Far-Infrared Therapy Improves Arteriovenous Fistula Patency and Decreases Plasma Asymmetric Dimethylarginine in Patients with Advanced Diabetic Kidney Disease: A Prospective Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
2.2. Study Protocol
2.3. Statistical Analysis
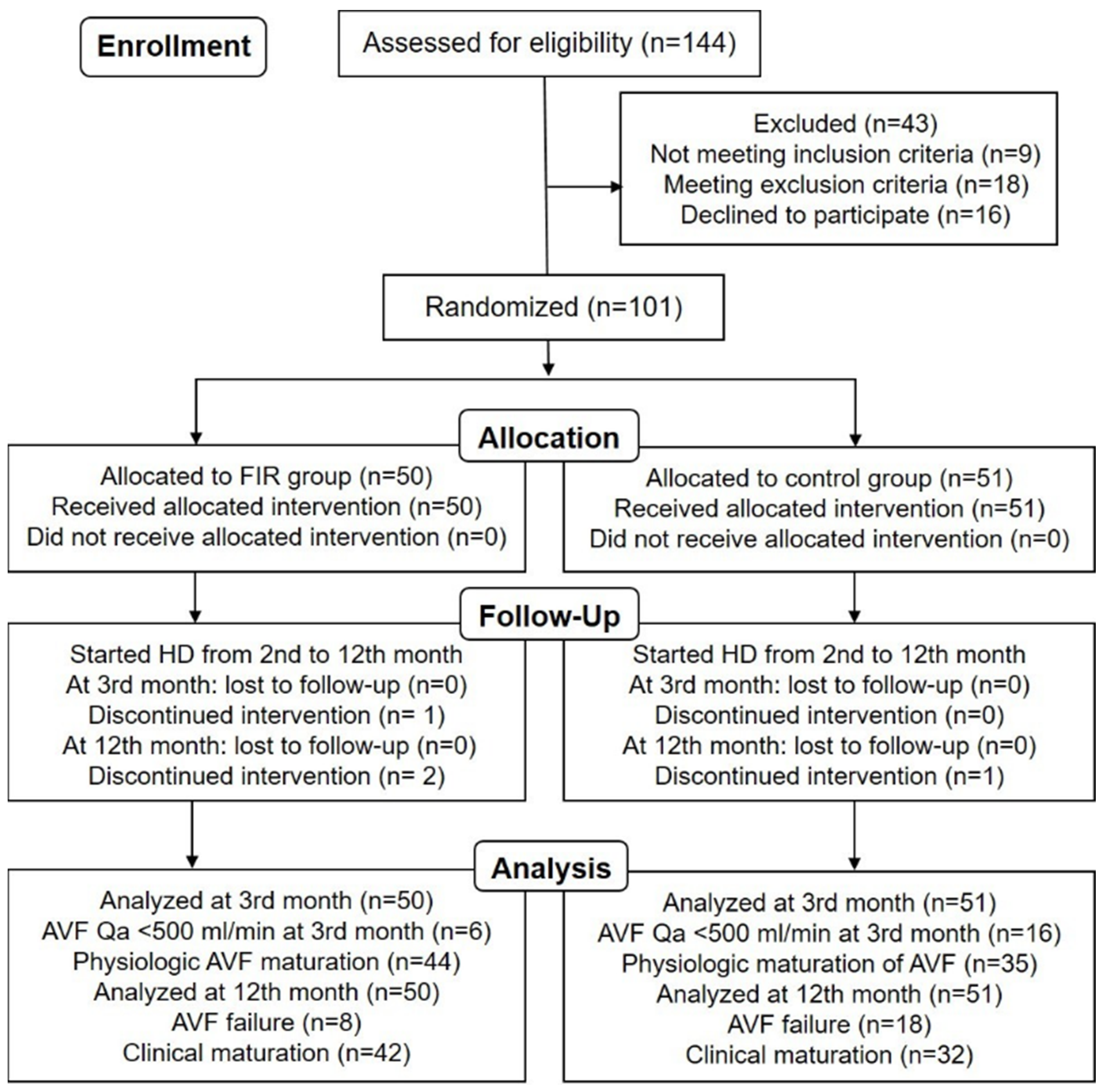
3. Results
3.1. Patient Characteristics
3.2. The Effect of 12-Month FIR Therapy on Qa and Plasma ADMA Concentrations in HD Patients
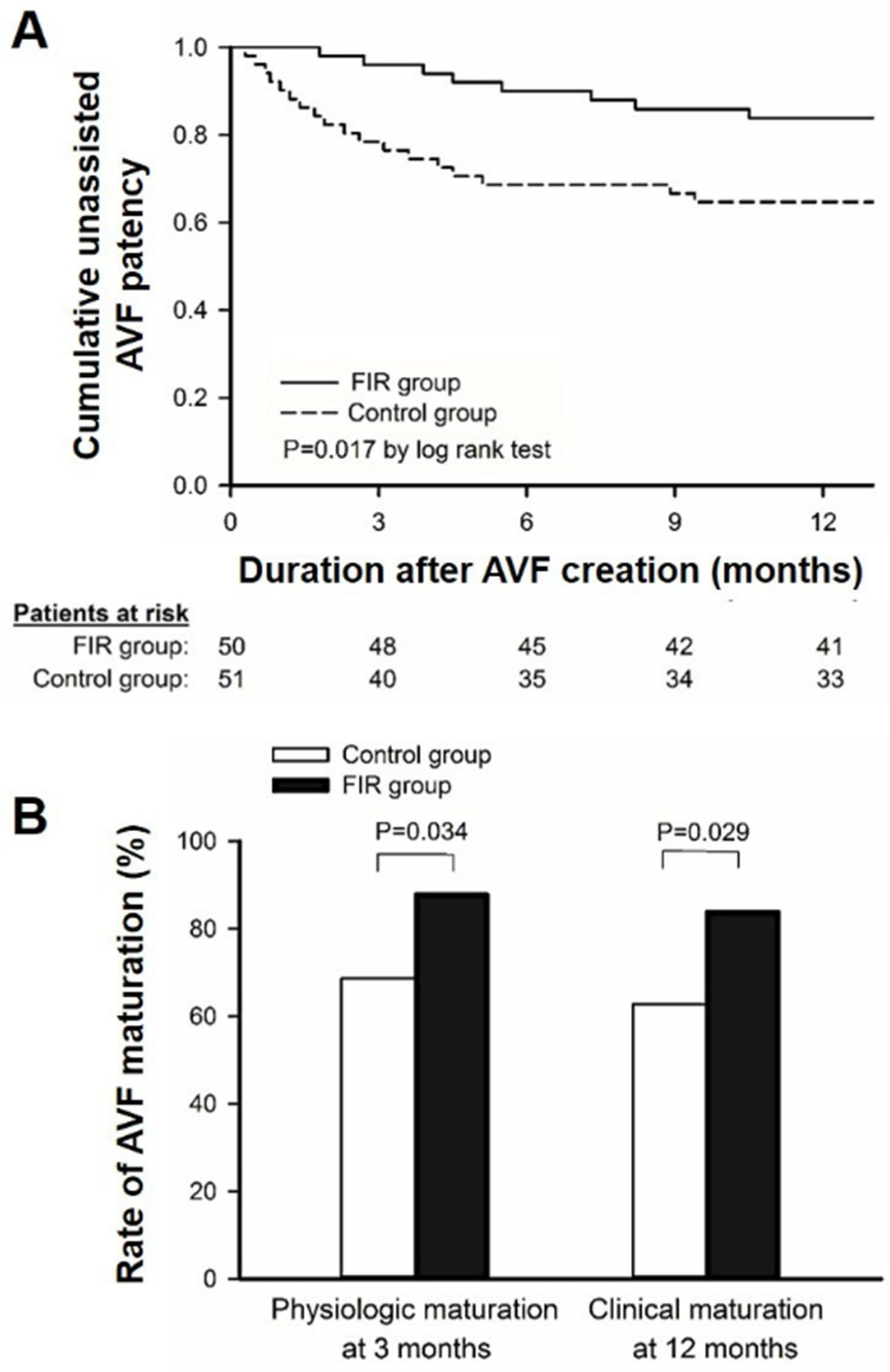
3.3. The Effect of FIR Therapy on AVF Maturation and Patency
3.4. AVF Prognosis and the Change in Plasma ADMA over Time
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zoccali, C.; Bode-Böger, S.; Mallamaci, F.; Benedetto, F.; Tripepi, G.; Malatino, L.; Cataliotti, A.; Bellanuova, I.; Fermo, I.; Frölich, J.; et al. Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: A prospective study. Lancet 2001, 358, 2113–2117. [Google Scholar] [CrossRef]
- Vallance, P.; Leone, A.; Calver, A.; Collier, J.; Moncada, S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet 1992, 339, 572–575. [Google Scholar] [PubMed]
- Jacobi, J.; Tsao, P.S. Asymmetrical dimethylarginine in renal disease: Limits of variation or variation limits? A systematic review. Am. J. Nephrol. 2008, 28, 224–237. [Google Scholar] [CrossRef] [Green Version]
- Mallamaci, F.; Zoccali, C. Clinical Implications of Elevated Asymmetric Dimethylarginine in Chronic Kidney Disease and End-Stage Renal Disease. J. Ren. Nutr. 2009, 19, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.-M.; Chung, M.-Y.; Lin, C.-C.; Hsu, C.-P.; Lin, S.-J. Asymmetric Dimethylarginine and Clinical Outcomes in Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 1566–1572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlesinger, S.; Sonntag, S.R.; Lieb, W.; Maas, R. Asymmetric and symmetric dimethylarginine as risk markers for total mortality and cardiovascular outcomes: A systematic review and meta-analysis of prospective studies. PLoS ONE 2016, 11, e0165811. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-C.; Wen, S.-C.; Yang, C.-W.; Pu, S.-Y.; Tsai, K.-C.; Chen, J.-W. Plasma ADMA Predicts Restenosis of Arteriovenous Fistula. J. Am. Soc. Nephrol. 2009, 20, 213–222. [Google Scholar] [CrossRef] [Green Version]
