Volumetric Changes after Coblation Ablation Tongue (CAT) in Obstructive Sleep Apnea Patients
Abstract
:1. Introduction
2. Methods
2.1. Ethics Statement
2.2. Study Population
2.3. Polysomnography
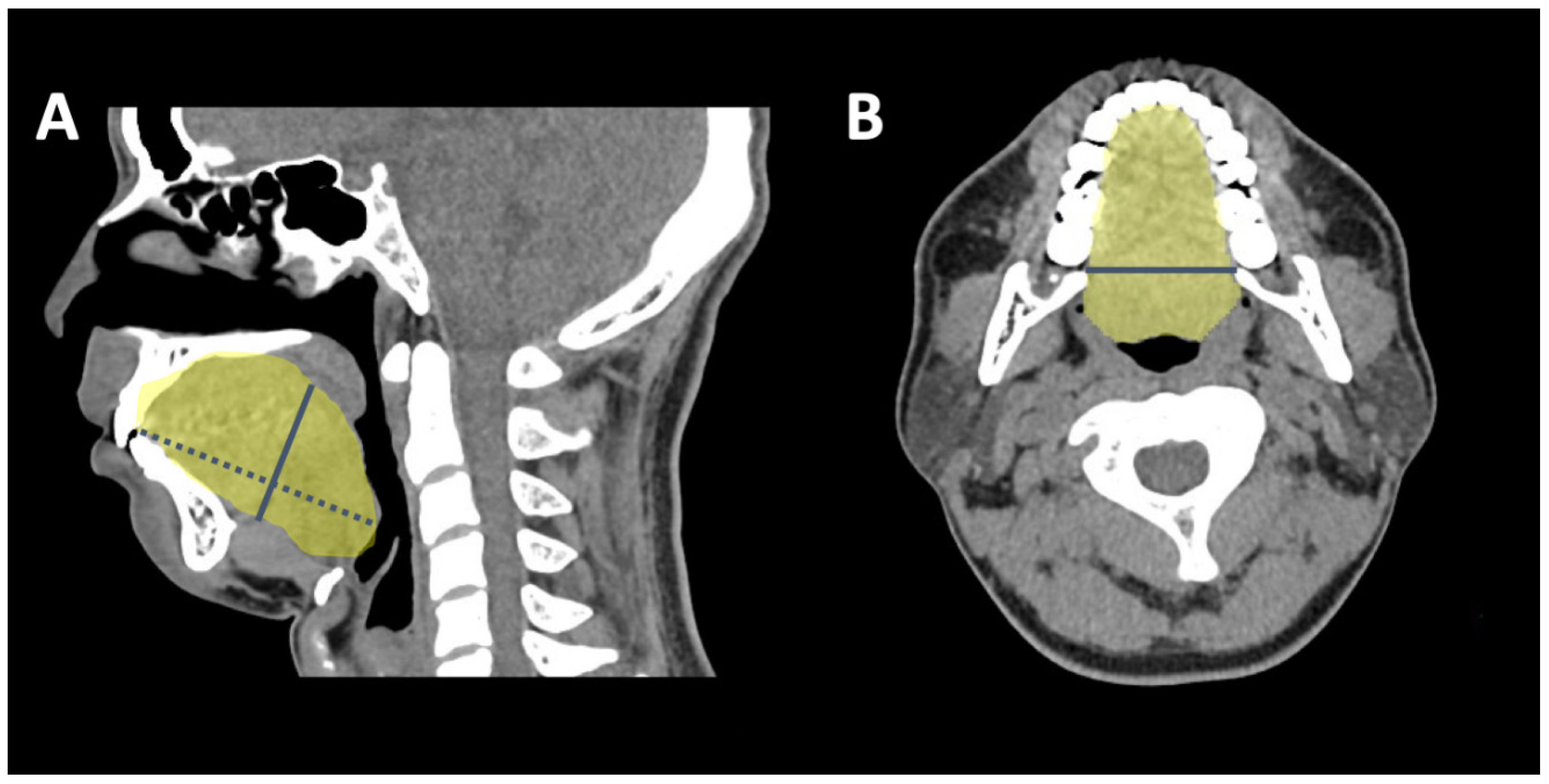
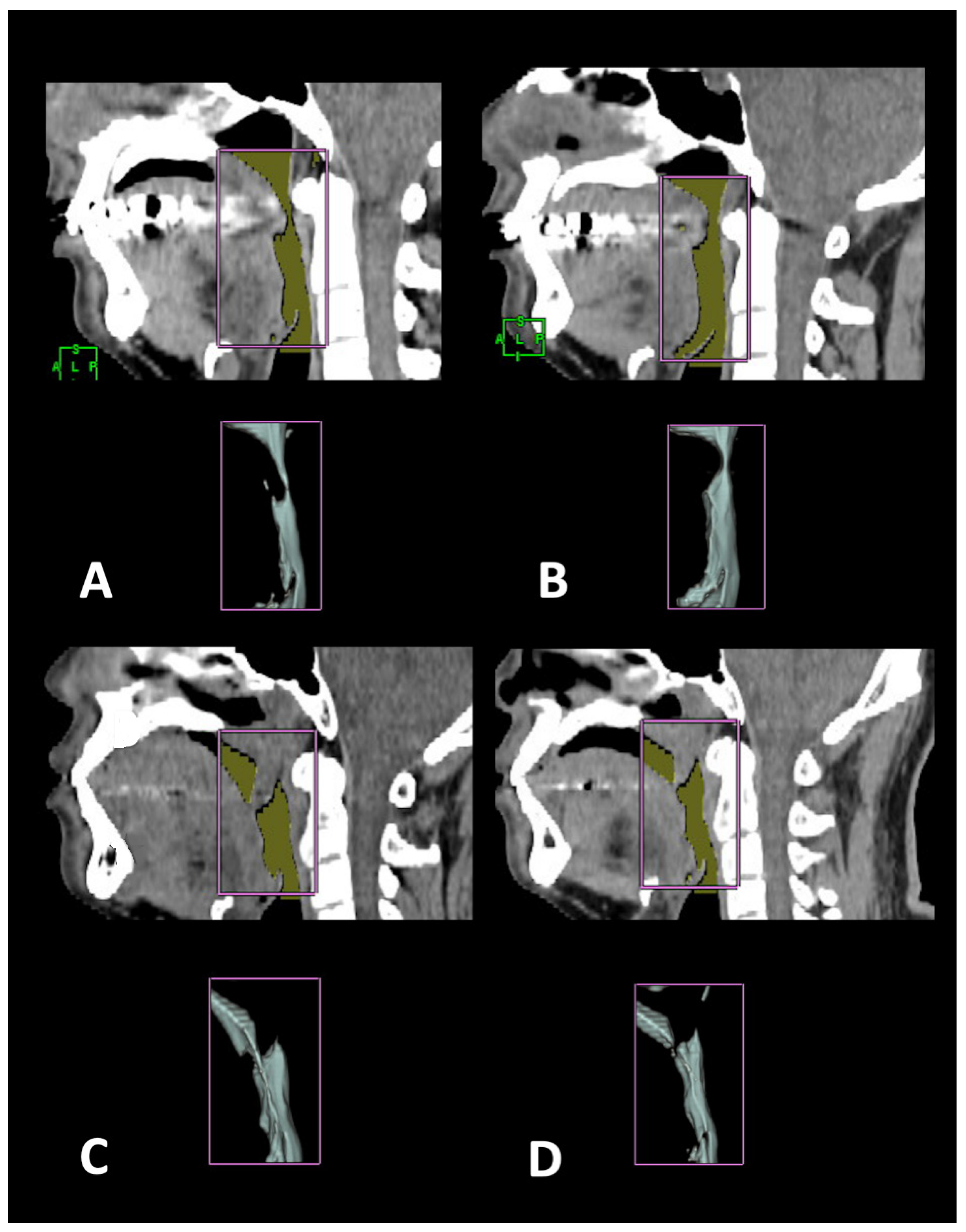
2.4. 3-D CT Measuring Algorithm
2.5. Surgical Plan and Design for Coblation Whole Tongue Surgery
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rotenberg, B.W.; Vicini, C.; Pang, E.B.; Pang, K.P. Reconsidering first-line treatment for obstructive sleep apnea: A systematic review of the literature. J. Otolaryngol. Head Neck Surg. 2016, 45, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Randerath, W.; Verbraecken, J.; de Raaff, C.A.; Hedner, J.; Herkenrath, S.; Hohenhorst, W.; Jakob, T.; Marrone, O.; Marklund, M.; McNicholas, W.T.; et al. European Respiratory Society guideline on non-CPAP therapies for obstructive sleep apnoea. Eur. Resp. Rev. 2021, 30, 210200. [Google Scholar] [CrossRef]
- Ephros, H.D.; Madani, M.; Yalamanchili, S.C. Surgical treatment of snoring & obstructive sleep apnoea. Indian J. Med. Res. 2010, 131, 267. [Google Scholar]
- Li, H.-Y. Palatal surgery for obstructive sleep apnea: From ablation to reconstruction. Sleep Med. Clin. 2019, 14, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Conway, W.; Zorick, F.; Roth, T. Surgical Correction of Anatomic Abnormalities in Obstructive Sleep Apnea Syndrome: Uvulopalatopharyngoplasty. Otolaryngol. Head Neck Surg. 1981, 89, 923–934. [Google Scholar] [CrossRef]
- DOS Riley, R.W.; DOS Powell, N.R. Maxillofacial surgery and obstructive sleep apnea syndrome. Otolaryngol. Clin. N. Am. 1990, 23, 809–826. [Google Scholar] [CrossRef]
- Caples, S.M.; Rowley, J.A.; Prinsell, J.R.; Pallanch, J.F.; Elamin, M.B.; Katz, S.G.; Harwick, J.D. Surgical modifications of the upper airway for obstructive sleep apnea in adults: A systematic review and meta-analysis. Sleep 2010, 33, 1396–1407. [Google Scholar] [CrossRef]
