Pleomorphic Adenoma of the Salivary Glands and Epithelial–Mesenchymal Transition
Abstract
:1. What Is Pleomorphic Adenoma (PA)?
2. Epithelial–Mesenchymal Transition (EMT) in Tumor Progression
2.1. EMT Marker
2.1.1. Major Epithelial Markers
E-Cadherin
Cytokeratin
2.1.2. Major Mesenchymal Markers
N-Cadherin
Vimentin
Fibronectin
3. EMT in PAs
4. EMT-Activating Transcription Factors (EMT-TFs) in PAs
4.1. TWIST and SLUG Are Expressed as EMT-TFs in PAs
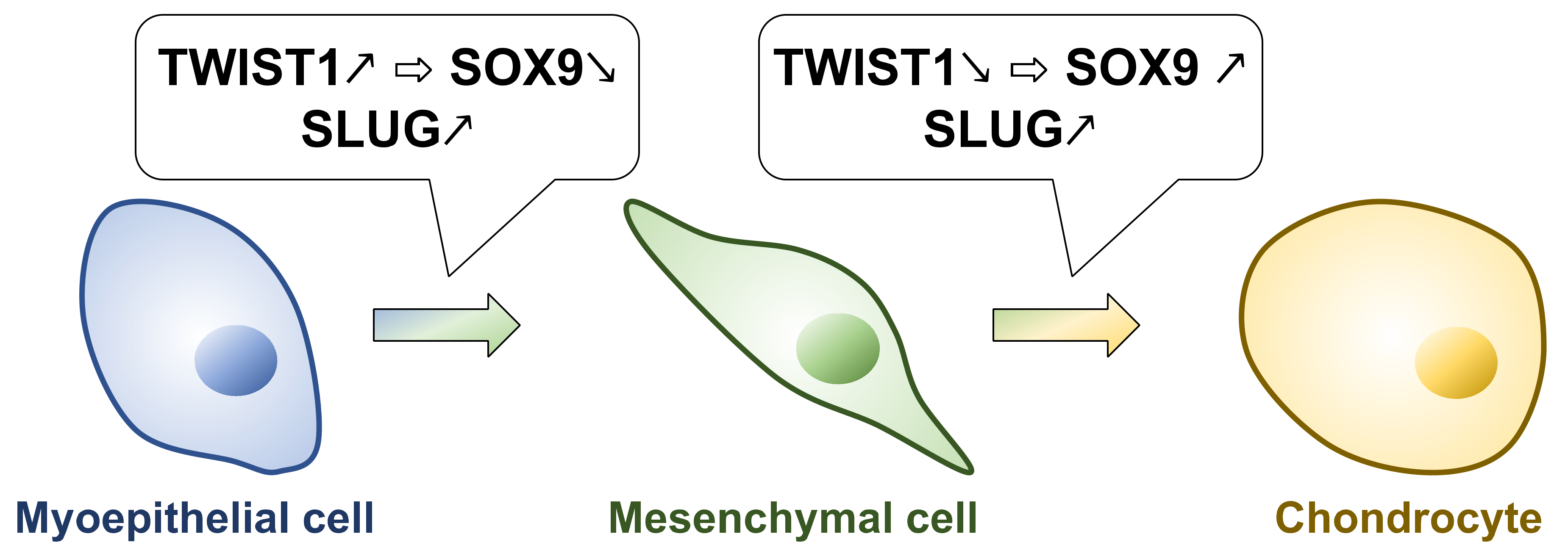
4.2. TWIST1 Inhibits Chondrocyte Differentiation in PAs
4.3. SLUG Is an Important EMT-TF for EMT Induction of Myoepithelial Cells in PA
5. Carcinoma Ex-Pleomorphic Adenoma (Ca ex PA) and EMT
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajendran, R. Shafer’s Textbook of Oral Pathology; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Regezi, J.A.; Batsakis, J.G. Histogenesis of Salivary Gland Neoplasms. Otolaryngol. Clin. N. Am. 1977, 10, 297–307. [Google Scholar] [CrossRef]
- Luna, M.A. Head and neck surgical pathology. In Salivary Glands; Pilch, B.Z., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001; pp. 284–349. [Google Scholar]
- Pons Vicente, O.; Almendros Marqués, N.; Berini Aytés, L.; Gay Escoda, C. Tumors of Minor Salivary Glands. Med. Oral Patol. Oral Cir. Bucal 2008, 13, 582–590. [Google Scholar]
- Almeslet, A.S. Pleomorphic Adenoma: A Systematic Review. Int. J. Clin. Pediatric Dent. 2020, 13, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Zarbo, R.J. Salivary Gland Neoplasia: A Review for the Practicing Pathologist. Mod. Pathol. 2002, 15, 298–323. [Google Scholar] [CrossRef] [Green Version]
- Mittal, D.G.; Aggrawal, D.A.; Garg, D.R.; Rathi, D.A.; Ganguly, D.A. Pleomorphic Adenoma: A Case Report. Int. J. Appl. Dent. Sci. 2017, 3, 154–155. [Google Scholar]
- Martinelli, M.; Martini, F.; Rinaldi, E.; Caramanico, L.; Magri, E.; Grandi, E.; Carinci, F.; Pastore, A.; Tognon, M. Simian Virus 40 Sequences and Expression of the Viral Large T Antigen Oncoprotein in Human Pleomorphic Adenomas of Parotid Glands. Am. J. Pathol. 2002, 161, 1127. [Google Scholar] [CrossRef] [Green Version]
- Friedrich, R.E.; Li, L.; Knop, J.; Giese, M.; Schmelzl, R. Pleomorphic Adenoma of the Salivary Glands: Analysis of 94 Patients. Anticancer Res. 2005, 25, 1703–1705. [Google Scholar]
- Nagaraj, H.; Raikar, R.; Rajalakshmi, T.A.; Asha, M.; Mutgi, A. The World’s Biggest Benign Parotid Tumour “Pleomorphic Adenoma”: A Rare Case Report. IOSR J. Dent. Med. Sci. 2014, 13, 8–12. [Google Scholar] [CrossRef]
- Sergi, B.; Limongelli, A.; Scarano, E.; Fetoni, A.R.; Paludetti, G. Giant Deep Lobe Parotid Gland Pleomorphic Adenoma Involving the Parapharyngeal Space. Report of Three Cases and Review of the Diagnostic and Therapeutic Approaches. Acta Otorhinolaryngol. Ital. 2008, 28, 261. [Google Scholar]
- Auclair, P.L.; Ellis, G.L. Atypical Features in Salivary Gland Mixed Tumors: Their Relationship to Malignant Transformation. Mod. Pathol. 1996, 9, 652–657. [Google Scholar]
