Symptom-Level Disability Status Assessed with an Electronic Unsupervised Patient-Reported Expanded Disability Status Scale (ePR-EDSS) in Multiple Sclerosis Patients—The Example of Croatia
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Procedures
2.2. Participants
2.3. Methods
2.4. Statystical Analysis
3. Results
3.1. Demographic Characteristics, Comorbidity, and MS Disease-Related Factors
3.2. MS Type and EDSS-Related Differences
3.3. ePR-EDSS Disability Status and Symptom-Level Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Howard, J.; Trevick, S.; Younger, D.S. Epidemiology of Multiple Sclerosis. Neurol. Clin. 2016, 34, 919–939. [Google Scholar] [CrossRef] [PubMed]
- MS International Federation. Available online: https://www.atlasofms.org/map/global/epidemiology/number-of-people-with-ms (accessed on 8 March 2022).
- Benjak, T.; Štefančić, V.; Draušnik, Ž.; Cerovečki, I.; Roginić, D.; Habek, M.; Mihel, S.; Stevanović, R. Prevalence of multiple sclerosis in Croatia: Data from national and non-governmental organization registries. Croat. Med. J. 2018, 59, 65–70. [Google Scholar] [CrossRef]
- Kamińska, J.; Koper, O.M.; Piechal, K.; Kemona, H. Multiple sclerosis—Etiology and diagnostic potential. Postep. Hig. Med. Dosw. 2017, 71, 551–563. [Google Scholar] [CrossRef]
- Ysrraelit, M.C.; Correale, J. Impact of sex hormones on immune function and multiple sclerosis development. Immunology 2019, 156, 9–22. [Google Scholar] [CrossRef] [Green Version]
- Goodin, D.S.; Khankhanian, P.; Gourraud, P.A.; Vince, N. The nature of genetic and environmental susceptibility to multiple sclerosis. PLoS ONE 2021, 16, e0246157. [Google Scholar] [CrossRef] [PubMed]
- Poser, C.M.; Brinar, V.V. Diagnostic criteria for multiple sclerosis. Clin. Neurol. Neurosurg. 2001, 103, 1–11. [Google Scholar] [CrossRef]
- Poser, C.M.; Paty, D.W.; Scheinberg, L.; McDonald, W.I.; Davis, F.A.; Ebers, G.C.; Johnson, K.P.; Sibley, W.A.; Silberberg, D.H.; Tourtellotte, W.W. New diagnostic criteria for multiple sclerosis: Guidelines for research protocols. Ann. Neurol. 1983, 13, 227–231. [Google Scholar] [CrossRef]
- McDonald, W.I.; Compston, A.; Edan, G.; Goodkin, D.; Hartung, H.P.; Lublin, F.D.; McFarland, H.F.; Paty, D.W.; Polman, C.H.; Reingold, S.C.; et al. Recommended diagnostic criteria for multiple sclerosis: Guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann. Neurol. 2001, 50, 121–127. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–14452. [Google Scholar] [CrossRef] [Green Version]
- Kurtzke, J.F. A new scale for evaluating disability in multiple sclerosis. Neurology 1955, 5, 580–583. [Google Scholar] [CrossRef] [PubMed]
- Glaser, A.; Stahmann, A.; Meissner, T.; Flachenecker, P.; Horáková, D.; Zaratin, P.; Brichetto, G.; Pugliatti, M.; Rienhoff, O.; Vukusic, S.; et al. Multiple sclerosis registries in Europe—An updated mapping survey. Mult. Scler. Relat. Disord. 2019, 27, 171–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, C.D.; Ivry, B.; Bowen, J.D.; Cheng, E.M.; Dobson, R.; Goodin, D.S.; Lechner-Scott, J.; Kappos, L.; Galea, I. A comparative analysis of Patient-Reported Expanded Disability Status Scale tools. Mult. Scler. J. 2016, 22, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Neurostatus. Available online: https://www.neurostatus.net/media/specimen/Definitions_0410-2_s.pdf (accessed on 10 February 2022).