- Verse, T.; Wenzel, S.; Brus, J. Multi-level surgery for obstructive sleep apnea. Lingual tonsillectomy vs. hyoid suspension in combination with radiofrequency of the tongue base. Sleep Breath. 2015, 19, 1361–1366. [Google Scholar] [CrossRef]
- Su, Y.-Y.; Lin, P.-W.; Lin, H.-C.; Chang, C.-T.; Lin, C.-Y.; Friedman, M.; Salapatas, A.M. Systematic review and updated meta-analysis of multi-level surgery for patients with OSA. Auris Nasus Larynx 2021, 49, 421–430. [Google Scholar] [CrossRef]
- Farrar, J.; Ryan, J.; Oliver, E.; Gillespie, M.B. Radiofrequency Ablation for the Treatment of Obstructive Sleep Apnea: A Meta-analysis. Laryngoscope 2008, 118, 1878–1883. [Google Scholar] [CrossRef]
- Baba, R.Y.; Mohan, A.; Metta, V.V.S.R.; Mador, M.J. Temperature controlled radiofrequency ablation at different sites for treatment of obstructive sleep apnea syndrome: A systematic review and meta-analysis. Sleep Breath. 2015, 19, 891–910. [Google Scholar] [CrossRef] [PubMed]
- Kezirian, E.J.; Hohenhorst, W.; de Vries, N. Drug-induced sleep endoscopy: The VOTE classification. Eur. Arch. Otorhinolaryngol. 2011, 268, 1233–1236. [Google Scholar] [CrossRef]
- Sher, A.E.; Schechtman, K.B.; Piccirillo, J.F. The Efficacy of Surgical Modifications of the Upper Airway in Adults With Obstructive Sleep Apnea Syndrome. Sleep 1996, 19, 156–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malhotra, R.K.; Kirsch, D.B.; Kristo, D.A.; Olson, E.J.; Aurora, R.N.; Carden, K.A.; Chervin, R.D.; Martin, J.L.; Ramar, K.; Rosen, C.L.; et al. Polysomnography for obstructive sleep apnea should include arousal-based scoring: An American Academy of Sleep Medicine Position Statement. J. Clin. Sleep Med. 2018, 14, 1245–1247. [Google Scholar] [CrossRef]