- De Marco Garcia, L.P.; Davila-Santini, L.R.; Feng, Q.; Calderin, J.; Krishnasastry, K.V.; Panetta, T.F. Primary balloon angioplasty plus balloon angioplasty maturation to upgrade small-caliber veins (<3 mm) for arteriovenous fistulas. J. Vasc. Surg. 2010, 52, 139–144. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-C.; Yang, W.-C.; Chen, M.-C.; Liu, W.-S.; Yang, C.-Y.; Lee, P.-C. Effect of Far Infrared Therapy on Arteriovenous Fistula Maturation: An Open-Label Randomized Controlled Trial. Am. J. Kidney Dis. 2013, 62, 304–311. [Google Scholar] [CrossRef]
- Robertson, V.J.; Low, J.; Ward, A. Electrotherapy Explained: Principles and Practice; Butterworth-Heinemann Elsevier: Edinburgh, UK; London, UK, 2008. [Google Scholar]
- Kipshidze, N.; Nikolaychik, V.; Muckerheidi, M.; Keelan, M.H.; Chekanov, V.; Maternowski, M.; Chawla, P.; Hernandez, I.; Iyer, S.; Dangas, G.; et al. Effect of short pulsed nonablative infrared laser irradiation on vascular cells in vitro and neointimal hyperplasia in a rabbit balloon injury model. Circulation 2001, 104, 1850–1855. [Google Scholar] [CrossRef] [Green Version]
- Masuda, A.; Miyata, M.; Kihara, T.; Minagoe, S.; Tei, C. Repeated Sauna Therapy Reduces Urinary 8-Epi-Prostaglandin F2.ALPHA. Jpn. Heart J. 2004, 45, 297–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.C.; Liu, X.M.; Peyton, K.; Wang, H.; Yang, W.C.; Lin, S.J.; Durante, W. Far infrared therapy inhibits vascular endothelial inflammation via the induction of heme oxygenase-1. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 739–745. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, Y.; Biro, S.; Kamogawa, Y.; Yoshifuku, S.; Eto, H.; Orihara, K.; Yu, B.; Kihara, T.; Miyata, M.; Hamasaki, S.; et al. Repeated Sauna Therapy Increases Arterial Endothelial Nitric Oxide Synthase Expression and Nitric Oxide Production in Cardiomyopathic Hamsters. Circ. J. 2005, 69, 722–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulze, F.; Wesemann, R.; Schwedhelm, E.; Sydow, K.; Albsmeier, J.; Cooke, J.; Böger, R.H. Determination of asymmetric dimethylarginine (ADMA) using a novel ELISA assay. Clin. Chem. Lab. Med. 2004, 42, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-C.; Zuo, L.; Chen, J.