Dual-Energy Computed Tomography-Based Quantitative Bone Marrow Imaging in Non-Hematooncological Subjects: Associations with Age, Gender and Other Variables
Abstract
:1. Introduction
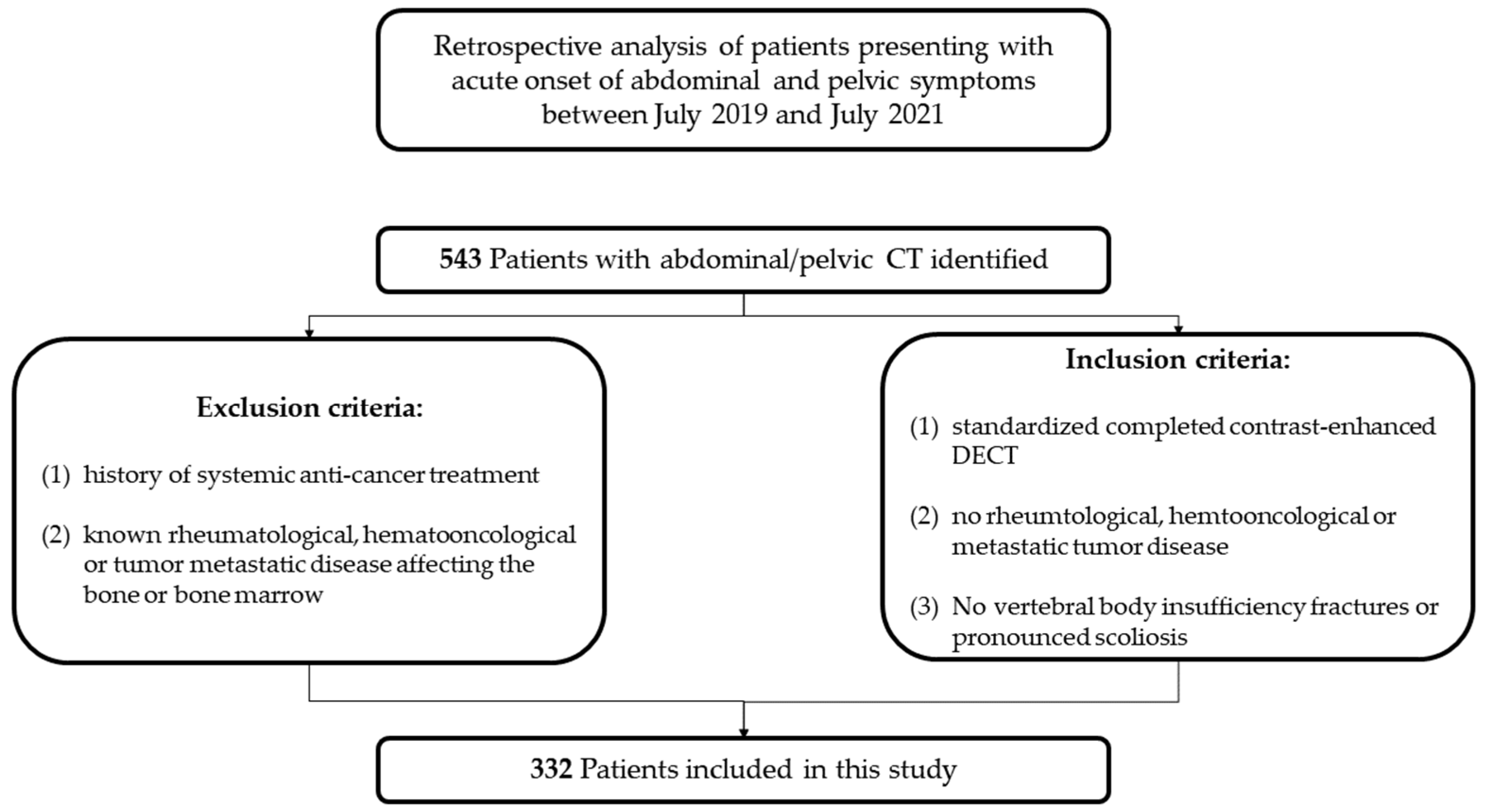
2. Materials and Methods
2.1. Patients
2.2. Computed Tomography Protocol and Post-Processing
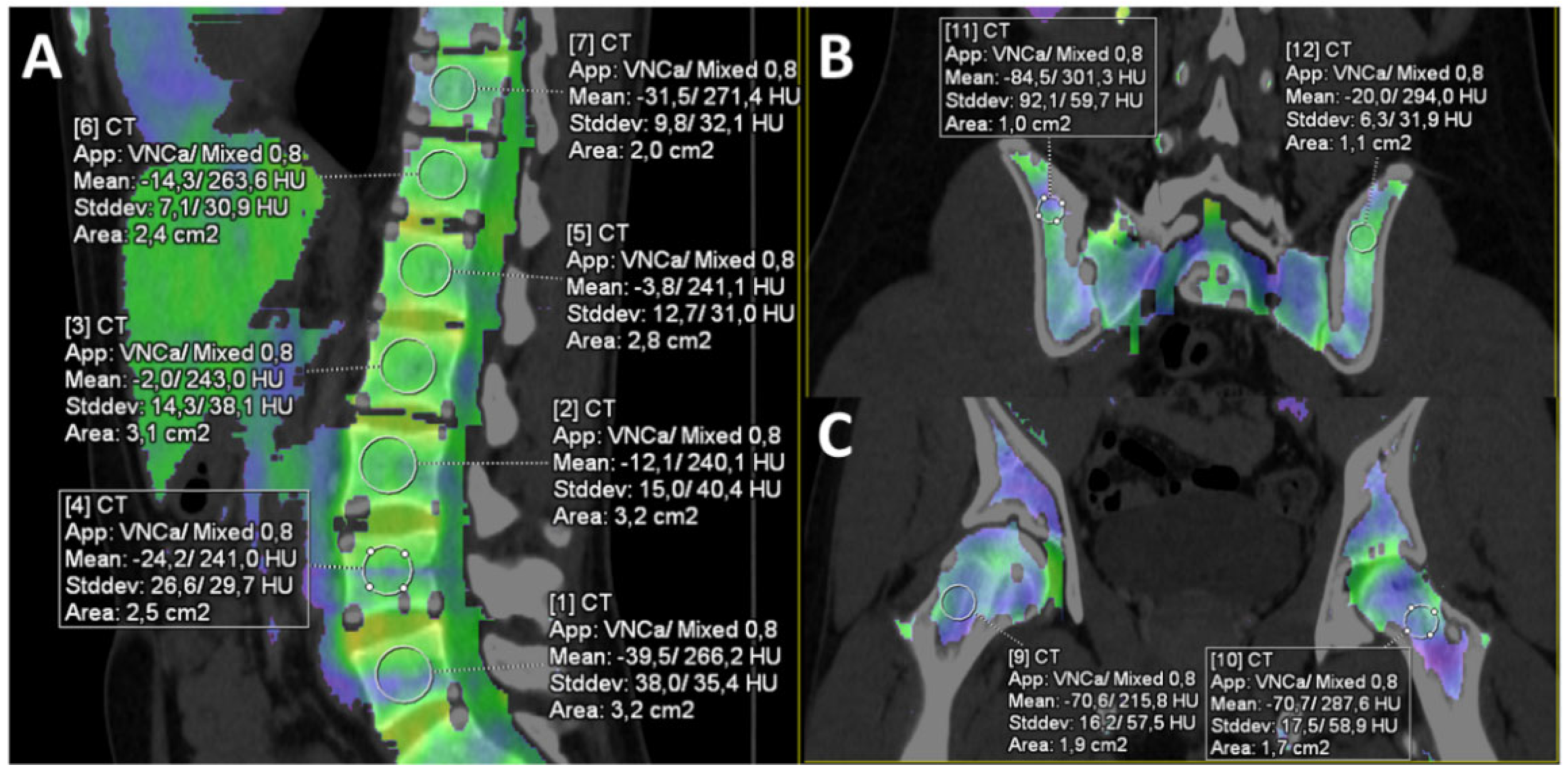
2.3. Image Analysis
2.4. Laboratory Data
2.5. Statistical Analysis
3. Results
3.1. Subjects
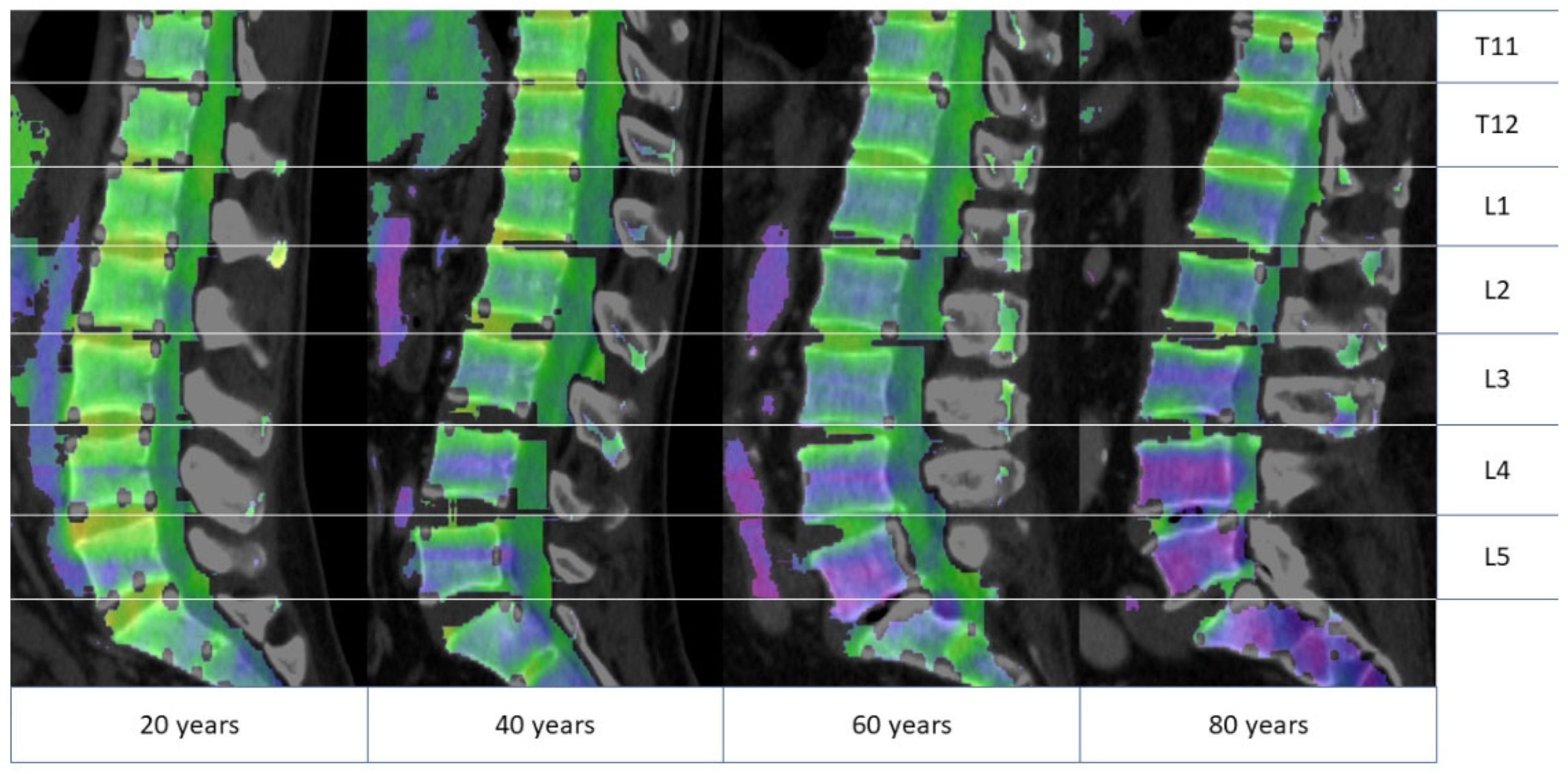
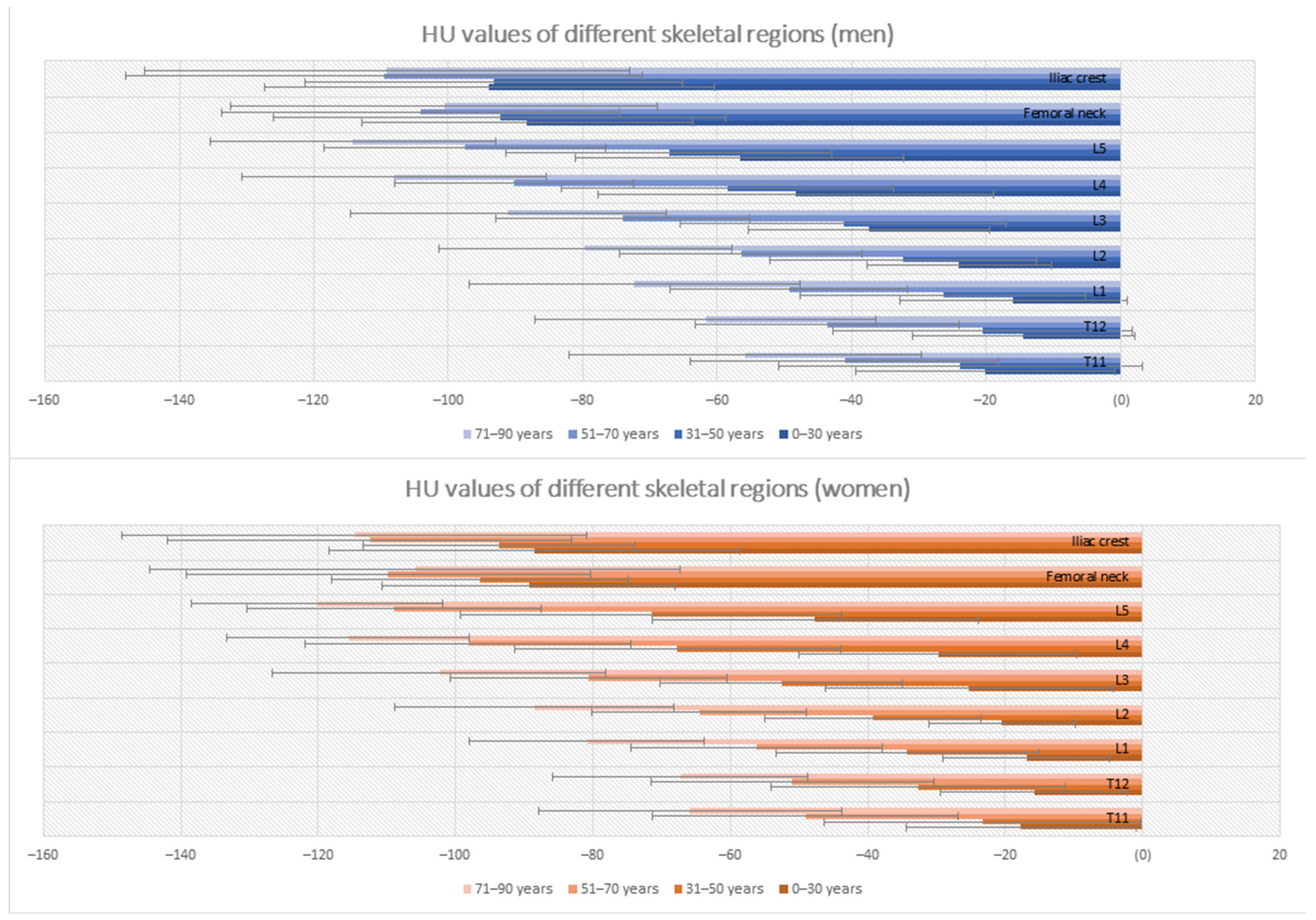
3.2. Differences in Bone Marrow Attenuation by Age and Gender
3.3. Differences in Bone Marrow Attenuation by Inflammation Markers
3.4. Differences in Bone Marrow Attenuation by Other Clinical and Habitual Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bathmann, J.; Sigmund, G. Stellenwert von Knochenmarkszintigraphie und Magnetresonanztomographie in der bildgebenden Diagnostik des Knochenmarks. Aktuelle Radiol. 1994, 4, 159–168. [Google Scholar] [PubMed]
- Mulé, S.; Reizine, E.; Blanc-Durand, P.; Baranes, L.; Zerbib, P.; Burns, R.; Nouri, R.; Itti, E.; Luciani, A. Whole-Body Functional MRI and PET/MRI in Multiple Myeloma. Cancers 2020, 12, 3155. [Google Scholar] [CrossRef] [PubMed]
- Person, A.; Janitz, E.; Thapa, M. Pediatric Bone Marrow: Normal and Abnormal MRI Appearance. Semin. Roentgenol. 2021, 56, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, X.; Zhang, L. Whole body FDG-PET/CT for the assessment of bone marrow infiltration in patients with newly diagnosed lymphoma. Med. Clin. 2020, 154, 61–65. [Google Scholar] [CrossRef]