- Hohol, M.J.; Orav, E.J.; Weiner, H.L. Disease steps in multiple sclerosis: A simple approach to evaluate disease progression. Neurology 1995, 45, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Lavorgna, L.; Miele, G.; Petruzzo, M.; Lanzillo, R.; Bonavita, S. Online validation of the Italian version of the patient determined disease steps scale (PDDS) in people with multiple sclerosis. Mult. Scler. Relat. Disord. 2018, 21, 108–109. [Google Scholar] [CrossRef]
- Romeo, A.R.; Rowles, W.M.; Schleimer, E.S.; Barba, P.; Hsu, W.Y.; Gomez, R.; Santaniello, A.; Zhao, C.; Pearce, J.R.; Jones, J.B.; et al. An electronic, unsupervised patient-reported Expanded Disability Status Scale for multiple sclerosis. Mult. Scler. J. 2021, 27, 1432–1441. [Google Scholar] [CrossRef]
- Çinar, B.P.; Yorgun, Y.G. What We Learned from The History of Multiple Sclerosis Measurement: Expanded Disability Status Scale. Noro. Psikiyatr. Ars. 2018, 55, S69–S75. [Google Scholar] [CrossRef]
- Harbo, H.F.; Gold, R.; Tintoré, M. Sex and gender issues in multiple sclerosis. Ther. Adv. Neurol. Disord. 2013, 6, 237–248. [Google Scholar] [CrossRef] [Green Version]
- Bergamaschi, R. Prognostic factors in multiple sclerosis. Int. Rev. Neurobiol. 2007, 79, 423–447. [Google Scholar] [CrossRef]
- Voskuhl, R.R.; Patel, K.; Paul, F.; Gold, S.M.; Scheel, M.; Kuchling, J.; Cooper, G.; Asseyer, S.; Chien, C.; Brandt, A.U.; et al. Sex differences in brain atrophy in multiple sclerosis. Biol. Sex Diff. 2020, 11, 49. [Google Scholar] [CrossRef]
- Tutuncu, M.; Tang, J.; Zeid, N.A.; Kale, N.; Crusan, D.J.; Atkinson, E.J.; Siva, A.; Pittock, S.J.; Pirko, I.; Keegan, B.M.; et al. Onset of progressive phase is an age-dependent clinical milestone in multiple sclerosis. Mult. Scler. J. 2013, 19, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Coret, F.; Pérez-Miralles, F.C.; Gascón, F.; Alcalá, C.; Navarré, A.; Bernad, A.; Boscá, I.; Escutia, M.; Gil-Perotin, S.; Casanova, B. Onset of secondary progressive multiple sclerosis is not influenced by current relapsing multiple sclerosis therapies. Mult. Scler. J. Exp. Transl. Clin. 2018, 4, 2055217318783347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeydan, B.; Kantarci, O.H. Progressive forms of multiple sclerosis: Distinct entity or age-dependent phenomena. Neurol. Clin. 2018, 36, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Hauer, L.; Perneczky, J.; Sellner, J. A global view of comorbidity in multiple sclerosis: A systematic review with a focus on regional differences, methodology, and clinical implications. J. Neurol. 