Abdominal Pain in Inflammatory Bowel Diseases: A Clinical Challenge
Abstract
:1. Introduction
2. Assessment and Consequences of Abdominal Pain in IBD
3. Mechanisms of Chronic Abdominal Pain
3.1. Direct Effect of Inflammation
3.2. Peripheral and Central Pain Dysregulation
3.3. Overlap between IBD and IBS
3.4. Impact of Psychological and Social Factors on Pain
3.5. Genetic Factors
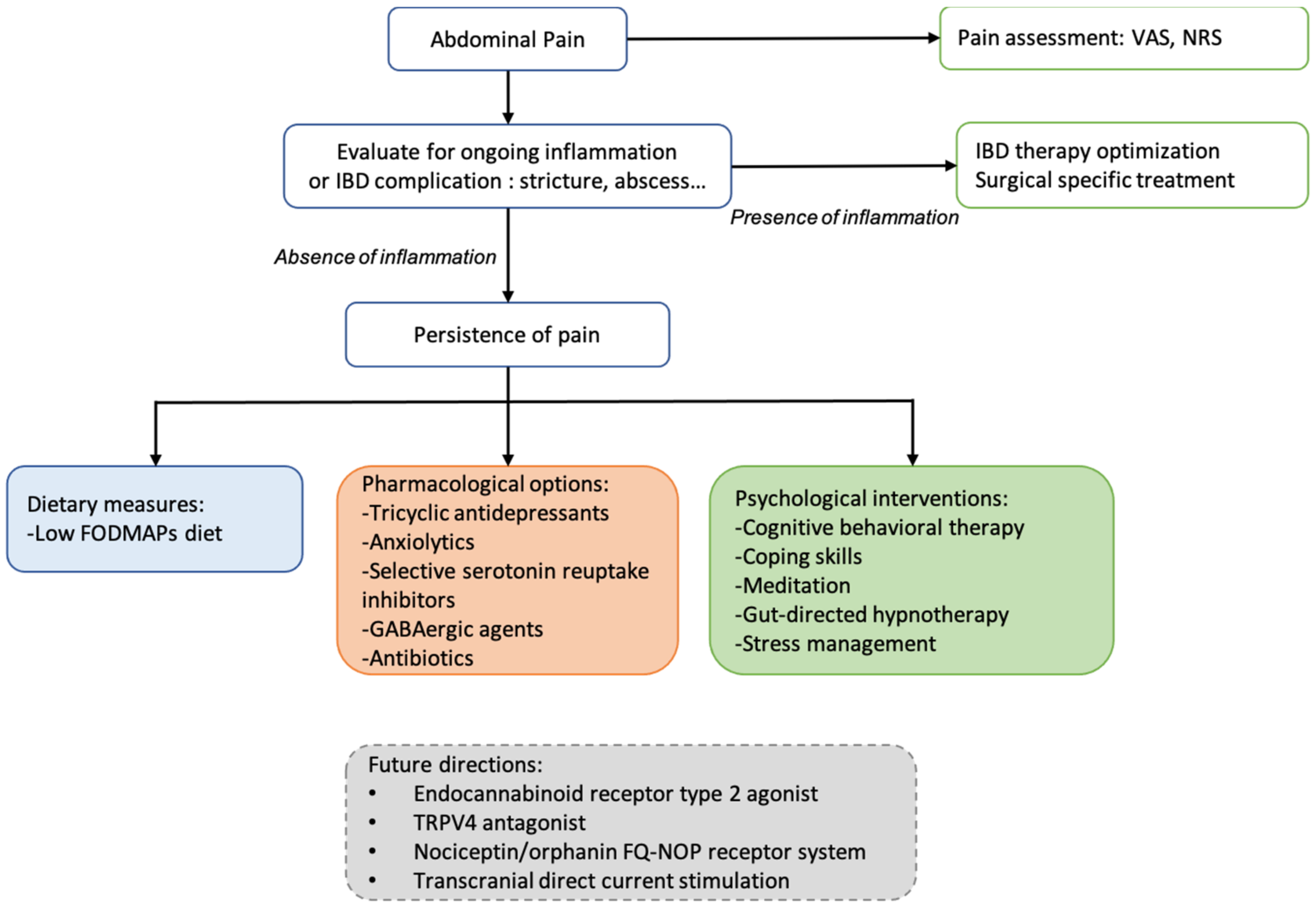
4. Pain Management
4.1. Current Pharmacological Options
4.2. Non-Pharmacological Interventions
4.2.1. Dietary Measures
4.2.2. Psychological Approaches
5. Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khanna, R.; Zou, G.; D’Haens, G.; Feagan, B.G.; Sandborn, W.J.; Vandervoort, M.K.; Rolleri, R.L.; Bortey, E.; Paterson, C.; Forbes, W.P.; et al. A Retrospective Analysis: The Development of Patient Reported Outcome Measures for the Assessment of Crohn’s Disease Activity. Aliment. Pharmacol. Ther. 2015, 41, 77–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, D.; Ricciuto, A.; Lewis, A.; D’Amico, F.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W.; et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target Strategies in IBD. Gastroenterology 2021, 160, 1570–1583. [Google Scholar] [CrossRef] [PubMed]
- Zeitz, J.; Ak, M.; Müller-Mottet, S.; Scharl, S.; Biedermann, L.; Fournier, N.; Frei, P.; Pittet, V.; Scharl, M.; Fried, M.; et al. Pain in IBD Patients: Very Frequent and Frequently Insufficiently Taken into Account. PLoS ONE 2016, 11, e0156666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coates, M.D.; Johri, A.; Gorrepati, V.S.; Maheshwari, P.; Dalessio, S.; Walter, V.; Stuart, A.; Koltun, W.; Bernasko, N.; Tinsley, A.; et al. Abdominal Pain in Quiescent Inflammatory Bowel Disease. Int. J. Colorectal Dis. 2021, 36, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Colombel, J.-F.; Shin, A.; Gibson, P.R. AGA Clinical Practice Update on Functional Gastrointestinal Symptoms in Patients with Inflammatory Bowel Disease: Expert Review. Clin. Gastroenterol. Hepatol. 2019, 17, 380–390.e1. [Google Scholar] [CrossRef] [Green Version]
- Srinath, A.I.; Walter, C.; Newara, M.C.; Szigethy, E.M. Pain Management in Patients with Inflammatory Bowel Disease: Insights for the Clinician. Ther. Adv. Gastroenterol. 2012, 5, 339–357. [Google Scholar] [CrossRef] [Green Version]
- Bakshi, N.; Hart, A.L.; Lee, M.C.; Williams, A.C.D.C.; Lackner, J.M.; Norton, C.; Croft, P. Chronic Pain in Patients with Inflammatory Bowel Disease. Pain 2021, 162, 2466–2471. [Google Scholar] [CrossRef]
- Bonaz, B.L.; Bernstein, C.N. Brain-Gut Interactions in Inflammatory Bowel Disease. Gastroenterology 2013, 144, 36–49. [Google Scholar] [CrossRef] [Green Version]