- Burke, M.C.; Garg, A.; Youngner, J.M.; Deshmukh, S.D.; Omar, I.M. Initial experience with dual-energy computed tomography-guided bone biopsies of bone lesions that are occult on monoenergetic CT. Skeletal Radiol. 2019, 48, 605–613. [Google Scholar] [CrossRef]
- Fervers, P.; Glauner, A.; Gertz, R.; Täger, P.; Kottlors, J.; Maintz, D.; Borggrefe, J. Virtual calcium-suppression in dual energy computed tomography predicts metabolic activity of focal MM lesions as determined by fluorodeoxyglucose positron-emission-tomography. Eur. J. Radiol. 2021, 135, 109502. [Google Scholar] [CrossRef]
- Poulton, T.B.; Murphy, W.D.; Duerk, J.L.; Chapek, C.C.; Feiglin, D.H. Bone marrow reconversion in adults who are smokers: MR Imaging findings. AJR Am. J. Roentgenol. 1993, 161, 1217–1221. [Google Scholar] [CrossRef]
- Duda, S.H.; Laniado, M.; Schick, F.; Strayle, M.; Claussen, C.D. Normal bone marrow in the sacrum of young adults: Differences between the sexes seen on chemical-shift MR imaging. AJR Am. J. Roentgenol. 1995, 164, 935–940. [Google Scholar] [CrossRef]
- Herrmann, J.; Krstin, N.; Schoennagel, B.P.; Sornsakrin, M.; Derlin, T.; Busch, J.D.; Petersen, K.U.; Graessner, J.; Adam, G.; Habermann, C.R. Age-related distribution of vertebral bone-marrow diffusivity. Eur. J. Radiol. 2012, 81, 4046–4049. [Google Scholar] [CrossRef]
- Mora-Bravo, F.; Muñoz, J. Impaired Reconversion of Bone Marrow in Nuclear Magnetic Resonance in Patients with Chronic Renal Disease. Curr. Med. Imaging 2021, 17, 1256–1261. [Google Scholar] [CrossRef]