2021, 268, 4066–4077. [Google Scholar] [CrossRef] [PubMed]
- Moss, B.P.; Rensel, M.R.; Hersh, C.M. Wellness and the Role of Comorbidities in Multiple Sclerosis. Neurotherapeutics 2017, 14, 999–1017. [Google Scholar] [CrossRef]
- Petruzzo, M.; Reia, A.; Maniscalco, G.T.; Luiso, F.; Lanzillo, R.; Russo, C.V.; Carotenuto, A.; Allegorico, L.; Palladino, R.; Brescia Morra, V.; et al. The Framingham cardiovascular risk score and 5-year progression of multiple sclerosis. Eur. J. Neurol. 2021, 28, 893–900. [Google Scholar] [CrossRef]
- Marrie, R.A.; Reider, N.; Cohen, J.; Stuve, O.; Trojano, M.; Cutter, G.; Reingold, S.; Sorensen, P.S. A systematic review of the incidence and prevalence of cardiac, cerebrovascular, and peripheral vascular disease in multiple sclerosis. Mult. Scler. J. 2015, 21, 318–331. [Google Scholar] [CrossRef]
- Meca-Lallana, V.; Berenguer-Ruiz, L.; Carreres-Polo, J.; Eichau-Madueño, S.; Ferrer-Lozano, J.; Forero, L.; Higueras, Y.; Téllez Lara, N.; Vidal-Jordana, A.; Pérez-Miralles, F.C. Deciphering Multiple Sclerosis Progression. Front. Neurol. 2021, 12, 608491. [Google Scholar] [CrossRef]
- Watson, C.; Scippa, K.; Barlev, A.; Kresa-Reahl, K.; Cole, J.C. Results from Patient Interviews on Fatigue in Progressive Multiple Sclerosis and Evaluation of Fatigue Patient-Reported Outcome (PRO) Instruments. Neurol. Ther. 2022, 11, 725–739. [Google Scholar] [CrossRef]
- Kos, D.; Kerckhofs, E.; Nagels, G.; D’hooghe, M.B.; Ilsbroukx, S. Origin of fatigue in multiple sclerosis: Review of the literature. Neurorehabil. Neural Repair 2008, 22, 91–100. [Google Scholar] [CrossRef]
- Hayes, H.A.; Gappmaier, E.; LaStayo, P.C. Effects of high-intensity resistance training on strength, mobility, balance, and fatigue in individuals with multiple sclerosis: A randomized controlled trial. J. Neurol. Phys. Ther. 2011, 35, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Jerković, A.; Proroković, A.; Matijaca, M.; Katić, A.Ć.; Košta, V.; Mihalj, M.; Dolić, K.; Đogaš, Z.; Vidaković, M.R. Validation of the fatigue severity scale in Croatian population of patients with multiple sclerosis disease: Factor structure, internal consistency, and correlates. Mult. Scler. Relat. Disord. 2022, 58, 103397. [Google Scholar] [CrossRef] [PubMed]