- Friedman, M.; Yalamanchali, S.; Gorelick, G.; Joseph, N.J.; Hwang, M.S. A standardized lingual tonsil grading system: Interex-aminer agreement. Otolaryngol. Head Neck Surg. 2015, 152, 667–672. [Google Scholar] [CrossRef]
- Janczy, J.; Woodson, T.; Garcia, G. Anatomical Factors Influencing the Collapsing Pressure in Obstructive Sleep Apnea. In b80-c. Getting Modeled: Bioengineering and Computational Methods; American Thoracic Society: New York, NY, USA, 2017; p. A4443. [Google Scholar]
- Li, H.-Y.; Chen, N.-H.; Wang, C.-R.; Shu, Y.-H.; Wang, P.-C. Use of 3-dimensional computed tomography scan to evaluate upper airway patency for patients undergoing sleep-disordered breathing surgery. Otolaryngol. Head Neck Surg. 2003, 129, 336–342. [Google Scholar] [CrossRef]
- Polo, O.J.; Tafti, M.; Fraga, J.; Porkka, K.V.; Déjean, Y.; Billiard, M. Why don’t all heavy snorers have obstructive sleep apnea? Am. Rev. Respir. Dis. 1991, 143, 1288–1293. [Google Scholar] [CrossRef]
- Kim, A.M.; Keenan, B.T.; Jackson, N.; Chan, E.L.; Staley, B.; Poptani, H.; Torigian, D.A.; Pack, A.I.; Schwab, R.J. Tongue Fat and its Relationship to Obstructive Sleep Apnea. Sleep 2014, 37, 1639–1648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwab, R.J.; Pasirstein, M.; Pierson, R.; Mackley, A.; Hachadoorian, R.; Arens, R.; Maislin, G.; Pack, A.I. Identification of Upper Airway Anatomic Risk Factors for Obstructive Sleep Apnea with Volumetric Magnetic Resonance Imaging. Am. J. Respir. Crit. Care Med. 2003, 168, 522–530. [Google Scholar] [CrossRef]
- Cavaliere, M.; Russo, F.; Iemma, M. Awake versus drug-induced sleep endoscopy: Evaluation of airway obstruction in obstruc-tive sleep apnea/hypopnoea syndrome. Laryngoscope 2013, 123, 2315–2318. [Google Scholar] [CrossRef]