- Harada, H.; Kawahara, A. Salivary Gland Tumors: Practical Learning with Consultation Cases, 1st ed.; Medical View Co., Ltd.: Tokyo, Japan, 2018; ISBN 978-4-7583-0397-2. [Google Scholar]
- Liu, X.; Yang, X.; Zhan, C.; Zhang, Y.; Hou, J.; Yin, X. Perineural Invasion in Adenoid Cystic Carcinoma of the Salivary Glands: Where We Are and Where We Need to Go. Front. Oncol. 2020, 10, 1493. [Google Scholar] [CrossRef]
- Morita, Y.; Kashima, K.; Suzuki, M.; Kinosada, H.; Teramoto, A.; Matsumiya, Y.; Uzawa, N. Differential Diagnosis between Oral Metastasis of Renal Cell Carcinoma and Salivary Gland Cancer. Diagnostics 2021, 11, 506. [Google Scholar] [CrossRef]
- Auclair, P.L.; Langloss, J.M.; Weiss, S.W.; Corio, R.L. Sarcomas and Sarcomatoid Neoplasms of the Major Salivary Gland Regions. A Clinicopathologic and Immunohistochemical Study of 67 Cases and Review of the Literature. Cancer 1986, 58, 1305–1315. [Google Scholar] [CrossRef]
- Ghosh, A.; Arundhati; Asthana, A.K. Pleomorphic Adenoma of the Parotid Gland Metastasizing to the Scapular Region: A Case Report. Acta Cytol. 2008, 52, 733–735. [Google Scholar] [CrossRef]
- Knight, J.; Ratnasingham, K. Metastasising Pleomorphic Adenoma: Systematic Review. Int. J. Surg. 2015, 19, 137–145. [Google Scholar] [CrossRef]
- Bradley, P.J. Recurrent Salivary Gland Pleomorphic Adenoma: Etiology, Management, and Results. Curr. Opin. Otolaryngol. Head Neck Surg. 2001, 9, 100–108. [Google Scholar] [CrossRef]
- Hay, E.D. An Overview of Epithelio-Mesenchymal Transformation. Acta Anat. 1995, 154, 8–20. [Google Scholar] [CrossRef]
- Nieto, M.A.; Huang, R.Y.Y.J.; Jackson, R.A.A.; Thiery, J.P.P. EMT: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.J.; Nieto, M.A. Epithelial-Mesenchymal Transitions in Development and Disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef]
- Arnoux, V.; Nassour, M.; L’Helgoualc’h, A.; Hipskind, R.A.; Savagner, P. Erk5 Controls Slug Expression and Keratinocyte Activation during Wound Healing. Mol. Biol. Cell 2008, 19, 4738–4749. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, N.; Maines-Bandiera, S.; Quinn, M.A.; Unger, W.G.; Dedhar, S.; Auersperg, N. Molecular Pathways Regulating EGF-Induced Epithelio-Mesenchymal Transition in Human Ovarian Surface Epithelium. Am. J. Physiol. Cell Physiol. 2006, 290, C1532–C1542. [Google Scholar] [CrossRef] [PubMed]
- McGranahan, N.; Swanton, C. Clonal Heterogeneity and Tumor Evolution: Past, Present, and the Future. Cell 2017, 168, 613–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brabletz, S.; Schuhwerk, H.; Brabletz, T.; Stemmler, M.P. Dynamic EMT: A Multi-Tool for Tumor Progression. EMBO J. 2021, 40, e108647. [Google Scholar] [CrossRef] [PubMed]
- Batlle, E.; Sancho, E.; Francí, C.; Domínguez, D.; Monfar, M.; Baulida, J.; García De Herreros, A. The Transcription Factor Snail Is a Repressor of E-Cadherin Gene Expression in Epithelial Tumour Cells. Nat. Cell Biol. 2000, 2, 84–89. [Google Scholar] [CrossRef]