- Armutlu, K.; Korkmaz, N.C.; Keser, I.; Sumbuloglu, V.; Akbiyik, D.I.; Guney, Z.; Karabudak, R. The validity and reliability of the Fatigue Severity Scale in Turkish multiple sclerosis patients. Int. J. Rehabil. Res. 2007, 30, 81–85. [Google Scholar] [CrossRef] [Green Version]
- Gavrilov, Y.V.; Shkilnyuk, G.G.; Valko, P.O.; Stolyarov, I.D.; Ivashkova, E.V.; Ilves, A.G.; Nikiforova, I.G.; Shchelkova, O.Y.; Vasserman, L.I.; Vais, E.E.; et al. Validation of the Russian version of the Fatigue Impact Scale and Fatigue Severity Scale in multiple sclerosis patients. Acta Neurol. Scand. 2018, 138, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Abbadessa, G.; Brigo, F.; Clerico, M.; De Mercanti, S.; Trojsi, F.; Tedeschi, G.; Bonavita, S.; Lavorgna, L. Digital therapeutics in neurology. J. Neurol. 2022, 269, 1209–1224. [Google Scholar] [CrossRef] [PubMed]
- Giunti, G.; Rivera-Romero, O.; Koo, J.; Bansi, J.; Sevillano, J.L.; Granja-Dominguez, A.; Izquierdo-Ayuso, G.; Giunta, D. Evaluation of More Stamina, a Mobile App for Fatigue Management in Persons with Multiple Sclerosis: Protocol for a Feasibility, Acceptability, and Usability Study. JMIR Res. Protoc. 2020, 9, e18196. [Google Scholar] [CrossRef]
- Rogić Vidaković, M.; Šimić, N.; Poljičanin, A.; Nikolić Ivanišević, M.; Ana, J.; Đogaš, Z. Psychometric properties of the Croatian version of the depression, anxiety, and stress scale-21 and multiple sclerosis impact scale-29 in multiple sclerosis patients. Mult. Scler. Relat. Disord. 2021, 50, 102850. [Google Scholar] [CrossRef]
- Jerković, A.; Matijaca, M.; Proroković, A.; Šikić, A.; Košta, V.; Ćurković Katić, A.; Dolić, K.; Duka Glavor, K.; Šoda, J.; Đogaš, Z.; et al. Information Processing Speed Assessed with Letter Digit Substitution Test in Croatian Sample of Multiple Sclerosis Patients. Diagnostics 2022, 12, 111. [Google Scholar] [CrossRef]
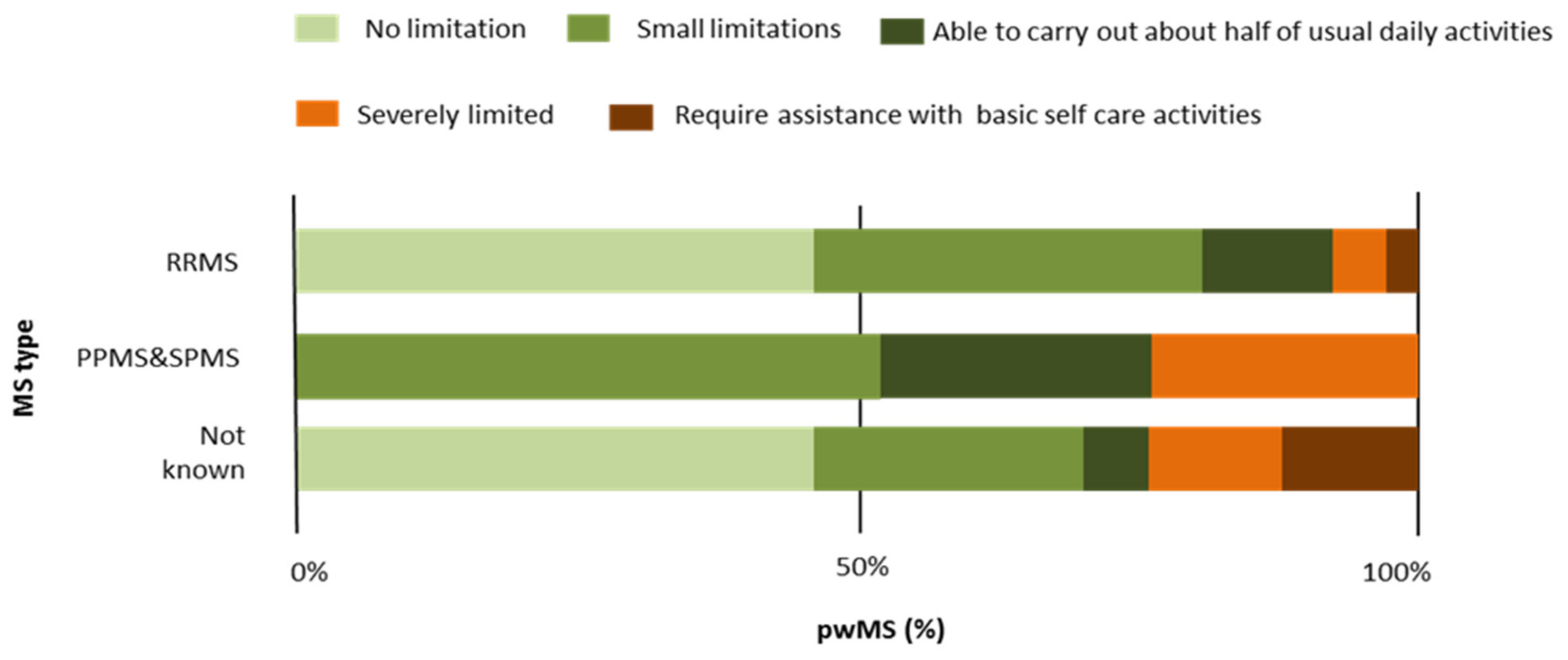
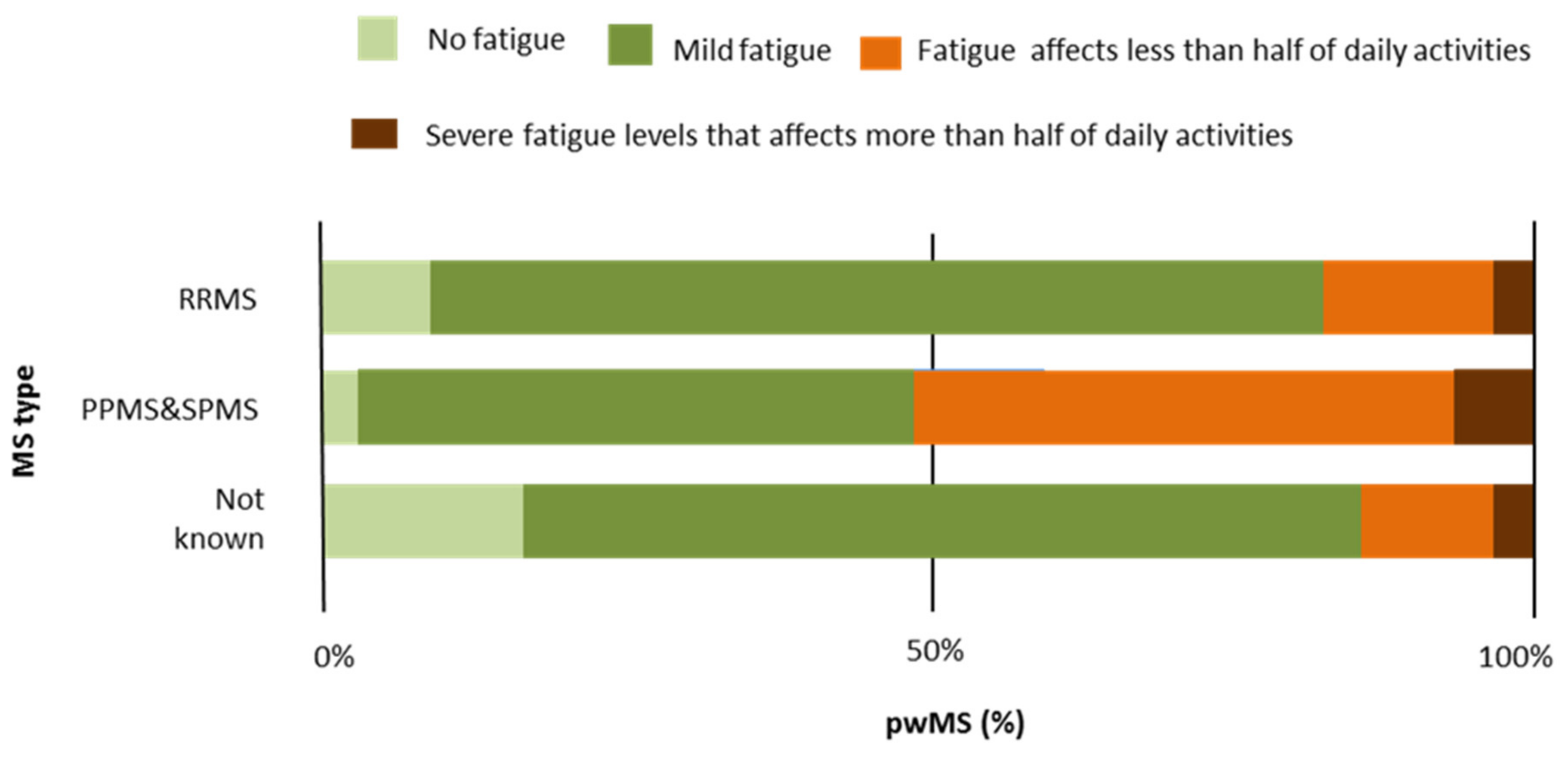
| RRMS | PPMS | SPMS | MS Type Not Known | Total | Test | p | |