- Vande Berg, B.C.; Malghem, J.; Lecouvet, F.E.; Maldague, B. La moelle osseuse normale: Aspects dynamiques en imagerie par résonance magnétique. J. Radiol. 2001, 82, 127–135. [Google Scholar] [PubMed]
- Vande Berg, B.C.; Lecouvet, F.E.; Galant, C.; Simoni, P.; Malghem, J. Normal variants of the bone marrow at MR imaging of the spine. Semin. Musculoskelet. Radiol. 2009, 13, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Ricci, C.; Cova, M.; Kang, Y.S.; Yang, A.; Rahmouni, A.; Scott, W.W.; Zerhouni, E.A. Normal age-related patterns of cellular and fatty bone marrow distribution in the axial skeleton: MR imaging study. Radiology 1990, 177, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Hynes, J.P.; Hughes, N.; Cunningham, P.; Kavanagh, E.C.; Eustace, S.J. Whole-body MRI of bone marrow: A review. J. Magn. Reson. Imaging 2019, 50, 1687–1701. [Google Scholar] [CrossRef] [PubMed]
- Federle, M.P. CT of the acute (emergency) abdomen. Eur. Radiol. 2005, 15 (Suppl. 4), D100–D104. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.R.; Hayashi, D.; Yonenaga, T.; Fukuda, K.; Genant, H.K.; Lin, C.; Rahmouni, A.; Guermazi, A. MRI of bone marrow abnormalities in hematological malignancies. Diagn. Interv. Radiol. 2013, 19, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Lecouvet, F.E.; Larbi, A.; Pasoglou, V.; Omoumi, P.; Tombal, B.; Michoux, N.; Malghem, J.; Lhommel, R.; Vande Berg, B.C. MRI for response assessment in metastatic bone disease. Eur. Radiol. 2013, 23, 1986–1997. [Google Scholar] [CrossRef]
- Teke, H.Ü.; Cansu, D.Ü.; Korkmaz, C. Indications for bone marrow examinations in rheumatology. Rheumatol. Int. 2019, 39, 1221–1228. [Google Scholar] [CrossRef]
