Clinical Significance of Antineutrophil Cytoplasmic Antibody Positivity in Patients Infected with SARS-CoV-2
Abstract
:1. Introduction
2. Patients and Methods
2.1. Study Subjects
2.2. Clinical Data
2.3. Blood Samples
2.4. ANCA Measurement
2.5. Application of the New Classification Criteria for AAV
2.6. Statistical Analysis
3. Results
3.1. Characteristics of Patients Infected with SARS-CoV-2
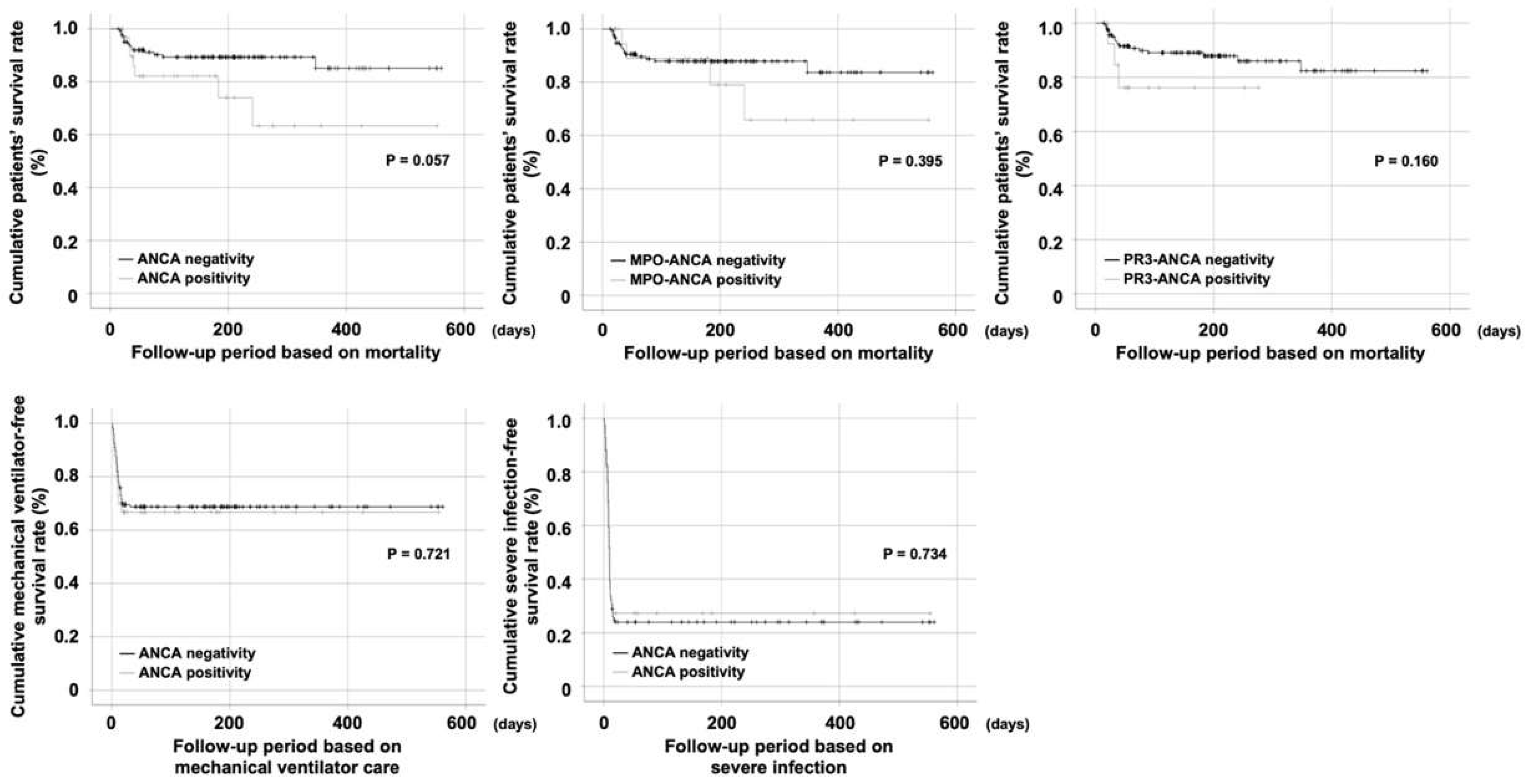
3.2. Comparison of Cumulative Survival Rates
3.3. Application of the New Classification Criteria for AAV
3.4. Classification of Each Patient Based on Items of the 2022 ACR/EULAR Criteria Met by at Least One Patient
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 4 July 2022).