- Hart, A.L.; Lomer, M.; Verjee, A.; Kemp, K.; Faiz, O.; Daly, A.; Solomon, J.; McLaughlin, J. What Are the Top 10 Research Questions in the Treatment of Inflammatory Bowel Disease? A Priority Setting Partnership with the James Lind Alliance. J. Crohns Colitis 2017, 11, 204–211. [Google Scholar] [CrossRef] [Green Version]
- Haapamäki, J.; Tanskanen, A.; Roine, R.P.; Blom, M.; Turunen, U.; Mäntylä, J.; Färkkilä, M.A.; Arkkila, P.E.T. Medication Use among Inflammatory Bowel Disease Patients: Excessive Consumption of Antidepressants and Analgesics. Scand. J. Gastroenterol. 2013, 48, 42–50. [Google Scholar] [CrossRef]
- Storr, M.; Devlin, S.; Kaplan, G.G.; Panaccione, R.; Andrews, C.N. Cannabis Use Provides Symptom Relief in Patients with Inflammatory Bowel Disease but Is Associated with Worse Disease Prognosis in Patients with Crohn’s Disease. Inflamm. Bowel Dis. 2014, 20, 472–480. [Google Scholar] [CrossRef]
- McNicol, E. Opioid Side Effects and Their Treatment in Patients with Chronic Cancer and Noncancer Pain. J. Pain Palliat. Care Pharmacother. 2008, 22, 270–281. [Google Scholar] [CrossRef]
- Targownik, L.E.; Nugent, Z.; Singh, H.; Bugden, S.; Bernstein, C.N. The Prevalence and Predictors of Opioid Use in Inflammatory Bowel Disease: A Population-Based Analysis. Am. J. Gastroenterol. 2014, 109, 1613–1620. [Google Scholar] [CrossRef]
- Treede, R.-D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. A Classification of Chronic Pain for ICD-11. Pain 2015, 156, 1003–1007. [Google Scholar] [CrossRef] [Green Version]
- Treede, R.-D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. Chronic Pain as a Symptom or a Disease: The IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2019, 160, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Melzack, R. The Short-Form McGill Pain Questionnaire. Pain 1987, 30, 191–197. [Google Scholar] [CrossRef]
- Erdemoglu, Â.K.; Koc, R. Brief Pain Inventory Score Identifying and Discriminating Neuropathic and Nociceptive Pain. Acta Neurol. Scand. 2013, 128, 351–358. [Google Scholar] [CrossRef]
- Knowles, S.R.; Graff, L.A.; Wilding, H.; Hewitt, C.; Keefer, L.; Mikocka-Walus, A. Quality of Life in Inflammatory Bowel Disease: A Systematic Review and Meta-Analyses—Part I. Inflamm. Bowel Dis. 2018, 24, 742–751. [Google Scholar] [CrossRef]
- Kim, E.S.; Cho, K.B.; Park, K.S.; Jang, B.I.; Kim, K.O.; Jeon, S.W.; Jung, M.K.; Kim, E.Y.; Yang, C.H. Predictive Factors of Impaired Quality of Life in Korean Patients with Inactive Inflammatory Bowel Disease: Association with Functional Gastrointestinal Disorders and Mood Disorders. J. Clin. Gastroenterol. 2013, 47, e38–e44. [Google Scholar] [CrossRef]