|---|---|---|---|---|---|---|---|
| N | 105 | 19 | 6 | 17 | 147 | - | - |
| Age in years (mean ± SD) | 41.1 ± 1.9 | 47.1 ± 7.7 | 49.6 ± 9.1 | 42.9 ± 9.3 | 41.1 ± 11.3 | F = 6.12 | 0.009 ** |
| Female/Male (%) | 80/20 | 84/16 | 100/0 | 95/5 | 84/16 | χ2 = 2.45 | 0.27 |
| Hand dominance-right hand (%) | 92.4 | 94.7 | 100 | 82.4 | 91.8 | - | - |
| BMI (mean ± SD) | 24.5 ± 4.2 | 24.6 ± 5.9 | 24.3 ± 4.8 | 21.3 ± 2.9 | 19.6 ± 10.4 | - | - |
| Years of MS disease (mean ± SD) | 7.1 ± 1.7 | 5.2 ± 5.5 | 14.8 ± 7.4 | 7.2 ± 8.4 | 8.5 ± 7.4 | F = 0.29 | 0.48 |
| EDSS (median(IQR), range 0–8 | 3(2.5) | 5.5(3) | 4.7(1.5) | 1.5(4.5) | 3.0(3.0) | χ2 = 18.5 | <0.001 ** |
| Corticosteroid treatment (%) | 90.4 | 89.5 | 100 | 94.1 | 91.2 | χ2 = 0.26 | 0.87 |
| Immunomodulatory therapy (%) | 70.5 | 78.9 | 16.7 | 58.8 | 68.0 | χ2 = 1.73 | 0.56 |
| Immunomodulation Medication | RRMS n = 64 n/% | PPMS n = 11 n/% | SPMS n = 1 n/% | MS Type Not Known, n = 9 n/% | Total n = 85 n/% |
|---|---|---|---|---|---|
| Ocrelizumab (Ocrevus) | 14/21.8 | 3/27.2 | - | 1/11.1 | 17/20.0 |
| Glatiramer-Acetate (Copaxone, Glatopa, Remurel) | 7/10.9 | 3/27.2 | - | 2/22.2 | 13/15.5 |
| Fingolimod (Gilenya) | 8/12.5 | 1/9.1 | - | 2/22.2 | 13/15.5 |
| Dimethyl Fumarate (Tecfidera) | 9/14.2 | - | 1/100 | 1/11.1 | 10/11.6 |
| Interferon (ifn beta-1a) (Rebif) | 7/10.9 | - | - | 1/11.1 | 10/11.6 |
| Peginterferon (beta-1a) (Plegridy) | 6/9.3 | 1/9.1 | - | - | 6/7.8 |
| Teriflunomid (Aubagio) | 2/3.1 | 2/18.2 | - | - | 4/4.6 |
| Interferon (ifn beta-1b) (Betaferon) | 3/4.7 | - | - | 1/11.1 | 4/4.6 |
| Natalizumab (Tysabri) | 3/4.7 | - | - | - | 3/3.3 |
| Cladribine (Leustatin, Mavenclad) | 2/3.1 | - | - | - | 3/3.3 |
| Alemtuzumab (Campath, Lemtrada) | - | 1/9.1 | 1/11.1 | 2/2.3 |
| Other Chronic Diseases (Not MS) | RRMS n = 35 n/% | PPMS n = 5 n/% | SPMS n = 2 n/% | MS Type Not Known, n = 1 n/% | Total n = 43 n/% |
|---|---|---|---|---|---|
| Endocrine, nutritional and metabolic diseases | 15/42.8 | 4/80.0 | - | - | 19/44.1 |
| Diseases of the circulatory system | 5/14.3 | 1/20.0 | - | - | 6/13.9 |
| Diseases of the nervous system | 4/11.4 | 1/20.0 | 1/50.0 | - | 5/11.6 |
| Diseases of the digestive system | 4/11.4 | - | - | - | 4/9.3 |
| Diseases of the respiratory system | 1/2.8 | - | 1/50.0 | 1/100 | 3/6.9 |