-H.; Luo, Q.; Yu, X.-Q.; Li, Y.; Xu, J.-S.; Huang, S.-M.; Wang, L.-N.; Huang, W.; et al. Modified Glomerular Filtration Rate Estimating Equation for Chinese Patients with Chronic Kidney Disease. J. Am. Soc. Nephrol. 2006, 17, 2937–2944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.-C.; Chang, C.-F.; Lai, M.-Y.; Chen, T.-W.; Lee, P.-C.; Yang, W.-C. Far-Infrared Therapy: A Novel Treatment to Improve Access Blood Flow and Unassisted Patency of Arteriovenous Fistula in Hemodialysis Patients. J. Am. Soc. Nephrol. 2007, 18, 985–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Y.; Ye, D.; Yang, L.; Ye, W.; Zhan, D.; Zhang, L.; Xiao, J.; Zeng, Y.; Chen, Q. A meta-analysis of the association between diabetic patients and AVF failure in dialysis. Ren. Fail. 2018, 40, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Oliva-Damaso, E.; Oliva-Damaso, N.; Rodriguez-Esparragon, F.; Payan, J.; Baamonde-Laborda, E.; Gonzalez-Cabrera, F.; Santana-Estupiñan, R.; Rodriguez-Perez, J.C. Asymmetric (ADMA) and Symmetric (SDMA) Dimethylarginines in Chronic Kidney Disease: A Clinical Approach. Int. J. Mol. Sci. 2019, 20, 3668. [Google Scholar] [CrossRef] [Green Version]
- Ronden, R.A.; Houben, A.J.H.M.; Teerlink, T.; Bakker, J.A.; Bierau, J.; Stehouwer, C.D.A.; De Leeuw, P.W.; Kroon, A.A. Reduced renal plasma clearance does not explain increased plasma asymmetric dimethylarginine in hypertensive subjects with mild to moderate renal insufficiency. Am. J. Physiol. Ren. Physiol. 2012, 303, F149–F156. [Google Scholar] [CrossRef] [Green Version]
- Lin, K.Y.; Ito, A.; Asagami, T.; Tsao, P.S.; Adimoolam, S.; Kimoto, M.; Tsuji, H.; Reaven, G.M.; Cooke, J.P. Impaired nitric oxide synthase pathway in diabetes mellitus: Role of asymmetric dimethylarginine and dimethylarginine dimethylaminohydrolase. Circulation 2002, 106, 987–992. [Google Scholar] [CrossRef] [Green Version]