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Soy, M.; Keser, G.; Atagündüz, P.; Tabak, F.; Atagündüz, I.; Kayhan, S. Cytokine storm in COVID-19: Pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020, 39, 2085–2094. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Brito-Zerón, P.; Mariette, X. Systemic and organ-specific immune-related manifestations of COVID-19. Nat. Rev. Rheumatol. 2021, 17, 315–332. [Google Scholar] [CrossRef] [PubMed]
- Ehrenfeld, M.; Tincani, A.; Andreoli, L.; Cattalini, M.; Greenbaum, A.; Kanduc, D.; Alijotas-Reig, J.; Zinserling, V.; Semenova, N.; Amital, H.; et al. Covid-19 and autoimmunity. Autoimmun. Rev. 2020, 19, 102597. [Google Scholar] [CrossRef]
- Damoiseaux, J.; Dotan, A.; Fritzler, M.J.; Bogdanos, D.P.; Meroni, P.L.; Roggenbuck, D.; Goldman, M.; Landegren, N.; Bastard, P.; Shoenfeld, Y.; et al. Autoantibodies and SARS-CoV2 infection: The spectrum from association to clinical implication: Report of the 15th Dresden Symposium on Autoantibodies. Autoimmun. Rev. 2022, 21, 103012. [Google Scholar] [CrossRef]
- Becker, R.C. COVID-19 update: Covid-19-associated coagulopathy. J. Thromb. Thrombolysis 2020, 50, 54–67. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Ackermann, M.; Anders, H.-J.; Bilyy, R.; Bowlin, G.L.; Daniel, C.; De Lorenzo, R.; Egeblad, M.; Henneck, T.; Hidalgo, A.; Hoffmann, M.; et al. Patients with COVID-19: In the dark-NETs of neutrophils. Cell Death Differ. 2021, 28, 3125–3139. [Google Scholar] [CrossRef]
- Jennette, J.C.; Falk, R.J.; Bacon, P.A.; Basu, N.; Cid, M.C.; Ferrario, F.; Flores-Suarez, L.F.; Gross, W.L.; Guillevin, L.; Hagen, E.C.; et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 2013, 65, 1–11. [Google Scholar] [CrossRef]
- Watts, R.; Lane, S.; Hanslik, T.; Hauser, T.; Hellmich, B.; Koldingsnes, W.; Mahr, A.; Segelmark, M.; Cohen-Tervaert, J.W.; Scott, D. Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann. Rheum. Dis. 2007, 66, 222–227. [Google Scholar] [CrossRef]
- Cornec, D.; Cornec-Le Gall, E.; Fervenza, F.C.; Specks, U. ANCA-associated vasculitis—Clinical utility of using ANCA specificity to classify patients. Nat. Rev. Rheumatol. 2016, 12, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Izci Duran, T.; Turkmen, E.; Dilek, M.; Sayarlioglu, H.; Arik, N. ANCA-associated vasculitis after COVID-19. Rheumatol. Int. 2021, 41, 1523–1529. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.; Patel, K.; Rahimi, O.; Sanyurah, O.; Iardino, A.; Khan, N. ANCA vasculitis: A manifestation of Post-Covid-19 Syndrome. Respir. Med. Case Rep. 2021, 34, 101549. [Google Scholar] [CrossRef] [PubMed]
- Kitching, A.R.; Anders, H.J.; Basu, N.; Brouwer, E.; Gordon, J.; Jayne, D.R.; Kullman, J.; Lyons, P.A.; Merkel, P.A.; Savage, C.O.S.; et al. ANCA-associated vasculitis. Nat. Rev. Dis. Primers 2020, 6, 71. [Google Scholar] [CrossRef]
- Apel, F.; Zychlinsky, A.; Kenny, E.F. The role of neutrophil extracellular traps in rheumatic diseases. Nat. Rev. Rheumatol. 2018, 14, 467–475. [Google Scholar] [CrossRef]
- Nakazawa, D.; Masuda, S.; Tomaru, U.; Ishizu, A. Pathogenesis and therapeutic interventions for ANCA-associated vasculitis. Nat. Rev. Rheumatol. 2019, 15, 91–101. [Google Scholar] [CrossRef]