- Cano, A.; Pérez-Moreno, M.A.; Rodrigo, I.; Locascio, A.; Blanco, M.J.; del Barrio, M.G.; Portillo, F.; Nieto, M.A. The Transcription Factor Snail Controls Epithelial-Mesenchymal Transitions by Repressing E-Cadherin Expression. Nat. Cell Biol. 2000, 2, 76–83. [Google Scholar] [CrossRef]
- Yang, J.; Mani, S.A.; Donaher, J.L.; Ramaswamy, S.; Itzykson, R.A.; Come, C.; Savagner, P.; Gitelman, I.; Richardson, A.; Weinberg, R.A. Twist, a Master Regulator of Morphogenesis, Plays an Essential Role in Tumor Metastasis. Cell 2004, 117, 927–939. [Google Scholar] [CrossRef] [Green Version]
- Eger, A.; Aigner, K.; Sonderegger, S.; Dampier, B.; Oehler, S.; Schreiber, M.; Berx, G.; Cano, A.; Beug, H.; Foisner, R. DeltaEF1 Is a Transcriptional Repressor of E-Cadherin and Regulates Epithelial Plasticity in Breast Cancer Cells. Oncogene 2005, 24, 2375–2385. [Google Scholar] [CrossRef] [Green Version]
- Comijn, J.; Berx, G.; Vermassen, P.; Verschueren, K.; van Grunsven, L.; Bruyneel, E.; Mareel, M.; Huylebroeck, D.; van Roy, F. The Two-Handed E Box Binding Zinc Finger Protein SIP1 Downregulates E-Cadherin and Induces Invasion. Mol. Cell 2001, 7, 1267–1278. [Google Scholar] [CrossRef] [Green Version]
- Dongre, A.; Weinberg, R.A. New Insights into the Mechanisms of Epithelial-Mesenchymal Transition and Implications for Cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef]
- Stemmler, M.P.; Eccles, R.L.; Brabletz, S.; Brabletz, T. Non-Redundant Functions of EMT Transcription Factors. Nat. Cell Biol. 2019, 21, 102–112. [Google Scholar] [CrossRef]
- Brabletz, T.; Kalluri, R.; Nieto, M.A.; Weinberg, R.A. EMT in Cancer. Nat. Rev. Cancer 2018, 18, 128–134. [Google Scholar] [CrossRef]
- Puisieux, A.; Brabletz, T.; Caramel, J. Oncogenic Roles of EMT-Inducing Transcription Factors. Nat. Cell Biol. 2014, 16, 488–494. [Google Scholar] [CrossRef]
- Morita, Y.; Hata, K.; Nakanishi, M.; Omata, T.; Morita, N.; Yura, Y.; Nishimura, R.; Yoneda, T. Cellular Fibronectin 1 Promotes VEGF-C Expression, Lymphangiogenesis and Lymph Node Metastasis Associated with Human Oral Squamous Cell Carcinoma. Clin. Exp. Metastasis 2015, 32, 739–753. [Google Scholar] [CrossRef]
- Aigner, T.; Neureiter, D.; Völker, U.; Belke, J.; Kirchner, T. Epithelial–Mesenchymal Transdifferentiation and Extracellular Matrix Gene Expression in Pleomorphic Adenomas of the Parotid Salivary Gland. J. Pathol. 1998, 186, 178–185. [Google Scholar] [CrossRef]
- Triantafyllou, A.; Thompson, L.D.R.; Devaney, K.O.; Bell, D.; Hunt, J.L.; Rinaldo, A.; Vander Poorten, V.; Ferlito, A. Functional Histology of Salivary Gland Pleomorphic Adenoma: An Appraisal. Head Neck Pathol. 2015, 9, 387–404. [Google Scholar] [CrossRef] [Green Version]
- Masson, P. Human Tumors: Histology Diagnosis and Technique, 2nd ed.; Wayne State University Press: Detroit, MI, USA, 1970; 1103p. [Google Scholar]
- Masson, P. The Rôle of the Neural Crests in the Embryonal Adenosarcomas of the Kidney1. Am. J. Cancer 1938, 33, 1–32. [Google Scholar] [CrossRef] [Green Version]