- Powell, N.B.; Riley, R.W.; Troell, R.J.; Blumen, M.B.; Guilleminault, C. Radiofrequency volumetric reduction of the tongue: A por-cine pilot study for the treatment of obstructive sleep apnea syndrome. Chest 1997, 111, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- Chousangsuntorn, K.; Bhongmakapat, T.; Apirakkittikul, N.; Sungkarat, W.; Supakul, N.; Laothamatas, J. Computed Tomography Characterization and Comparison With Polysomnography for Obstructive Sleep Apnea Evaluation. J. Oral Maxillofac. Surg. 2018, 76, 854–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, H.H.; Jeong, W.S.; Choi, J.W.; Jeong, S.E.; Nam, S.Y.; Choi, S.H.; Lee, Y.S.; Kim, H.A.; Kim, Y.J. 3D computer simulation analysis of the flap volume change in total tongue reconstruction flaps. J. Cranio-Maxillofac. Surg. 2018, 46, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Hotwani, K.; Sharma, K.; Jaiswal, A. Evaluation of tongue/mandible volume ratio in children with obstructive sleep apnea. Dent. Press J. Orthod. 2018, 23, 72–78. [Google Scholar] [CrossRef]
- Sillo, T.O.; Lloyd-Owen, S.; White, E.; Abolghasemi-Malekabadi, K.; Lock-Pullan, P.; Ali, M.; Perry, A.; Robinson, S.J.; Wadley, M.S. The impact of bariatric surgery on the resolution of obstructive sleep apnoea. BMC Res. Notes 2018, 11, 385. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Qin, W.; Liu, D. Coblation Channeling for the Tongue. Otolaryngol. Head Neck Surg. 2011, 145 (Suppl. S2), 270. [Google Scholar] [CrossRef]
- MacKay, S.G.; Carney, A.S.; Woods, C.; Antic, N.; McEvoy, R.D.; Chia, M.; Sands, T.; Jones, A.; Hobson, J.; Robinson, S. Modified uvulopa-latopharyngoplasty and coblation channeling of the tongue for obstructive sleep apnea: A multicentre Australian trial. J. Clin. Sleep Med. 2013, 9, 117–124. [Google Scholar] [CrossRef] [Green Version]
- Vicini, C.; Montevecchi, F. Transoral robotic surgery for obstructive sleep apnea: Past, present, and future. Sleep Med. Clin. 2019, 14, 67–72. [Google Scholar] [CrossRef]