- Gracie, D.J.; Williams, C.J.M.; Sood, R.; Mumtaz, S.; Bholah, M.H.; Hamlin, P.J.; Ford, A.C. Negative Effects on Psychological Health and Quality of Life of Genuine Irritable Bowel Syndrome-Type Symptoms in Patients with Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2017, 15, 376–384.e5. [Google Scholar] [CrossRef] [Green Version]
- Schirbel, A.; Reichert, A.; Roll, S.; Baumgart, D.C.; Büning, C.; Wittig, B.; Wiedenmann, B.; Dignass, A.; Sturm, A. Impact of Pain on Health-Related Quality of Life in Patients with Inflammatory Bowel Disease. World J. Gastroenterol. 2010, 16, 3168–3177. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.L.; Nguyen, G.C.; Benchimol, E.I.; Bernstein, C.N.; Bitton, A.; Kaplan, G.G.; Murthy, S.K.; Lee, K.; Cooke-Lauder, J.; Otley, A.R. The Impact of Inflammatory Bowel Disease in Canada 2018: Quality of Life. J. Can. Assoc. Gastroenterol. 2019, 2, S42–S48. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Mitchell, R. Impact of Inflammatory Bowel Disease on Quality of Life: Results of the European Federation of Crohn’s and Ulcerative Colitis Associations (EFCCA) Patient Survey. J. Crohns Colitis 2007, 1, 10–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gracie, D.J.; Hamlin, J.P.; Ford, A.C. Longitudinal Impact of IBS-Type Symptoms on Disease Activity, Healthcare Utilization, Psychological Health, and Quality of Life in Inflammatory Bowel Disease. Am. J. Gastroenterol. 2018, 113, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Lönnfors, S.; Vermeire, S.; Greco, M.; Hommes, D.; Bell, C.; Avedano, L. IBD and Health-Related Quality of Life—Discovering the True Impact. J. Crohns Colitis 2014, 8, 1281–1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Limsrivilai, J.; Stidham, R.W.; Govani, S.M.; Waljee, A.K.; Huang, W.; Higgins, P.D.R. Factors That Predict High Health Care Utilization and Costs for Patients with Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2017, 15, 385–392.e2. [Google Scholar] [CrossRef] [Green Version]
- Bielefeldt, K.; Davis, B.; Binion, D.G. Pain and Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2009, 15, 778–788. [Google Scholar] [CrossRef]
- Best, W.R.; Becktel, J.M.; Singleton, J.W.; Kern, F. Development of a Crohn’s Disease Activity Index. National Cooperative Crohn’s Disease Study. Gastroenterology 1976, 70, 439–444. [Google Scholar] [CrossRef]
- Fairbrass, K.M.; Costantino, S.J.; Gracie, D.J.; Ford, A.C. Prevalence of Irritable Bowel Syndrome-Type Symptoms in Patients with Inflammatory Bowel Disease in Remission: A Systematic Review and Meta-Analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 1053–1062. [Google Scholar] [CrossRef]