- Tronc, F.; Wassef, M.; Esposito, B.; Henrion, D.; Glagov, S.; Tedgui, A. Role of NO in Flow-Induced Remodeling of the Rabbit Common Carotid Artery. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Osanai, T.; Saitoh, M.; Sasaki, S.; Tomita, H.; Matsunaga, T.; Okumura, K. Effect of shear stress on asymmetric dimethylarginine release from vascular endothelial cells. Hypertension 2003, 42, 985–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soares, M.P.; Seldon, M.P.; Gregoire, I.P.; Vassilevskaia, T.; Berberat, P.O.; Yu, J.; Tsui, T.-Y.; Bach, F.H. Heme Oxygenase-1 Modulates the Expression of Adhesion Molecules Associated with Endothelial Cell Activation. J. Immunol. 2004, 172, 3553–3563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yerra, V.G.; Negi, G.; Sharma, S.S.; Kumar, A. Potential therapeutic effects of the simultaneous targeting of the Nrf2 and NF-kappaB pathways in diabetic neuropathy. Redox Biol. 2013, 1, 394–397. [Google Scholar] [CrossRef] [Green Version]
- Ito, A.; Tsao, P.S.; Adimoolam, S.; Kimoto, M.; Ogawa, T.; Cooke, J.P. Novel mechanism for endothelial dysfunction: Dysregulation of dimethylarginine dimethylaminohydrolase. Circulation 1999, 99, 3092–3095. [Google Scholar] [CrossRef] [Green Version]
- Zoccali, C. Asymmetric dimethylarginine (ADMA): A cardiovascular and renal risk factor on the move. J. Hypertens. 2006, 24, 611–619. [Google Scholar] [CrossRef]
- Thum, T.; Tsikas, D.; Stein, S.; Schultheiss, M.; Eigenthaler, M.; Anker, S.D.; Poole-Wilson, P.A.; Ertl, G.; Bauersachs, J. Suppression of Endothelial Progenitor Cells in Human Coronary Artery Disease by the Endogenous Nitric Oxide Synthase Inhibitor Asymmetric Dimethylarginine. J. Am. Coll. Cardiol. 2005, 46, 1693–1701. [Google Scholar] [CrossRef] [Green Version]
- Lai, C.-C.; Fang, H.-C.; Mar, G.-Y.; Liou, J.-C.; Tseng, C.-J.; Liu, C.-P. Post-angioplasty Far Infrared Radiation Therapy Improves 1-Year Angioplasty-Free Hemodialysis Access Patency of Recurrent Obstructive Lesions. Eur. J. Vasc. Endovasc. Surg. 2013, 46, 726–732. [Google Scholar] [CrossRef] [Green Version]

| Characteristics | Control Group (n = 51) | FIR Group (n = 50) | p |