- Lee, S.H.; Choi, J.H.; Shin, C.; Lee, H.M.; Kwon, S.Y.; Lee, S.H. How does open-mouth breathing influence upper airway anatomy. Laryngoscope 2007, 117, 1102–1106. [Google Scholar] [CrossRef] [Green Version]


| Parameter | r Value | p Value |
|---|---|---|
| Basic assessment | ||
| BMI (kg/m2) | 0.61 | 0.02 * |
| Age (year) | 0.23 | 0.41 |
| Airway minimal area and diameter | ||
| RP area (cm2) | −0.19 | 0.49 |
| RG area (cm2) | −0.01 | 0.99 |
| Airway volume | ||
| Total volume (cm3) | −0.33 | 0.23 |
| RP volume (cm3) | 0.42 | 0.12 |
| RG volume (cm3) | −0.43 | 0.11 |
| Tongue volume and diameter | ||
| Tongue volume (cm3) | 0.35 | 0.20 |
| Tongue length (cm) | 0.60 | 0.02 * |
| Tongue height (cm) | −0.03 | 0.90 |
| Tongue width (cm) | 0.91 | 0.26 |
| Pre-Surgery | Post-Surgery | ||
|---|---|---|---|
| Parameter | Mean ± Std. | Mean ± Std. | p Value |
| Basic assessment | |||
| AHI (event/h) | 43.8 ± 16.9 | 23.7 ± 13.4 | 0.008 ** |
| AI (event/h) | 28.4 ± 15.6 | 11.6 ± 16.8 | 0.01 * |
| ESS (0–24) | 12.5 ± 5.1 | 7.3 ± 2.6 | 0.005 ** |
| Airway minimal area and diameter | |||
| RP area (cm2) | 27.4 ± 26.7 | 32.7 ± 44.0 | 0.66 |
| RG area (cm2) | 96.2 ± 63.7 | 131.9 ± 72.9 | 0.12 |
| Airway volume | |||
| Total volume (cm3) | 11.6 ± 5.3 | 12.9 ± 3.3 | 0.21 |
| RP volume (cm3) | 2.7 ± 1.9 | 2.8 ± 2.4 | 0.88 |
| RG volume (cm3) | 8.2 ± 4.2 | 9.5 ± 2.8 | 0.24 |
| Tongue volume and diameter | |||
| Tongue volume (cm3) | 91.3 ± 12.6 | 85.6 ± 10.4 | 0.02 * |
| Tongue length (cm) | 71.8 ± 6.4 | 71.2 ± 6.3 | 0.46 |
| Tongue height (cm) | 43.4 ± 6.5 | 44.5 ± 5.9 | 0.21 |
| Tongue width (cm) | 46.5 ± 3.6 | 45.2 ± 5.0 | 0.70 |
| Pre-Surgery | Post-Surgery | |||||
|---|---|---|---|---|---|---|
| Parameter | Success Group (n = 8) | Failure Group (n = 4) | p Value | Success Group (n = 8) | Failure Group (n = 4) | p Value |