- Abdullayev, N.; Große Hokamp, N.; Lennartz, S.; Holz, J.A.; Romman, Z.; Pahn, G.; Neuhaus, V.; Maintz, D.; Krug, B.; Borggrefe, J. Improvements of diagnostic accuracy and visualization of vertebral metastasis using multi-level virtual non-calcium reconstructions from dual-layer spectral detector computed tomography. Eur. Radiol. 2019, 29, 5941–5949. [Google Scholar] [CrossRef]
- Hua, C.-H.; Shapira, N.; Merchant, T.E.; Klahr, P.; Yagil, Y. Accuracy of electron density, effective atomic number, and iodine concentration determination with a dual-layer dual-energy computed tomography system. Med. Phys. 2018, 45, 2486–2497. [Google Scholar] [CrossRef]
- Müller, F.C.; Gosvig, K.K.; Mikkel, Ø.; Bjarne, R.; Rodell, A.; Henrik, B.; Krauss, B.; Gade, J.S.; Boesen, M. Quantifying the bone marrow composition of the healthy adult wrist with dual-energy CT. Eur. J. Radiol. 2021, 139, 109725. [Google Scholar] [CrossRef] [PubMed]
- Shabshin, N.; Schweitzer, M.E. Age dependent T2 changes of bone marrow in pediatric wrist MRI. Skeletal Radiol. 2009, 38, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Vogler, J.B.; Murphy, W.A. Bone marrow imaging. Radiology 1988, 168, 679–693. [Google Scholar] [CrossRef]
- Małkiewicz, A.; Dziedzic, M. Bone marrow reconversion—Imaging of physiological changes in bone marrow. Pol. J. Radiol. 2012, 77, 45–50. [Google Scholar] [CrossRef]