- Falk, R.J.; Terrell, R.S.; Charles, L.A.; Jennette, J.C. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc. Natl. Acad. Sci. USA 1990, 87, 4115–4119. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.H.; Kronbichler, A.; Park, D.D.; Park, Y.; Moon, H.; Kim, H.; Choi, J.H.; Choi, Y.; Shim, S.; Lyu, I.S.; et al. Neutrophil extracellular traps (NETs) in autoimmune diseases: A comprehensive review. Autoimmun. Rev. 2017, 16, 1160–1173. [Google Scholar] [CrossRef]
- Nakazawa, D.; Shida, H.; Tomaru, U.; Yoshida, M.; Nishio, S.; Atsumi, T.; Ishizu, A. Enhanced formation and disordered regulation of NETs in myeloperoxidase-ANCA-associated microscopic polyangiitis. J. Am. Soc. Nephrol. 2014, 25, 990–997. [Google Scholar] [CrossRef]
- Kadkhoda, K.; Laurita, K. Antineutrophil cytoplasmic antibodies and their association with clinical outcomes in hospitalized COVID-19 patients. Cell Death Discov. 2021, 7, 277. [Google Scholar] [CrossRef] [PubMed]
- Grayson, P.C.; Ponte, C.; Suppiah, R.; Robson, J.C.; Craven, A.; Judge, A.; Khalid, S.; Hutchings, A.; Luqmani, R.A.; Watts, R.A.; et al. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology Classification Criteria for Eosinophilic Granulomatosis with Polyangiitis. Ann. Rheum. Dis. 2022, 81, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Suppiah, R.; Robson, J.C.; Grayson, P.C.; Ponte, C.; Craven, A.; Khalid, S.; Judge, A.; Hutchings, A.; Merkel, P.A.; Luqmani, R.A.; et al. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for microscopic polyangiitis. Ann. Rheum. Dis. 2022, 81, 321–326. [Google Scholar] [CrossRef]
- Robson, J.C.; Grayson, P.C.; Ponte, C.; Suppiah, R.; Craven, A.; Judge, A.; Khalid, S.; Hutchings, A.; Watts, R.A.; Merkel, P.A.; et al. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for granulomatosis with polyangiitis. Ann. Rheum. Dis. 2022, 81, 315–320. [Google Scholar] [CrossRef]
- Choi, H.; Park, Y.B.; Song, J.; Lee, S.W. Unclassifiable repeated antineutrophil cytoplasmic antibody (ANCA) positivity in diseases other than ANCA-associated vasculitis. Z. Rheumatol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, H.; Fukui, S.; Nakai, T.; Kidoguchi, G.; Kawaai, S.; Ozawa, H.; Ikeda, Y.; Koido, A.; Ohara, Y.; Shimizu, H.; et al. AB0533 Anti-neutrophil cytoplasmic antibody (ANCA) in general population without anca associated vasculitis. Ann. Rheum. Dis. 2020, 79, 1563. [Google Scholar] [CrossRef]
- Choi, C.B.; Park, Y.B.; Lee, S.W. Antineutrophil Cytoplasmic Antibody-Associated Vasculitis in Korea: A Narrative Review. Yonsei Med. J. 2019, 60, 10–21. [Google Scholar] [CrossRef]
- McAdoo, S.P.; Pusey, C.D. Is there a role for TNFα blockade in ANCA-associated vasculitis and glomerulonephritis? Nephrol. Dial. Transplant. 2017, 32, i80–i88. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, E.C.; Abdulahad, W.H.; Rutgers, A.; Huitema, M.G.; O’Reilly, V.P.; Coughlan, A.M.; Harrington, M.; Heeringa, P.; Little, M.A.; Hickey, F.B. Intermediate monocytes in ANCA vasculitis: Increased surface expression of ANCA autoantigens and IL-1β secretion in response to anti-MPO antibodies. Sci. Rep. 2015, 5, 11888. [Google Scholar] [CrossRef] [Green Version]