| Airway minimal area | ||||||
| RP area (cm2) | 31.3 ± 27.2 | 19.5 ± 27.8 | 0.50 | 44.3 ± 50.4 | 9.5 ± 10.2 | 0.21 |
| RG area (cm2) | 99.3 ± 54.8 | 90.0 ± 88.3 | 0.83 | 161.0 ± 70.4 | 73.8 ± 33.9 | 0.04 * |
| Airway volume | ||||||
| Total volume (cm3) | 12.4 ± 4.0 | 10.2 ± 7.9 | 0.53 | 14.4 ± 3.0 | 7.0 ± 1.2 | 0.02 * |
| RP volume (cm3) | 2.9 ± 2.3 | 2.0 ± 0.8 | 0.50 | 2.8 ± 2.4 | 2.1 ± 0.6 | 0.61 |
| RG volume (cm3) | 9.1 ± 3.8 | 6.5 ± 5.0 | 0.32 | 11.1 ± 1.8 | 6.3 ± 1.2 | 0.001 ** |
| Tongue volume and diameter | ||||||
| Tongue volume (cm3) | 89.3 ± 11.2 | 95.4 ± 16.0 | 0.45 | 82.1 ± 8.4 | 92.8 ± 11.5 | 0.09 |
| Tongue length (cm) | 70.0 ± 5.9 | 75.4 ± 6.4 | 0.18 | 68.8 ± 5.0 | 76.1 ± 6.2 | 0.05 |
| Tongue height (cm) | 44.6 ± 6.6 | 41.0 ± 6.6 | 0.39 | 45.1 ± 6.7 | 43.2 ± 4.4 | 0.62 |
| Tongue width (cm) | 46.7 ± 4.2 | 46.1 ± 2.3 | 0.79 | 43.5 ± 4.8 | 48.7 ± 3.8 | 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.-A.; Wang, C.-J.; Chiang, Y.-T.; Li, H.-Y. Volumetric Changes after Coblation Ablation Tongue (CAT) in Obstructive Sleep Apnea Patients. J. Clin. Med. 2022, 11, 4186. https://doi.org/10.3390/jcm11144186
Lu Y-A, Wang C-J, Chiang Y-T, Li H-Y. Volumetric Changes after Coblation Ablation Tongue (CAT) in Obstructive Sleep Apnea Patients. Journal of Clinical Medicine. 2022; 11(14):4186. https://doi.org/10.3390/jcm11144186
Chicago/Turabian StyleLu, Yi-An, Chao-Jan Wang, Yen-Ting Chiang, and Hsueh-Yu Li. 2022. "Volumetric Changes after Coblation Ablation Tongue (CAT) in Obstructive Sleep Apnea Patients" Journal of Clinical Medicine 11, no. 14: 4186. https://doi.org/10.3390/jcm11144186
APA StyleLu, Y.-A., Wang, C.-J., Chiang, Y.-T., & Li, H.-Y. (2022). Volumetric Changes after Coblation Ablation Tongue (CAT) in Obstructive Sleep Apnea Patients. Journal of Clinical Medicine, 11(14), 4186. https://doi.org/10.3390/jcm11144186