- Cristy, M. Active bone marrow distribution as a function of age in humans. Phys. Med. Biol. 1981, 26, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Alfrey, C.P., Jr.; Lynch, E.C.; Hettig, R.A. Studies of iron kinetics using a linear scanner. I. Distribution of sites of uptake of plasma iron in hematological disorders. J. Lab. Clin. Med. 1969, 73, 405–417. [Google Scholar] [PubMed]
- Schneider, C.; Montz, R. Die quantitative Verteilung des erythropoetischen Knochenmarks beim Menschen gemessen mit Radioeisen. Klin. Wochenschr. 1966, 44, 969–973. [Google Scholar] [CrossRef]
- Vande Berg, B.C.; Malghem, J.; Lecouvet, F.E.; Maldague, B. Magnetic resonance imaging of the normal bone marrow. Skeletal Radiol. 1998, 27, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Maltais, S.; Perrault, L.P.; Ly, H.Q. The bone marrow-cardiac axis: Role of endothelial progenitor cells in heart failure. Eur. J. Cardiothorac. Surg. 2011, 39, 368–374. [Google Scholar] [CrossRef]
- Hoffmann, J.; Luxán, G.; Abplanalp, W.T.; Glaser, S.-F.; Rasper, T.; Fischer, A.; Muhly-Reinholz, M.; Potente, M.; Assmus, B.; John, D.; et al. Post-myocardial infarction heart failure dysregulates the bone vascular niche. Nat. Commun. 2021, 12, 3964. [Google Scholar] [CrossRef]
- Sheu, Y.; Amati, F.; Schwartz, A.V.; Danielson, M.E.; Li, X.; Boudreau, R.; Cauley, J.A. Vertebral bone marrow fat, bone mineral density and diabetes: The Osteoporotic fractures in Men (MrOS) study. Bone 2017, 97, 299–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karampinos, D.C.; Ruschke, S.; Dieckmeyer, M.; Diefenbach, M.; Franz, D.; Gersing, A.S.; Krug, R.; Baum, T. Quantitative MRI and spectroscopy of bone marrow. J. Magn. Reson. Imaging 2018, 