- Harrison, J.D.; Auger, D.W. Mucosubstance Histochemistry of Pleomorphic Adenoma of Parotid and Submandibular Salivary Glands of Man: Light and Electron Microscopy. Histochem. J. 1991, 23, 293–302. [Google Scholar] [CrossRef]
- Zhao, M.; Takata, T.; Kudo, Y.; Sato, S.; Ogawa, I.; Wakida, K.; Uchida, T.; Nikai, H. Biosynthesis of Glycosaminoglycans and Aggrecan by Tumor Cells in Salivary Pleomorphic Adenoma: Ultrastructural Evidence. J. Oral Pathol. Med. 1999, 28, 442–450. [Google Scholar] [CrossRef]
- Economopoulou, P.; Hanby, A.; Odell, E.W. Expression of E-Cadherin, Cellular Differentiation and Polarity in Epithelial Salivary Neoplasms. Oral Oncol. 2000, 36, 515–518. [Google Scholar] [CrossRef]
- Franchi, A.; Santoro, R.; Paglierani, M.; Bondi, R. Immunolocalization of Alpha 2, Alpha 5, and Alpha 6 Integrin Subunits in Salivary Tissue and Adenomas of the Parotid Gland. J. Oral Pathol. Med. 1994, 23, 457–460. [Google Scholar] [CrossRef]
- Neureiter, D.; Böhmer, J.; Kirchner, T.; Aigner, T. Pleomorphic Adenomas of the Parotid Express Different Mesenchymal Phenotypes: Demonstration of Matrix Gene Expression Products Characteristic of the Fibroblastic and Chondrocytic Cell Lineages. Histopathology 1999, 35, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P. Epithelial-Mesenchymal Transitions in Development and Pathologies. Curr. Opin. Cell Biol. 2003, 15, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Morgan, P.R.; Harrison, D.L.; Waseem, A.; Lane, E.B. Expression of Keratin MRNAs and Proteins in Normal Salivary Epithelia and Pleomorphic Adenomas. J. Pathol. 1993, 171, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Kusafuka, K.; Yamaguchi, A.; Kayano, T.; Takemura, T. Immunohistochemical Localization of Members of the Transforming Growth Factor (TGF)-Beta Superfamily in Normal Human Salivary Glands and Pleomorphic Adenomas. J. Oral Pathol. Med. 2001, 30, 413–420. [Google Scholar] [CrossRef]
- Nawshad, A.; Lagamba, D.; Polad, A.; Hay, E.D. Transforming Growth Factor-Beta Signaling during Epithelial-Mesenchymal Transformation: Implications for Embryogenesis and Tumor Metastasis. Cells Tissues Organs 2005, 179, 11–23. [Google Scholar] [CrossRef]
- Seifert, G.; Donath, K.; Schäfer, R. Lipomatous Pleomorphic Adenoma of the Parotid Gland. Classification of Lipomatous Tissue in Salivary Glands. Pathol. Res. Pract. 1999, 195, 247–252. [Google Scholar] [CrossRef]
- Haskell, H.D.; Butt, K.M.; Woo, S.-B. Pleomorphic Adenoma with Extensive Lipometaplasia: Report of Three Cases. Am. J. Surg. Pathol. 2005, 29, 1389–1393. [Google Scholar] [CrossRef]
- Garrett, J.R.; Lenninger, S.; Ohlin, P. Concerning Possible Contractile Mechanisms in the Pancreas–Myoepithelial Cells. Experientia 1970, 26, 741. [Google Scholar] [CrossRef]
- Langman, G.; Andrews, C.L.; Weissferdt, A. WT1 Expression in Salivary Gland Pleomorphic Adenomas: A Reliable Marker of the Neoplastic Myoepithelium. Mod. Pathol. 2011, 24, 168–174. [Google Scholar] [CrossRef]