- Regueiro, M.; Greer, J.B.; Szigethy, E. Etiology and Treatment of Pain and Psychosocial Issues in Patients with Inflammatory Bowel Diseases. Gastroenterology 2017, 152, 430–439.e4. [Google Scholar] [CrossRef] [Green Version]
- Arendt-Nielsen, L.; Morlion, B.; Perrot, S.; Dahan, A.; Dickenson, A.; Kress, H.G.; Wells, C.; Bouhassira, D.; Drewes, A.M. Assessment and Manifestation of Central Sensitisation across Different Chronic Pain Conditions. Eur. J. Pain 2018, 22, 216–241. [Google Scholar] [CrossRef] [Green Version]
- Lakhan, S.E.; Kirchgessner, A. Neuroinflammation in Inflammatory Bowel Disease. J. Neuroinflamm. 2010, 7, 37. [Google Scholar] [CrossRef] [Green Version]
- Mika, J. Modulation of Microglia Can Attenuate Neuropathic Pain Symptoms and Enhance Morphine Effectiveness. Pharmacol. Rep. 2008, 60, 297–307. [Google Scholar]
- Drewes, A.M.; Olesen, A.E.; Farmer, A.D.; Szigethy, E.; Rebours, V.; Olesen, S.S. Gastrointestinal Pain. Nat. Rev. Dis. Primers 2020, 6, 1. [Google Scholar] [CrossRef]
- Beyak, M.J.; Vanner, S. Inflammation-Induced Hyperexcitability of Nociceptive Gastrointestinal DRG Neurones: The Role of Voltage-Gated Ion Channels. Neurogastroenterol. Motil. 2005, 17, 175–186. [Google Scholar] [CrossRef]
- Bao, C.-H.; Liu, P.; Liu, H.-R.; Wu, L.-Y.; Jin, X.-M.; Wang, S.-Y.; Shi, Y.; Zhang, J.-Y.; Zeng, X.-Q.; Ma, L.-L.; et al. Differences in Regional Homogeneity between Patients with Crohn’s Disease with and without Abdominal Pain Revealed by Resting-State Functional Magnetic Resonance Imaging. Pain 2016, 157, 1037–1044. [Google Scholar] [CrossRef]
- Graff, L.A.; Walker, J.R.; Bernstein, C.N. Depression and Anxiety in Inflammatory Bowel Disease: A Review of Comorbidity and Management. Inflamm. Bowel Dis. 2009, 15, 1105–1118. [Google Scholar] [CrossRef]
- Thomann, A.K.; Griebe, M.; Thomann, P.A.; Hirjak, D.; Ebert, M.P.; Szabo, K.; Reindl, W.; Wolf, R.C. Intrinsic Neural Network Dysfunction in Quiescent Crohn’s Disease. Sci. Rep. 2017, 7, 11579. [Google Scholar] [CrossRef]
- Lovell, R.M.; Ford, A.C. Global Prevalence of and Risk Factors for Irritable Bowel Syndrome: A Meta-Analysis. Clin. Gastroenterol. Hepatol. 2012, 10, 712–721.e4. [Google Scholar] [CrossRef]
- Halpin, S.J.; Ford, A.C. Prevalence of Symptoms Meeting Criteria for Irritable Bowel Syndrome in Inflammatory Bowel Disease: Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2012, 107, 1474–1482. [Google Scholar] [CrossRef]
- Larsson, M.B.O.; Tillisch, K.; Craig, A.D.; Engström, M.; Labus, J.; Naliboff, B.; Lundberg, P.; Ström, M.; Mayer, E.A.; Walter, S.A. Brain Responses to Visceral Stimuli Reflect Visceral Sensitivity Thresholds in Patients with Irritable Bowel Syndrome. Gastroenterology 2012, 142, 463–472.e3. [Google Scholar] [CrossRef] [Green Version]