|---|---|---|---|
| Age (years) | 62.3 ± 14.2 | 62.4 ± 18.9 | 0.980 |
| Females | 45.1 | 50 | 0.622 |
| Comorbidities | |||
| Hypertension | 66.7 | 68 | 0.886 |
| Coronary artery disease | 17.6 | 18 | 0.963 |
| Peripheral artery disease | 5.9 | 8 | 0.678 |
| Cerebrovascular disease | 3.9 | 6 | 0.630 |
| Biochemistry | |||
| Hematocrit (%) | 30.9 ± 4.7 | 30.5 ± 4.8 | 0.782 |
| Serum creatinine (mg/dL) | 7.42 ± 4.41 | 7.35 ± 3.51 | 0.927 |
| eGFR (ml/min/1.73 m2) | 9.02 ± 5.06 | 9.03 ± 5.69 | 0.997 |
| Serum calcium (mg/dL) | 8.3 ± 1.1 | 8.4 ± 1.2 | 0.517 |
| Serum phosphate (mg/dL) | 5.3 ± 1.8 | 5.1 ± 1.6 | 0.374 |
| Parathyroid hormone (pg/mL) | 252.2 ± 211.7 | 279.7 ± 247.2 | 0.587 |
| Urine protein creatinine ratio | 5.7 ± 3.1 | 5.9 ± 3.3 | 0.526 |
| Medications | |||
| Calcium channel blocker | 51 | 40 | 0.268 |
| Angiotensin II receptor blocker | 47.1 | 46 | 0.915 |
| HMG-CoA reductase inhibitor | 35.3 | 34 | 0.891 |
| Antiplatelet agents | 66.7 | 56 | 0.271 |
| Pentoxifylline | 62.7 | 54 | 0.373 |
| Aspirin | 21.6 | 20 | 0.846 |
| Clopidogrel | 5.9 | 6 | 0.980 |
| Characteristics | Control Group (n = 51) | FIR Group (n = 50) | p |
|---|---|---|---|
| Pre-operative diameter of vein for AVF (mm) | 3.64 ± 0.96 | 3.56 ± 0.92 | 0.756 |
| Radiocephalic AVF | 74.5 | 78 | 0.680 |
| Renal function and clearance | |||
| eGFR0 (mL/min/1.73 m2) | 9.02 ± 5.06 | 9.03 ± 5.69 | 0.997 |
| eGFR3 (mL/min/1.73 m2) | 7.51 ± 3.82 | 7.69 ± 3.91 | 0.825 |
| eGFR12 (mL/min/1.73 m2) | 3.54 ± 1.79 | 3.85 ± 1.96 | 0.676 |
| Kt/V12 | 1.52 ± 0.15 | 1.63 ± 0.16 | 0.513 |
| Plasma ADMA concentrations | |||
| ADMA0 (µM) | 1.001 ± 0.230 | 1.026 ± 0.233 | 0.597 |
| ADMA3 (µM) | 1.021 ± 0.268 | 0.990 ± 0.245 | 0.549 |
| ∆ADMA3-0 (µM) | 0.019 ± 0.050 | −0.036 ± 0.047 | 0.01 |
| ADMA12 (µM) | 1.061 ± 0.313 | 0.956 ± 0.288 | 0.082 |
| ∆ADMA12-0 (µM) | 0.060 ± 0.096 | −0.070 ± 0.087 | 0.001 |
| Qa rates | |||
| Qa0 (mL/min) | 301.0 ± 70.0 | 302.2 ± 110.1 | 0.947 |
| Qa1 (mL/min) | 596.5 ± 216.0 | 761.8 ± 276.9 | 0.001 |
| Qa3 (mL/min) | 787.5 ± 259.1 | 982.2 ± 299.4 | 0.001 |