47, 332–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurevitch, O.; Slavin, S.; Feldman, A.G. Conversion of red bone marrow into yellow—Cause and mechanisms. Med. Hypotheses 2007, 69, 531–536. [Google Scholar] [CrossRef] [PubMed]

| Male (197) | Female (135) | p-Value | |
|---|---|---|---|
| Age in years (mean ± SD) | 62 ± 17 | 67 ± 18 | 0.006 * |
| Weight in kg (mean ± SD) | 81 ± 14 | 69 ± 14 | <0.001 * |
| Size in cm (mean ± SD) | 176 ± 7 | 165 ± 6 | <0.001 * |
| (mean ± SD) | 26 ± 4 | 25 ± 5 | 0.045 * |
| Heavy smoking history (n (%)) | 74 (37.6%) | 24 (17.8%) | <0.001 ** |
| Diabetes (n (%)) | 23 (18.8%) | 17 (20.0%) | 0.556 ** |
| Heart failure (NYHA I-IV) (n (%)) | 21 (10.7%) | 12 (8.9%) | 0.596 ** |
| Renal failure (n (%)) | 22 (11.2%) | 11 (8.1%) | 0.366 ** |
| Alcohol abuse (n (%)) | 30 (15.2%) | 4 (3.0%) | <0.001 |
| (mean ± SD) | 6.8 ± 8.5 | 6.6 ± 9.7 | 0.814 * |
| (mean ± SD) | 260.6 ± 501.7 | 235.4 ± 166.7 | 0.575 * |
| Anemia (n (%)) | 68 (34.5%) | 64 (47.4%) | 0.018 ** |
| 0–30 Years | 31–50 Years | 51–70 Years | 71–90 Years | |||||
|---|---|---|---|---|---|---|---|---|
| Vertebral Body | Men | Women | Men | Women | Men | Women | Men | Women |
| T11 | −20.15 ± 19.27 | −17.73 ± 16.73 | −23.81 ± 27.07 | −23.32 ± 23.06 | −42.05 ± 22.93 | −49.11 ± 22.28 | −55.83 ± 26.25 | −65.86 ± 22.08 |
| T12 | −14.44 ± 16.52 | −15.85 ± 13.67 | −20.53 ± 22.23 | −32.74 ± 21.42 | −43.61 ± 19.60 | −50.99 ± 20.62 | −61.72 ± 25.41 | −67.30 ± 18.61 |
| L1 | −15.93 ± 16.88 | −16.93 ± 12.10 | −26.41 ± 21.26 | −34.31 ± 19.13 | −49.32 ± 17.65 | −56.20 ± 18.36 | −72.30 ± 24.59 | −80.92 ± 17.18 |