- Zamani, M.; Alizadeh-Tabari, S.; Zamani, V. Systematic Review with Meta-Analysis: The Prevalence of Anxiety and Depression in Patients with Irritable Bowel Syndrome. Aliment. Pharmacol. Ther. 2019, 50, 132–143. [Google Scholar] [CrossRef] [Green Version]
- Khan, I.; Ullah, N.; Zha, L.; Bai, Y.; Khan, A.; Zhao, T.; Che, T.; Zhang, C. Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): Cause or Consequence? IBD Treatment Targeting the Gut Microbiome. Pathogens 2019, 8, 126. [Google Scholar] [CrossRef] [Green Version]
- Shutkever, O.; Gracie, D.J.; Young, C.; Wood, H.M.; Taylor, M.; John Hamlin, P.; Ford, A.C.; Quirke, P. No Significant Association Between the Fecal Microbiome and the Presence of Irritable Bowel Syndrome-Type Symptoms in Patients with Quiescent Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2018, 24, 1597–1605. [Google Scholar] [CrossRef]
- Pérez-Berezo, T.; Pujo, J.; Martin, P.; Le Faouder, P.; Galano, J.-M.; Guy, A.; Knauf, C.; Tabet, J.C.; Tronnet, S.; Barreau, F.; et al. Identification of an Analgesic Lipopeptide Produced by the Probiotic Escherichia Coli Strain Nissle 1917. Nat. Commun. 2017, 8, 1314. [Google Scholar] [CrossRef] [Green Version]
- Shah, A.; Morrison, M.; Burger, D.; Martin, N.; Rich, J.; Jones, M.; Koloski, N.; Walker, M.M.; Talley, N.J.; Holtmann, G.J. Systematic Review with Meta-Analysis: The Prevalence of Small Intestinal Bacterial Overgrowth in Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2019, 49, 624–635. [Google Scholar] [CrossRef]
- Van Tilburg, M.A.L.; Claar, R.L.; Romano, J.M.; Langer, S.L.; Drossman, D.A.; Whitehead, W.E.; Abdullah, B.; Levy, R.L. Psychological Factors May Play an Important Role in Pediatric Crohn’s Disease Symptoms and Disability. J. Pediatr. 2017, 184, 94–100.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neuendorf, R.; Harding, A.; Stello, N.; Hanes, D.; Wahbeh, H. Depression and Anxiety in Patients with Inflammatory Bowel Disease: A Systematic Review. J. Psychosom. Res. 2016, 87, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Barberio, B.; Zamani, M.; Black, C.J.; Savarino, E.V.; Ford, A.C. Prevalence of Symptoms of Anxiety and Depression in Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Lancet Gastroenterol. Hepatol. 2021, 6, 359–370. [Google Scholar] [CrossRef]
- Gracie, D.J.; Guthrie, E.A.; Hamlin, P.J.; Ford, A.C. Bi-Directionality of Brain-Gut Interactions in Patients with Inflammatory Bowel Disease. Gastroenterology 2018, 154, 1635–1646.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, A.H.; Maletic, V.; Raison, C.L. Inflammation and Its Discontents: The Role of Cytokines in the Pathophysiology of Major Depression. Biol. Psychiatry 2009, 65, 732–741. [Google Scholar] [CrossRef] [Green Version]