- Berti, A.; Cavalli, G.; Campochiaro, C.; Guglielmi, B.; Baldissera, E.; Cappio, S.; Sabbadini, M.G.; Doglioni, C.; Dagna, L. Interleukin-6 in ANCA-associated vasculitis: Rationale for successful treatment with tocilizumab. Semin. Arthritis Rheum. 2015, 45, 48–54. [Google Scholar] [CrossRef]
- Noonan, T.P.; Konstantinov, K.N.; Echevarria, L. Epstein-Barr virus reactivation induced myeloperoxidase-specific antineutrophil cytoplasmic antibody (MPO-ANCA)-associated vasculitis. BMJ Case Rep. 2021, 14, e245059. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, M.C.; Tamiazzo, S.; Stobbione, P.; Agatea, L.; De Gaspari, P.; Stecca, A.; Lauritano, E.C.; Roveta, A.; Tozzoli, R.; Guaschino, R.; et al. SARS-CoV-2 infection as a trigger of autoimmune response. Clin. Transl. Sci. 2021, 14, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lee, J.Y.; Yang, J.W.; Lee, K.H.; Effenberger, M.; Szpirt, W.; Kronbichler, A.; Shin, J.I. Immunopathogenesis and treatment of cytokine storm in COVID-19. Theranostics 2021, 11, 316–329. [Google Scholar] [CrossRef]
- Lamprecht, P.; Kerstein, A.; Klapa, S.; Schinke, S.; Karsten, C.M.; Yu, X.; Ehlers, M.; Epplen, J.T.; Holl-Ulrich, K.; Wiech, T.; et al. Pathogenetic and Clinical Aspects of Anti-Neutrophil Cytoplasmic Autoantibody-Associated Vasculitides. Front. Immunol. 2018, 9, 680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jennette, J.C.; Falk, R.J. Pathogenesis of antineutrophil cytoplasmic autoantibody-mediated disease. Nat. Rev. Rheumatol. 2014, 10, 463–473. [Google Scholar] [CrossRef] [PubMed]
| Variables | Values |
|---|---|
| Demographic data | |
| Age (years) | 65.0 (20.0) |
| Male sex (N (%)) | 111 (62.4) |
| ANCA positivity (N (%)) | |
| Any ANCA positivity | 33 (18.5) |
| MPO-ANCA positivity | 22 (12.4) |
| PR3-ANCA positivity | 14 (7.9) |
| Both ANCA positivity | 3 (0.2) |
| ANCA negativity | 145 (81.5) |
| Glucocorticoid use | |
| Cumulative dose equivalent to methylprednisolone (mg) | 656.4 (691.5) |
| Usage duration (days) | 13.0 (10.0) |
| Clinical outcomes (N (%)) | |
| Mortality | 22 (12.4) |
| Mechanical ventilator care | 54 (30.3) |
| HFNC | 80 (44.9) |
| Severe infection (Mechanical ventilator care + HFNC) | 134 (75.3) |
| Follow-up period (days) | |
| Follow-up period based on mortality | 166.0 (192.0) |
| Follow-up period based on mechanical ventilator care | 71.5 (182.0) |
| Follow-up period based on severe infection | 9.0 (12.0) |
| Lag time from symptom onset to blood sampling (days) | 21.0 (7.0) |
| Variables | Number of Patients |
|---|---|
| At the time of first symptom | |
| Clinical criteria | |
| Nasal involvement | 0 |
| Cartilaginous involvement | 0 |
| Conductive or sensorineural hearing loss | 0 |
| Obstructive airway disease | 3 (9.1) |
| Nasal polyp | 0 |
| Mononeuritis multiplex | 0 |
| Laboratory criteria | |
| PR3-ANCA positivity | 14 (42.4) |
| MPO-ANCA positivity | 22 (66.7) |
| Serum eosinophil ≥1000/µL | 2 (6.1) |
| Haematuria | 20 (60.6) |
| Biopsy criteria | |
| Granuloma, granulomatous inflammation, or giant cells | N/A |