| L2 | −24.00 ± 13.79 | −20.45 ± 10.74 | −32.41 ± 19.82 | −39.27 ± 15.75 | −56.42 ± 18.03 | −64.55 ± 15.58 | −79.63 ± 21.81 | −88.59 ± 20.30 |
| L3 | −37.42 ± 17.91 | −25.23 ± 20.92 | −41.26 ± 24.18 | −52.56 ± 17.64 | −74.12 ± 18.87 | −80.59 ± 20.10 | −91.10 ± 23.44 | −102.43 ± 24.28 |
| L4 | −48.35 ± 29.41 | −29.75 ± 20.21 | −58.43 ± 24.69 | −67.77 ± 23.72 | −90.17 ± 17.77 | −98.20 ± 23.70 | −108.04 ± 22.65 | −115.61 ± 17.62 |
| L5 | −56.62 ± 24.43 | −47.70 ± 23.72 | −67.13 ± 24.23 | −71.54 ± 27.81 | −97.51 ± 20.91 | −108.97 ± 21.40 | −114.92 ± 21.21 | −120.16 ± 18.28 |
| Femoral neck * | −88.27 ± 24.54 | −89.36 ± 21.28 | −92.37 ± 33.67 | −96.52 ± 21.62 | −104.18 ± 29.59 | −109.80 ± 29.42 | −100.62 ± 31.75 | −105.92 ± 38.65 |
| Iliac crest | −93.90 ± 33.42 | −88.50 ± 29.89 | −93.19 ± 28.10 | −93,71 ± 19.18 | −109.60 ± 38.45 | −112.47 ± 29.40 | −109.14 ± 36.03 | −114.73 ± 33.86 |
| Variables | Thoracic Spine | Lumbar Spine | Femoral Bone | Iliac Crest | ||||
|---|---|---|---|---|---|---|---|---|
| Standardized Beta Coefficient (β) | p-Value | Standardized Beta Coefficient (β) | p-Value | Standardized Beta Coefficient (β) | p-Value | Standardized Beta Coefficient (β) | p-Value | |
| LDH | 0.122 | 0.006 | 0.107 | 0.003 | 0.162 | 0.003 | 0.145 | 0.007 |
| CRP | 0.038 | 0.392 | 0.107 | 0.008 | 0.099 | 0.078 | 0.019 | 0.724 |
| Anemia | 0.015 | 0.739 | 0.042 | 0.257 | 0.156 | 0.006 | 0.083 | 0.128 |
| Variables | Thoracic Spine | Lumbar Spine | Femoral Bone | Iliac Crest | ||||
|---|---|---|---|---|---|---|---|---|