- Harrison, N.A.; Brydon, L.; Walker, C.; Gray, M.A.; Steptoe, A.; Critchley, H.D. Inflammation Causes Mood Changes through Alterations in Subgenual Cingulate Activity and Mesolimbic Connectivity. Biol. Psychiatry 2009, 66, 407–414. [Google Scholar] [CrossRef] [Green Version]
- Fuller-Thomson, E.; Sulman, J. Depression and Inflammatory Bowel Disease: Findings from Two Nationally Representative Canadian Surveys. Inflamm. Bowel Dis. 2006, 12, 697–707. [Google Scholar] [CrossRef]
- Murphy, L.K.; de la Vega, R.; Kohut, S.A.; Kawamura, J.S.; Levy, R.L.; Palermo, T.M. Systematic Review: Psychosocial Correlates of Pain in Pediatric Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2021, 27, 697–710. [Google Scholar] [CrossRef]
- Mawdsley, J.E.; Rampton, D.S. Psychological Stress in IBD: New Insights into Pathogenic and Therapeutic Implications. Gut 2005, 54, 1481–1491. [Google Scholar] [CrossRef]
- Sweeney, L.; Moss-Morris, R.; Czuber-Dochan, W.; Meade, L.; Chumbley, G.; Norton, C. Systematic Review: Psychosocial Factors Associated with Pain in Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2018, 47, 715–729. [Google Scholar] [CrossRef] [Green Version]
- Sweeney, L.; Moss-Morris, R.; Czuber-Dochan, W.; Murrells, T.; Norton, C. Developing a Better Biopsychosocial Understanding of Pain in Inflammatory Bowel Disease: A Cross-Sectional Study. Eur. J. Gastroenterol. Hepatol. 2020, 32, 335–344. [Google Scholar] [CrossRef]
- Diatchenko, L.; Slade, G.D.; Nackley, A.G.; Bhalang, K.; Sigurdsson, A.; Belfer, I.; Goldman, D.; Xu, K.; Shabalina, S.A.; Shagin, D.; et al. Genetic Basis for Individual Variations in Pain Perception and the Development of a Chronic Pain Condition. Hum. Mol. Genet. 2005, 14, 135–143. [Google Scholar] [CrossRef] [Green Version]
- de Lange, K.M.; Moutsianas, L.; Lee, J.C.; Lamb, C.A.; Luo, Y.; Kennedy, N.A.; Jostins, L.; Rice, D.L.; Gutierrez-Achury, J.; Ji, S.-G.; et al. Genome-Wide Association Study Implicates Immune Activation of Multiple Integrin Genes in Inflammatory Bowel Disease. Nat. Genet. 2017, 49, 256–261. [Google Scholar] [CrossRef] [Green Version]
- Akbar, A.; Yiangou, Y.; Facer, P.; Brydon, W.G.; Walters, J.R.F.; Anand, P.; Ghosh, S. Expression of the TRPV1 Receptor Differs in Quiescent Inflammatory Bowel Disease with or without Abdominal Pain. Gut 2010, 59, 767–774. [Google Scholar] [CrossRef]
- Ek, W.E.; Reznichenko, A.; Ripke, S.; Niesler, B.; Zucchelli, M.; Rivera, N.V.; Schmidt, P.T.; Pedersen, N.L.; Magnusson, P.; Talley, N.J.; et al. Exploring the Genetics of Irritable Bowel Syndrome: A GWA Study in the General Population and Replication in Multinational Case-Control Cohorts. Gut 2015, 64, 1774–1782. [Google Scholar] [CrossRef] [Green Version]