- Leader, R.; Deol-Poonia, R.K.; Sheard, J.; Triantafyllou, A. Immunohistochemical Localization of WT1 in Epithelial Salivary Tumors. Pathol. Res. Pract. 2014, 210, 726–732. [Google Scholar] [CrossRef]
- Hohenstein, P.; Hastie, N.D. The Many Facets of the Wilms’ Tumour Gene, WT1. Hum. Mol. Genet. 2006, 15, R196–R201. [Google Scholar] [CrossRef]
- Tsuneki, M.; Maruyama, S.; Yamazaki, M.; Essa, A.; Abé, T.; Babkair, H.A.; Ahsan, M.S.; Cheng, J.; Saku, T. Podoplanin Is a Novel Myoepithelial Cell Marker in Pleomorphic Adenoma and Other Salivary Gland Tumors with Myoepithelial Differentiation. Virchows Arch. 2013, 462, 297–305. [Google Scholar] [CrossRef]
- Cheuk, W.; Chan, J.K. Salivary Gland Tumors. In Diagnostic Histopathology of Tumors; Churchill Livingstone Elsevier: Philadelphia, PA, USA, 2007; pp. 239–325. [Google Scholar]
- Enescu, A.; Enescu, A.Ş.; Florou, C.; Petrescu, F. E-Cadherin and α-SMA Expression in the Epithelial-Mesenchymal Transition of Salivary Glands Pleomorphic Adenomas. Rom. J. Morphol. Embryol. 2014, 55, 1383–1387. [Google Scholar]
- Devi, A.; Yadav, A.B.; Kamboj, M.; Narwal, A.; Kumar, V.; Singh, V. Potential Immmunohistochemical Markers to Characterize Epithelial-Mesenchymal Transition in Pleomorphic Adenoma. J. Exp. Ther. Oncol. 2019, 13, 1–7. [Google Scholar]
- Pardis, S.; Zare, R.; Jaafari-Ashkavandi, Z.; Ashraf, M.J.; Khademi, B. Twist Expression in Pleomorphic Adenoma, Adenoid Cystic Carcinoma and Mucoepidermoid Carcinoma of Salivary Glands. Turk. Patoloji. Derg. 2016, 32, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, Y.; Sato, S.; Maeda, T.; Kishino, M.; Toyosawa, S.; Usami, Y.; Iwai, S.; Nakazawa, M.; Yura, Y.; Ogawa, Y. Transcription Factors Related to Chondrogenesis in Pleomorphic Adenoma of the Salivary Gland: A Mechanism of Mesenchymal Tissue Formation. Lab. Investig. 2016, 96, 16–24. [Google Scholar] [CrossRef]
- Shen, M.; Wen, Y.; Hua, C.; Xiao, J. The Expression of Twist in Salivary Adenoid Cystic Carcinoma and Its Clinicopathological Significance. Chin. Ger. J. Clin. Oncol. 2010, 9, 187–192. [Google Scholar] [CrossRef]
- Kim, H.; Lee, S.B.; Myung, J.K.; Park, J.H.; Park, E.; Kim, D.I.; Lee, C.; Kim, Y.; Park, C.-M.; Kim, M.B.; et al. SLUG Is a Key Regulator of Epithelial-Mesenchymal Transition in Pleomorphic Adenoma. Lab. Investig. 2022, 102, 631–640. [Google Scholar] [CrossRef]
- Yuen, H.-F.; Chua, C.-W.; Chan, Y.-P.; Wong, Y.-C.; Wang, X.; Chan, K.-W. Significance of TWIST and E-Cadherin Expression in the Metastatic Progression of Prostatic Cancer. Histopathology 2007, 50, 648–658. [Google Scholar] [CrossRef]
- Fendrich, V.; Waldmann, J.; Feldmann, G.; Schlosser, K.; König, A.; Ramaswamy, A.; Bartsch, D.K.; Karakas, E. Unique Expression Pattern of the EMT Markers Snail, Twist and E-Cadherin in Benign and Malignant Parathyroid Neoplasia. Eur. J. Endocrinol. 2009, 160, 695–703. [Google Scholar] [CrossRef] [Green Version]