| Pauci-immune glomerulonephritis | N/A |
| Extravascular eosinophilic-predominant inflammation | N/A |
| Imaging criteria | |
| Pulmonary nodules, mass, or cavitation on chest imaging | 3 (9.1) |
| Fibrosis or ILD on chest imaging | 4 (12.1) |
| Nasal/paranasal sinusitis or mastoiditis on imaging | 6 (18.2) |
| Number of patients with total score ≥5 for GPA = 12 Number of patients with total score ≥5 for MPA = 21 Number of patients with total score ≥6 for EGPA = 0 |
| Patients Number | Classification Criteria | Score for GPA | Score for MPA | Score for EGPA | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Obstructive Airway Disease | PR-3 ANCA Positivity | MPO ANCA Positivity | Serum Eosinophil ≥1000/µL | Haematuria | Pulmonary Nodules, Mass, or Cavitation on Chest Imaging | Fibrosis or ILD on Chest Imaging | Nasal/Paranasal Sinusitis or Mastoiditis on Imaging | ||||
| 1 | + | + | + | 4 | 5 | −4 | |||||
| 2 | + | −1 | 6 | 0 | |||||||
| 3 | + | + | + | + | + | + | −2 | 5 | 4 | ||
| 4 | + | + | −1 | 6 | −1 | ||||||
| 5 | + | + | + | + | −1 | 9 | −1 | ||||
| 6 | + | −1 | 6 | 0 | |||||||
| 7 | + | + | + | 5 | 2 | −4 | |||||
| 8 | + | + | −1 | 6 | 3 | ||||||
| 9 | + | + | −1 | 6 | −1 | ||||||
| 10 | + | + | 5 | −1 | −4 | ||||||
| 11 | + | + | 5 | −1 | −4 | ||||||
| 12 | + | + | + | + | 5 | 5 | −4 | ||||
| 13 | + | 5 | −1 | −3 | |||||||
| 14 | + | + | + | + | 2 | 6 | −1 | ||||
| 15 | + | + | 5 | −1 | −4 | ||||||
| 16 | + | + | + | −4 | 2 | 5 | |||||
| 17 | + | + | 5 | −1 | −4 | ||||||
| 18 | + | 5 | −1 | −3 | |||||||
| 19 | + | + | + | −1 | 6 | 2 | |||||
| 20 | + | −1 | 6 | 0 | |||||||
| 21 | + | 5 | −1 | −3 | |||||||
| 22 | + | −1 | 6 | 0 | |||||||
| 23 | + | −1 | 6 | 0 | |||||||
| 24 | + | + | −1 | 6 | −1 | ||||||
| 25 | + | 5 | −1 | −3 | |||||||
| 26 | + | + | + | −1 | 9 | −1 | |||||
| 27 | + | + | 1 | 6 | 0 | ||||||
| 28 | + | + | + | 4 | 5 | −4 | |||||
| 29 | + | + | 5 | −1 | −4 | ||||||
| 30 | + | + | + | 0 | 6 | −1 | |||||
| 31 | + | 5 | −1 | −3 | |||||||
| 32 | + | + | + | −1 | 6 | 2 | |||||
| 33 | + | + | + | 0 | 6 | −1 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, L.E.; Jeong, W.; Park, Y.-B.; Jeong, S.J.; Lee, S.-W. Clinical Significance of Antineutrophil Cytoplasmic Antibody Positivity in Patients Infected with SARS-CoV-2. J. Clin. Med. 2022, 11, 4152. https://doi.org/10.3390/jcm11144152
Lee LE, Jeong W, Park Y-B, Jeong SJ, Lee S-W. Clinical Significance of Antineutrophil Cytoplasmic Antibody Positivity in Patients Infected with SARS-CoV-2. Journal of Clinical Medicine. 2022; 11(14):4152. https://doi.org/10.3390/jcm11144152
Chicago/Turabian StyleLee, Lucy Eunju, Wooyong Jeong, Yong-Beom Park, Su Jin Jeong, and Sang-Won Lee. 2022. "Clinical Significance of Antineutrophil Cytoplasmic Antibody Positivity in Patients Infected with SARS-CoV-2" Journal of Clinical Medicine 11, no. 14: 4152. https://doi.org/10.3390/jcm11144152
APA StyleLee, L. E., Jeong, W., Park, Y.-B., Jeong, S. J., & Lee, S.-W. (2022). Clinical Significance of Antineutrophil Cytoplasmic Antibody Positivity in Patients Infected with SARS-CoV-2. Journal of Clinical Medicine, 11(14), 4152. https://doi.org/10.3390/jcm11144152






