparoscopic Living Donor Nephrectomy: A Systematic Review. Biomed. Res. Int. 2019, 2019, 1729138. [Google Scholar] [CrossRef]
- Sagnelli, C.; Sica, A.; Gallo, M.; Peluso, G.; Varlese, F.; D’Alessandro, V.; Ciccozzi, M.; Crocetto, F.; Garofalo, C.; Fiorelli, A.; et al. Renal involvement in COVID-19: Focus on kidney transplant sector. Infection 2021, 49, 1265–1275. [Google Scholar] [CrossRef] [PubMed]

| Immunosuppressants | CV Risk Factors Exacerbated | |
|---|---|---|
| Corticosteroids | Prednisone | Hyperglycemia (+++), Arterial hypertension (++), Triglycerides (++), LDL (++). |
| Deflazacort | Hyperglycemia (+), Arterial hypertension (++), Triglycerides (+), LDL (+). | |
| Calcineurin Inhibitors | Cyclosporine | Hyperglycemia (+), Arterial hypertension (+++), Triglycerides (++), LDL (+++). |
| Tacrolimus | Hyperglycemia (+), Arterial hypertension (+++), Triglycerides (+), LDL (++). | |
| mTOR Inhibitors | Sirolimus | Triglycerides (+++), LDL (+++). |
| Everolimus | Triglycerides (+++), LDL (+++). | |
| Antiproliferative Agents | Azathioprine | No significant increase |
| Mycophenolate Mofetil | No significant increase | |
| Drugs | Dangerous Interaction | Benefit | |
|---|---|---|---|
| Statins | Atorvastatin | Co-administered with CsA or Tac increase statin exposure and risk for myopathy | LDL and Tg reduction CVD mortality reduction Plaque stabilization |
| Lovastatin | Co-administered with CsA or Tac increase statin exposure and risk for myopathy | ||
| Simvastatin | Co-administered with CsA or Tac increase statin exposure and risk for myopathy | ||
| Rosuvastatin | Co-administered with CsA or Tac increase statin exposure and risk for myopathy | ||
| Fibrates | Gemfibrozil | Can reduce plasma levels of CsA and mTORi. Increased risk for myopathy in coadministration with statins | LDL reduction HDL increase |
| Fenofibrate | Can reduce plasma levels of CsA and mTORi. Increased risk for myopathy in coadministration with statins | ||
| Ezetimibe | Co-administered with CsA can increase ezetimibe and CsA levels and risk for side effects | LDL reduction | |
| Bile acid sequestrants | Can reduce MMF, CsA, Tac and mTORi levels, their administration should be delayed by 4 h from bile acid sequestrants | LDL reduction | |
| Lomitapide | Can increase plasma levels of CsA, Tac and mTORi | LDL reduction |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iannuzzo, G.; Cuomo, G.; Di Lorenzo, A.; Tripaldella, M.; Mallardo, V.; Iaccarino Idelson, P.; Sagnelli, C.; Sica, A.; Creta, M.; Baltar, J.; et al. Dyslipidemia in Transplant Patients: Which Therapy? J. Clin. Med. 2022, 11, 4080. https://doi.org/10.3390/jcm11144080
Iannuzzo G, Cuomo G, Di Lorenzo A, Tripaldella M, Mallardo V, Iaccarino Idelson P, Sagnelli C, Sica A, Creta M, Baltar J, et al. Dyslipidemia in Transplant Patients: Which Therapy? Journal of Clinical Medicine. 2022; 11(14):4080. https://doi.org/10.3390/jcm11144080
Chicago/Turabian StyleIannuzzo, Gabriella, Gianluigi Cuomo, Anna Di Lorenzo, Maria Tripaldella, Vania Mallardo, Paola Iaccarino Idelson, Caterina Sagnelli, Antonello Sica, Massimiliano Creta, Javier Baltar, and et al. 2022. "Dyslipidemia in Transplant Patients: Which Therapy?" Journal of Clinical Medicine 11, no. 14: 4080. https://doi.org/10.3390/jcm11144080
APA StyleIannuzzo, G., Cuomo, G., Di Lorenzo, A., Tripaldella, M., Mallardo, V., Iaccarino Idelson, P., Sagnelli, C., Sica, A., Creta, M., Baltar, J., Crocetto, F., Bresciani, A., Gentile, M., Calogero, A., & Giallauria, F. (2022). Dyslipidemia in Transplant Patients: Which Therapy? Journal of Clinical Medicine, 11(14), 4080. https://doi.org/10.3390/jcm11144080