- Merikallio, H.; Pääkkö, P.; Salmenkivi, K.; Kinnula, V.; Harju, T.; Soini, Y. Expression of Snail, Twist, and Zeb1 in Malignant Mesothelioma. APMIS 2013, 121, 1–10. [Google Scholar] [CrossRef]
- De Freitas Silva, B.-S.; Yamamoto, F.-P.; Corrêa Pontes, F.-S.; Cury, S.-E.; Fonseca, F.-P.; Rebelo-Pontes, H.; Pinto-Júnior, D.S. TWIST and P-Akt Immunoexpression in Normal Oral Epithelium Oral Dysplasia and in Oral Squamous Cell Carcinoma. Med. Oral Patol. Oral Cir. Bucal. 2012, 17, e29–e34. [Google Scholar] [CrossRef]
- Savera, A.T.; Zarbo, R.J. Defining the Role of Myoepithelium in Salivary Gland Neoplasia. Adv. Anat. Pathol. 2004, 11, 69–85. [Google Scholar] [CrossRef]
- Jang, B.G.; Kim, H.S.; Chang, W.Y.; Bae, J.M.; Kim, W.H.; Kang, G.H. Expression Profile of LGR5 and Its Prognostic Significance in Colorectal Cancer Progression. Am. J. Pathol. 2018, 188, 2236–2250. [Google Scholar] [CrossRef] [Green Version]
- Maruyama, S.; Cheng, J.; Shingaki, S.; Tamura, T.; Asakawa, S.; Minoshima, S.; Shimizu, Y.; Shimizu, N.; Saku, T. Establishment and Characterization of Pleomorphic Adenoma Cell Systems: An in-Vitro Demonstration of Carcinomas Arising Secondarily from Adenomas in the Salivary Gland. BMC Cancer 2009, 9, 247. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Kang, K.; Lee, S.B.; Seo, D.; Yoon, S.; Kim, S.J.; Jang, K.; Jung, Y.K.; Lee, K.G.; Factor, V.M.; et al. Small Molecule-Mediated Reprogramming of Human Hepatocytes into Bipotent Progenitor Cells. J. Hepatol. 2019, 70, 97–107. [Google Scholar] [CrossRef]
- Park, J.-H.; Kim, Y.-H.; Shim, S.; Kim, A.; Jang, H.; Lee, S.-J.; Park, S.; Seo, S.; Jang, W.I.; Lee, S.B.; et al. Radiation-Activated PI3K/AKT Pathway Promotes the Induction of Cancer Stem-Like Cells via the Upregulation of SOX2 in Colorectal Cancer. Cells 2021, 10, 135. [Google Scholar] [CrossRef]
- De Brito, B.S.; Giovanelli, N.; Egal, E.S.; Sánchez-Romero, C.; do Nascimento, J.D.S.; Martins, A.S.; Tincani, Á.J.; Negro, A.D.; Gondak, R.D.O.; Almeida, O.P.D.; et al. Loss of Expression of Plag1 in Malignant Transformation from Pleomorphic Adenoma to Carcinoma Ex Pleomorphic Adenoma. Hum. Pathol. 2016, 57, 152–159. [Google Scholar] [CrossRef]
- Voz, M.L.; Mathys, J.; Hensen, K.; Pendeville, H.; Van Valckenborgh, I.; Van Huffel, C.; Chavez, M.; Van Damme, B.; De Moor, B.; Moreau, Y.; et al. Microarray Screening for Target Genes of the Proto-Oncogene PLAG1. Oncogene 2004, 23, 179–191. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.; Keckesova, Z.; Donaher, J.L.; Shibue, T.; Tischler, V.; Reinhardt, F.; Itzkovitz, S.; Noske, A.; Zürrer-Härdi, U.; Bell, G.; et al. Slug and Sox9 Cooperatively Determine the Mammary Stem Cell State. Cell 2012, 148, 1015–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, C.; Wu, Y.; Yao, J.; Wang, Y.; Yu, Y.; Rychahou, P.G.; Evers, B.M.; Zhou, B.P. G9a Interacts with Snail and Is Critical for Snail-Mediated E-Cadherin Repression in Human Breast Cancer. J. Clin. Investig. 