| Qa12 (mL/min) | 842.0 ± 330.1 | 1089.4 ± 376.2 | 0.006 |
| Primary outcomes | |||
| AVF failure within 12 months | 35.3 | 16 | 0.027 |
| AVF occlusion on 12th month | 13.7 | 2 | 0.029 |
| Intervention for AVF within 12 months | 21.6 | 14 | 0.32 |
| Secondary outcomes | |||
| Unassisted AVF patency on 12th month | 64.7 | 84 | 0.017 |
| Physiologic AVF maturation on 3rd month | 68.6 | 88 | 0.034 |
| Clinical AVF maturation within 12 months | 62.7 | 84 | 0.029 |
| Plasma ADMA Concentration | AVF Prognosis | p | ||
|---|---|---|---|---|
| Occlusion Group (n = 8) | Intervention Group (n = 18) | Stable Group (n = 75) | ||
| ADMA0 (µM) | 1.142 ± 0.318 | 1.078 ± 0.299 | 0.984 ± 0.274 | 0.042 |
| ADMA3 (µM) | 1.193 ± 0.352 | 1.104 ± 0.306 | 0.962 ± 0.265 | 0.026 |
| ADMA12 (µM) | 1.261 ± 0.373 | 1.146 ± 0.332 | 0.949 ± 0.247 | 0.011 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-F.; Lee, C.-Y.; Chen, F.-A.; Yang, C.-Y.; Chen, T.-H.; Ou, S.-M.; Lee, K.-H.; Li, C.-P.; Chan, C.-H.; Lee, P.-C.; et al. Far-Infrared Therapy Improves Arteriovenous Fistula Patency and Decreases Plasma Asymmetric Dimethylarginine in Patients with Advanced Diabetic Kidney Disease: A Prospective Randomized Controlled Trial. J. Clin. Med. 2022, 11, 4168. https://doi.org/10.3390/jcm11144168
Chen C-F, Lee C-Y, Chen F-A, Yang C-Y, Chen T-H, Ou S-M, Lee K-H, Li C-P, Chan C-H, Lee P-C, et al. Far-Infrared Therapy Improves Arteriovenous Fistula Patency and Decreases Plasma Asymmetric Dimethylarginine in Patients with Advanced Diabetic Kidney Disease: A Prospective Randomized Controlled Trial. Journal of Clinical Medicine. 2022; 11(14):4168. https://doi.org/10.3390/jcm11144168
Chicago/Turabian StyleChen, Chun-Fan, Chiu-Yang Lee, Fu-An Chen, Chih-Yu Yang, Tz-Heng Chen, Shuo-Ming Ou, Kuo-Hua Lee, Ching-Po Li, Chia-Hao Chan, Pui-Ching Lee, and et al. 2022. "Far-Infrared Therapy Improves Arteriovenous Fistula Patency and Decreases Plasma Asymmetric Dimethylarginine in Patients with Advanced Diabetic Kidney Disease: A Prospective Randomized Controlled Trial" Journal of Clinical Medicine 11, no. 14: 4168. https://doi.org/10.3390/jcm11144168