- The Swiss IBD Cohort Study Group; Ledergerber, M.; Lang, B.M.; Heinrich, H.; Biedermann, L.; Begré, S.; Zeitz, J.; Krupka, N.; Rickenbacher, A.; Turina, M.; et al. Abdominal Pain in Patients with Inflammatory Bowel Disease: Association with Single-Nucleotide Polymorphisms Prevalent in Irritable Bowel Syndrome and Clinical Management. BMC Gastroenterol. 2021, 21, 53. [Google Scholar] [CrossRef]
- Panés, J.; Vermeire, S.; Lindsay, J.O.; Sands, B.E.; Su, C.; Friedman, G.; Zhang, H.; Yarlas, A.; Bayliss, M.; Maher, S.; et al. Tofacitinib in Patients with Ulcerative Colitis: Health-Related Quality of Life in Phase 3 Randomised Controlled Induction and Maintenance Studies. J. Crohns Colitis 2018, 12, 145–156. [Google Scholar] [CrossRef]
- Ghosh, S.; Sanchez Gonzalez, Y.; Zhou, W.; Clark, R.; Xie, W.; Louis, E.; Loftus, E.V.; Panes, J.; Danese, S. Upadacitinib Treatment Improves Symptoms of Bowel Urgency and Abdominal Pain, and Correlates with Quality of Life Improvements in Patients with Moderate to Severe Ulcerative Colitis. J. Crohns Colitis 2021, 15, 2022–2030. [Google Scholar] [CrossRef]
- Zielińska, A.; Sałaga, M.; Włodarczyk, M.; Fichna, J. Focus on Current and Future Management Possibilities in Inflammatory Bowel Disease-Related Chronic Pain. Int. J. Colorectal Dis. 2019, 34, 217–227. [Google Scholar] [CrossRef] [Green Version]
- Norton, C.; Czuber-Dochan, W.; Artom, M.; Sweeney, L.; Hart, A. Systematic Review: Interventions for Abdominal Pain Management in Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2017, 46, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Makharia, G.K. Understanding and Treating Abdominal Pain and Spasms in Organic Gastrointestinal Diseases: Inflammatory Bowel Disease and Biliary Diseases. J. Clin. Gastroenterol. 2011, 45, S89–S93. [Google Scholar] [CrossRef]
- Camilleri, M.; Lembo, A.; Katzka, D.A. Opioids in Gastroenterology: Treating Adverse Effects and Creating Therapeutic Benefits. Clin. Gastroenterol. Hepatol. 2017, 15, 1338–1349. [Google Scholar] [CrossRef] [Green Version]
- Burr, N.E.; Smith, C.; West, R.; Hull, M.A.; Subramanian, V. Increasing Prescription of Opiates and Mortality in Patients with Inflammatory Bowel Diseases in England. Clin. Gastroenterol. Hepatol. 2018, 16, 534–541.e6. [Google Scholar] [CrossRef] [Green Version]
- Iskandar, H.N.; Cassell, B.; Kanuri, N.; Gyawali, C.P.; Gutierrez, A.; Dassopoulos, T.; Ciorba, M.A.; Sayuk, G.S. Tricyclic Antidepressants for Management of Residual Symptoms in Inflammatory Bowel Disease. J. Clin. Gastroenterol. 2014, 48, 423–429. [Google Scholar] [CrossRef] [Green Version]