2012, 122, 1469–1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.-Q.; Yuan, Y.; Jiang, S.; Jiang, H. Promoter Methylation and Expression of CDH1 and Susceptibility and Prognosis of Eyelid Squamous Cell Carcinoma. Tumour Biol. 2016, 37, 9521–9526. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Cui, L.; Wang, C.; Guo, Y.; Shen, S.; Kuang, G.; Dong, Z. Decreased Expression of RASSF1A and Up-Regulation of RASSF1C Is Associated with Esophageal Squamous Cell Carcinoma. Clin. Exp. Metastasis 2014, 31, 521–533. [Google Scholar] [CrossRef]
- Bai, J.; Zhang, X.; Hu, K.; Liu, B.; Wang, H.; Li, A.; Lin, F.; Zhang, L.; Sun, X.; Du, Z.; et al. Silencing DNA Methyltransferase 1 (DNMT1) Inhibits Proliferation, Metastasis and Invasion in ESCC by Suppressing Methylation of RASSF1A and DAPK. Oncotarget 2016, 7, 44129–44141. [Google Scholar] [CrossRef] [Green Version]
- Contaldo, M.; Domenico, M.D.; Caraglia, M.; Giordano, A.; Tombolini, V.; Giovane, A.; Papagerakis, S.; Rubini, C.; Rosa, A.D.; Serpico, R.; et al. The Role of E-Cadherin Down-Regulation in Oral Cancer: CDH1 Gene Expression and Epigenetic Blockage. Curr. Cancer Drug Targets 2014, 14, 115–127. [Google Scholar]
- Hu, Y.-H.; Zhang, C.-Y.; Tian, Z.; Wang, L.-Z.; Li, J. Aberrant Protein Expression and Promoter Methylation of P16 Gene Are Correlated with Malignant Transformation of Salivary Pleomorphic Adenoma. Arch. Pathol. Lab. Med. 2011, 135, 882–889. [Google Scholar] [CrossRef]
- Li, J.; El-Naggar, A.; Mao, L. Promoter Methylation of P16INK4a, RASSF1A, and DAPK Is Frequent in Salivary Adenoid Cystic Carcinoma. Cancer 2005, 104, 771–776. [Google Scholar] [CrossRef]
- Shargh, S.A.; Sakizli, M.; Khalaj, V.; Movafagh, A.; Yazdi, H.; Hagigatjou, E.; Sayad, A.; Mansouri, N.; Mortazavi-Tabatabaei, S.A.; Khorram Khorshid, H.R. Downregulation of E-Cadherin Expression in Breast Cancer by Promoter Hypermethylation and Its Relation with Progression and Prognosis of Tumor. Med. Oncol. 2014, 31, 250. [Google Scholar] [CrossRef]
- Li, G.; Liu, Y.; Yin, H.; Zhang, X.; Mo, X.; Tang, J.; Chen, W. E-Cadherin Gene Promoter Hypermethylation May Contribute to the Risk of Bladder Cancer among Asian Populations. Gene 2014, 534, 48–53. [Google Scholar] [CrossRef]
- Xia, L.; Hu, Y.; Gu, T.; Wang, L.; Tian, Z. Promoter Hypermethylation May Contribute to E-cadherin Repression in the Human Salivary Carcinoma Ex Pleomorphic Adenoma. Int. J. Oncol. 2018, 52, 496–504. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.-Y.; Mao, L.; Li, L.; Tian, Z.; Zhou, X.-J.; Zhang, Z.-Y.; Li, J. Promoter Methylation as a Common Mechanism for Inactivating E-Cadherin in Human Salivary Gland Adenoid Cystic Carcinoma. Cancer 2007, 110, 87–95. [Google Scholar] [CrossRef]
- Yan, F.; Shen, N.; Pang, J.; Molina, J.R.; Yang, P.; Liu, S. The DNA Methyltransferase DNMT1 and Tyrosine-Protein Kinase KIT Cooperatively Promote Resistance to 5-Aza-2′-Deoxycytidine (Decitabine) and Midostaurin (PKC412) in Lung Cancer Cells. J. Biol. Chem. 2015, 290, 18480–18494. [Google Scholar] [CrossRef] [Green Version]