| Musculoskelet disorders | 2/5.7 | - | 1/50.0 | - | 3/6.9 |
| Diseases of the eye | 3/8.5 | - | - | - | 3/6.9 |
| Mental and behavioural disorders | 2/5.7 | - | 1/50.0 | - | 3/6.9 |
| Neoplasms | 2/5.7 | - | - | - | 2/4.6 |
| Diseases of the genitourinary system | 1/2.8 | - | - | - | 1/2.3 |
| Diseases of the skin | 1/2.8 | - | - | - | 1/2.3 |
| EDSS Range | EDSS 0–3.5 | EDSS 4–8 | Test | p |
|---|---|---|---|---|
| N (%) | 69 (58.4) | 49 (41.6) | - | - |
| Age (mean ± SD) | 39.7 ± 8.5 | 46.0 ± 12.5 | t = −3.51 | <0.001 ** |
| Male/female (%) | 8.7/91.3 | 28.6/71.4 | χ2 = 8.04 | 0.01 ** |
| Years of MS disease (mean ± SD) | 8.8 ± 7.6 | 10.4 ± 7.2 | t = −1.71 | 0.33 |
| Corticosteroid treatment (y/n, %) | 91.3/8.7 | 97.9/2.1 | χ2 = 2.27 | 0.13 |
| Immunomodulatory drug (y/n, %) | 79.7/20.3 | 59.1/40.9 | χ2 = 5.89 | 0.02 * |
| RRMS n = 105 | PPMS and SPMS n = 25 | Ms Type Not Know n = 17 | Total n = 147 | Test (H Value) | p | |
|---|---|---|---|---|---|---|
| Overall wellness—functional abilities (1–5) * | 2.0 (1.0) | 2.0 (2.0) | 2.0 (2.0) | 2.0 (2.0) | 18.6 | <0.001 |
| Mobility-ability to walk | ||||||
| Ability to walk (1–4) | 1.0 (1.0) | 2.0 (0) | 1.0 1.(0) | 2.0 1.(0) | 21.3 | <0.001 |
| Ability to walk without aid (1–4) | 2.0 (3.0) | 5.0 (2.0) | 5.0 (0) | 3.0 (4.0) | 9.17 | 0.01 ** |
| Ability to walk without aid (% of time) | 1.0 (0.25) | 0.2 (0.75) | 1.0 (0.7) | 1.0 (0.5) | 18.02 | <0.001 |
| Use a cane, a single crutch, or hold onto another person (% of time) | 0 (0.3) | 0.8 (0.7) | 0 (1.0) | 0.1(0.8) | 17.36 | <0.001 |
| Use a walker or other bilateral support (% of time) | 0 (0) | 0.5 (1.0) | 0 (0.9) | 0 (0.3) | 12.38 | <0.001 |
| Use a wheelchair (% of time) | 0 (0) | 0 (0.3) | 0 (0) | 0 (0) | 7.12 | <0.02 * |
| Sensation | ||||||
| Right hand or arm (1–6) | 2.0 (1.0) | 2.0 (2.0) | 2.0 (2.0) | 2.0 (1.0) | 6.04 | 0.05 |
| Left hand or arm (1–6) | 1.0 (1.0) | 2.0 (1.0) | 2.0 (1.0) | 2.0 (1.0) | 2.72 | 0.25 |
| Right foot or leg (1–6) | 2.0 (1.0) | 2.0 (1.0) | 2.0 (1.0) | 2.0 (2.0) | 7.13 | 0.028 * |
| Left foot or leg (1–6) | 2.0 (1.0) | 2.0 (1.0) | 2.0 (1.0) | 2.0 (1.0) | 6.07 | 0.04 * |
| Strength † | ||||||
| Right arm (1–6) | 1.0 (1.0) | 2.0 (1.0) | 2.0 (2.0) | 1.0 (1.0) | 13.64 | 0.01 ** |