| Standardized Beta Coefficient (β) | p-Value | Standardized Beta Coefficient (β) | p-Value | Standardized Beta Coefficient (β) | p-Value | Standardized Beta Coefficient (β) | p-Value | |
| Smoking History | 0.010 | 0.833 | −0.020 | 0.610 | 0.013 | 0.826 | 0.017 | 0.771 |
| Alcohol abuse | −0.033 | 0.484 | −0.046 | 0.231 | 0.057 | 0.330 | −0.001 | 0.981 |
| Diabetes | −0.057 | 0.211 | −0.042 | 0.261 | −0.034 | 0.555 | −0.049 | 0.374 |
| Heart failure (NYHA I-IV) | −0.103 | 0.029 | 0.017 | 0.661 | −0.020 | 0.734 | −0.016 | 0.981 |
| Renal Insufficiency | 0.059 | 0.217 | 0.002 | 0.963 | -0.042 | 0.476 | 0.007 | 0.909 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hagen, F.; Fritz, J.; Mair, A.; Horger, M.; Bongers, M.N. Dual-Energy Computed Tomography-Based Quantitative Bone Marrow Imaging in Non-Hematooncological Subjects: Associations with Age, Gender and Other Variables. J. Clin. Med. 2022, 11, 4094. https://doi.org/10.3390/jcm11144094
Hagen F, Fritz J, Mair A, Horger M, Bongers MN. Dual-Energy Computed Tomography-Based Quantitative Bone Marrow Imaging in Non-Hematooncological Subjects: Associations with Age, Gender and Other Variables. Journal of Clinical Medicine. 2022; 11(14):4094. https://doi.org/10.3390/jcm11144094
Chicago/Turabian StyleHagen, Florian, Jan Fritz, Antonia Mair, Marius Horger, and Malte N. Bongers. 2022. "Dual-Energy Computed Tomography-Based Quantitative Bone Marrow Imaging in Non-Hematooncological Subjects: Associations with Age, Gender and Other Variables" Journal of Clinical Medicine 11, no. 14: 4094. https://doi.org/10.3390/jcm11144094