- Lombaerts, M.; van Wezel, T.; Philippo, K.; Dierssen, J.W.F.; Zimmerman, R.M.E.; Oosting, J.; van Eijk, R.; Eilers, P.H.; van de Water, B.; Cornelisse, C.J.; et al. E-Cadherin Transcriptional Downregulation by Promoter Methylation but Not Mutation Is Related to Epithelial-to-Mesenchymal Transition in Breast Cancer Cell Lines. Br. J. Cancer 2006, 94, 661–671. [Google Scholar] [CrossRef] [Green Version]
- Nass, S.J.; Herman, J.G.; Gabrielson, E.; Iversen, P.W.; Parl, F.F.; Davidson, N.E.; Graff, J.R. Aberrant Methylation of the Estrogen Receptor and E-Cadherin 5′ CpG Islands Increases with Malignant Progression in Human Breast Cancer. Cancer Res. 2000, 60, 4346–4348. [Google Scholar]
- Liu, J.; Sun, X.; Qin, S.; Wang, H.; Du, N.; Li, Y.; Pang, Y.; Wang, C.; Xu, C.; Ren, H. CDH1 Promoter Methylation Correlates with Decreased Gene Expression and Poor Prognosis in Patients with Breast Cancer. Oncol. Lett. 2016, 11, 2635–2643. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.-X.; Lu, Y.; Li, C.-Y.; Yuan, P.; Lin, S.-S. Role of CDH1 Promoter Methylation in Colorectal Carcinogenesis: A Meta-Analysis. DNA Cell Biol. 2014, 33, 455–462. [Google Scholar] [CrossRef]
- Sato, T.; Tanigami, A.; Yamakawa, K.; Akiyama, F.; Kasumi, F.; Sakamoto, G.; Nakamura, Y. Allelotype of Breast Cancer: Cumulative Allele Losses Promote Tumor Progression in Primary Breast Cancer. Cancer Res. 1990, 50, 7184–7189. [Google Scholar] [PubMed]
- Cui, H.; Wang, L.; Gong, P.; Zhao, C.; Zhang, S.; Zhang, K.; Zhou, R.; Zhao, Z.; Fan, H. Deregulation between MiR-29b/c and DNMT3A Is Associated with Epigenetic Silencing of the CDH1 Gene, Affecting Cell Migration and Invasion in Gastric Cancer. PLoS ONE 2015, 10, e0123926. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Wu, Y.; Wang, Y.; Wang, C.; Kang, T.; Rychahou, P.G.; Chi, Y.-I.; Evers, B.M.; Zhou, B.P. Interaction with Suv39H1 Is Critical for Snail-Mediated E-Cadherin Repression in Breast Cancer. Oncogene 2013, 32, 1351–1362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsumiya-Matsumoto, Y.; Morita, Y.; Uzawa, N. Pleomorphic Adenoma of the Salivary Glands and Epithelial–Mesenchymal Transition. J. Clin. Med. 2022, 11, 4210. https://doi.org/10.3390/jcm11144210
Matsumiya-Matsumoto Y, Morita Y, Uzawa N. Pleomorphic Adenoma of the Salivary Glands and Epithelial–Mesenchymal Transition. Journal of Clinical Medicine. 2022; 11(14):4210. https://doi.org/10.3390/jcm11144210
Chicago/Turabian StyleMatsumiya-Matsumoto, Yuka, Yoshihiro Morita, and Narikazu Uzawa. 2022. "Pleomorphic Adenoma of the Salivary Glands and Epithelial–Mesenchymal Transition" Journal of Clinical Medicine 11, no. 14: 4210. https://doi.org/10.3390/jcm11144210
APA StyleMatsumiya-Matsumoto, Y., Morita, Y., & Uzawa, N. (2022). Pleomorphic Adenoma of the Salivary Glands and Epithelial–Mesenchymal Transition. Journal of Clinical Medicine, 11(14), 4210. https://doi.org/10.3390/jcm11144210