| Left arm (1–6) | 1.0 (1.0) | 1.0 (1.0) | 1.0 (1.0) | 1.0 (1.0) | 2.44 | 0.29 |
| Right leg (1–6) | 2.0 (1.0) | 2.0 (2.0) | 2.0 (2.0) | 2.0 (1.0) | 16.93 | <0.001 |
| Left leg (1–6) | 2.0 (1.0) | 2.0 (1.0) | 2.0 (1.0) | 2.0 (1.0) | 12.16 | <0.01 ** |
| Strength †† | ||||||
| Right arm (1–5) | 1.0 (1.0) | 2.0 (1.0) | 2.0 (1.0) | 1.0 (1.0) | 2.78 | 0.24 |
| Left arm (1–5) | 1.0 (1.0) | 1.0 (1.0) | 1.0 (1.0) | 1.0 (1.0) | 0.65 | 0.71 |
| Right leg (1–5) | 2.0 (1.0) | 2.0 (1.0) | 2.0 (1.0) | 2.0 (2.0) | 14.96 | <0.001 |
| Left leg (1–5) | 2.0 (1.0) | 2.0 (1.0) | 2.0 (1.0) | 2.0 (1.0) | 9.51 | 0.01 ** |
| Coordination | ||||||
| Arms (1–5) | 2.0 (2.0) | 2.0 (1.0) | 2.0 (1.0) | 2.0 (2.0) | 4.08 | 0.13 |
| Legs (1–5) | 2.0 (2.0) | 3.0 (2.0) | 3.0 (2.0) | 2.0 (2.0) | 14.36 | <0.001 |
| Balance when standing (1–5) | 2.0 (1.0) | 3.0 (1.0) | 3.0 (1.0) | 2.0 (2.0) | 13.85 | <0.001 |
| Balance when walking (1–5) | 2.0 (2.0) | 3.0 (1.0) | 3.0 (1.0) | 2.0 (2.0) | 21.97 | <0.001 |
| Balance when sitting (1–5) | 1.0 (0) | 1.0 (1.0) | 1.0 (1.0) | 1.0 (1.0) | 5.38 | 0.06 |
| Vision | ||||||
| Double vision (1–4) | 1.0 (1.0) | 1.0 (1.0) | 1.0 (1.0) | 1.0 (1.0) | 2.84 | 0.24 |
| Vision problems—right eye (1–4) | 1.0 (1.0) | 2.0 (1.0) | 2.0 (1.0) | 1.0 (1.0) | 8.46 | 0.01 * |
| Vision problems—left eye (1–4) | 1.0 (1.0) | 2.0 (2.0) | 2.0 (2.0) | 2.0 (1.0) | 7.21 | 0.02 * |
| Blind spot in vision (1–4) | 1.0 (0) | 1.0 (0) | 1.0 (0) | 1.0 (0) | 2.65 | 0.26 |
| Face and neck | ||||||
| Muscle weakness—right side (1–4) | 1.0 (0) | 1.0 (1.0) | 1.0 (1.0) | 1.0 (0) | 11.18 | <0.01 |
| Muscle weakness—left side (1–4) | 1.0 (0) | 1.0 (1.0) | 1.0 (1.0) | 1.0 (0) | 7.73 | 0.02 * |
| Feeling—right side (1–5) | 1.0 (0) | 1.0 (2.0) | 1.0 (2.0) | 2.0 (0) | 6.9 | 0.03 * |
| Feeling—left side (1–5) | 1.0 (0) | 1.0 (1.0) | 1.0 (1.0) | 2.0 (0) | 3.81 | 0.14 |
| Ability to speak (1–6) | 2.0 (1.0) | 1.0 (1.0) | 1.0 (1.0) | 1.0 (1.0) | 3.27 | 0.19 |
| Ability to swallow liquids and solids (1–4) | 1.0 (0) | 1.0 (1.0) | 1.0 (1.0) | 1.0 (0) | 2.94 | 0.22 |
| Hearing problems (1–4) | 1.0 (0) | 1.0 (1.0) | 1.0 (1.0) | 1.0 (0) | 4.15 | 0.12 |
| Bowel and bladder | ||||||
| Urinary urgency (1–5) | 2.0 (3.0) | 4.0 (1.0) | 4.0 (1.0) | 3.0 (3.0) | 12.69 | <0.001 |
| Urine leak (1–5) | 1.0 (1.0) | 2.0 (1.0) | 2.0 (1.0) | 1.0 (1.0) | 3.03 | 0.21 |
| Wearing a pad or use a urinal (yes/no) ¶ | 26/79 | 9/16 | 4/17 | 39/112 | χ2 = 1.10 | 0.49 |
| Start to urinate (1–6) | 1.0 (1.0) | 2.0 (2.0) | 2.0 (2.0) | 2.0 (1.0) | 4.65 | 0.11 |
| Constipation (1–3) | 2.0 (1.0) | 2.0 (1.0) | 2.0 (1.0) | 2.0 (1.0) | 1.55 | 0.45 |
| Bowel frequency (1–4) | 1.0 (1.0) | 1.0 (1.0) | 1.0 (1.0) | 1.0 (1.0) | 1.38 | 0.06 |
| Mood and thinking ability | ||||||
| Cognitive abilities (1–5) | 2.0 (1.0) | 2.0 (0) | 2.0 (0) | 2.0 (1.0) | 4.73 | 0.09 |
| Fatigue (1–4) | 2.0 (0) | 3.0 (1.0) | 3.0 (1.0) | 2.0 (1.0) | 9.37 | 0.01 ** |
| Little interest or pleasure in doing things (1–4) | 2.0 (1.0) | 2.0 (2.0) | 2.0 (2.0) | 2.0 (1.0) | 3.71 | 0.15 |
| Feeling down, depressed, or hopeless (1–4) | 2.0 (1.0) | 2.0 (2.0) | 2.0 (2.0) | 2.0 (1.0) | 3.54 | 0.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jerković, A.; Pavelin, S.; Šoda, J.; Vujović, I.; Rogić Vidaković, M. Symptom-Level Disability Status Assessed with an Electronic Unsupervised Patient-Reported Expanded Disability Status Scale (ePR-EDSS) in Multiple Sclerosis Patients—The Example of Croatia. J. Clin. Med. 2022, 11, 4081. https://doi.org/10.3390/jcm11144081
Jerković A, Pavelin S, Šoda J, Vujović I, Rogić Vidaković M. Symptom-Level Disability Status Assessed with an Electronic Unsupervised Patient-Reported Expanded Disability Status Scale (ePR-EDSS) in Multiple Sclerosis Patients—The Example of Croatia. Journal of Clinical Medicine. 2022; 11(14):4081. https://doi.org/10.3390/jcm11144081
Chicago/Turabian StyleJerković, Ana, Sanda Pavelin, Joško Šoda, Igor Vujović, and Maja Rogić Vidaković. 2022. "Symptom-Level Disability Status Assessed with an Electronic Unsupervised Patient-Reported Expanded Disability Status Scale (ePR-EDSS) in Multiple Sclerosis Patients—The Example of Croatia" Journal of Clinical Medicine 11, no. 14: 4081. https://doi.org/10.3390/jcm11144081
APA StyleJerković, A., Pavelin, S., Šoda, J., Vujović, I., & Rogić Vidaković, M. (2022). Symptom-Level Disability Status Assessed with an Electronic Unsupervised Patient-Reported Expanded Disability Status Scale (ePR-EDSS) in Multiple Sclerosis Patients—The Example of Croatia. Journal of Clinical Medicine, 11(14), 4081. https://doi.org/10.3390/jcm11144081