- Walker, E.A.; Gelfand, M.D.; Gelfand, A.N.; Creed, F.; Katon, W.J. The Relationship of Current Psychiatric Disorder to Functional Disability and Distress in Patients with Inflammatory Bowel Disease. Gen. Hosp. Psychiatry 1996, 18, 220–229. [Google Scholar] [CrossRef]
- Frolkis, A.D.; Vallerand, I.A.; Shaheen, A.-A.; Lowerison, M.W.; Swain, M.G.; Barnabe, C.; Patten, S.B.; Kaplan, G.G. Depression Increases the Risk of Inflammatory Bowel Disease, Which May Be Mitigated by the Use of Antidepressants in the Treatment of Depression. Gut 2019, 68, 1606–1612. [Google Scholar] [CrossRef] [PubMed]
- Castiglione, F.; Rispo, A.; Di Girolamo, E.; Cozzolino, A.; Manguso, F.; Grassia, R.; Mazzacca, G. Antibiotic Treatment of Small Bowel Bacterial Overgrowth in Patients with Crohn’s Disease. Aliment. Pharmacol. Ther. 2003, 18, 1107–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gatta, L.; Scarpignato, C. Systematic Review with Meta-Analysis: Rifaximin Is Effective and Safe for the Treatment of Small Intestine Bacterial Overgrowth. Aliment. Pharmacol. Ther. 2017, 45, 604–616. [Google Scholar] [CrossRef] [Green Version]
- Ducrotté, P.; Sawant, P.; Jayanthi, V. Clinical Trial: Lactobacillus Plantarum 299v (DSM 9843) Improves Symptoms of Irritable Bowel Syndrome. World J. Gastroenterol. 2012, 18, 4012–4018. [Google Scholar] [CrossRef]
- Guglielmetti, S.; Mora, D.; Gschwender, M.; Popp, K. Randomised Clinical Trial: Bifidobacterium Bifidum MIMBb75 Significantly Alleviates Irritable Bowel Syndrome and Improves Quality of Life—A Double-Blind, Placebo-Controlled Study. Aliment. Pharmacol. Ther. 2011, 33, 1123–1132. [Google Scholar] [CrossRef]
- Pullan, R.D.; Rhodes, J.; Ganesh, S.; Mani, V.; Morris, J.S.; Williams, G.T.; Newcombe, R.G.; Russell, M.A.; Feyerabend, C.; Thomas, G.A. Transdermal Nicotine for Active Ulcerative Colitis. N. Engl. J. Med. 1994, 330, 811–815. [Google Scholar] [CrossRef]
- Van Outryve, M.; Toussaint, J. Loperamide Oxide for the Treatment of Chronic Diarrhoea in Crohn’s Disease. J. Int. Med. Res. 1995, 23, 335–341. [Google Scholar] [CrossRef]
- Swaminath, A.; Berlin, E.P.; Cheifetz, A.; Hoffenberg, E.; Kinnucan, J.; Wingate, L.; Buchanan, S.; Zmeter, N.; Rubin, D.T. The Role of Cannabis in the Management of Inflammatory Bowel Disease: A Review of Clinical, Scientific, and Regulatory Information. Inflamm. Bowel Dis. 2019, 25, 427–435. [Google Scholar] [CrossRef] [Green Version]
- Naftali, T.; Bar-Lev Schleider, L.; Dotan, I.; Lansky, E.P.; Sklerovsky Benjaminov, F.; Konikoff, F.M. Cannabis Induces a Clinical Response in Patients with Crohn’s Disease: A Prospective Placebo-Controlled Study. Clin. Gastroenterol. Hepatol. 2013, 11, 1276–1280.e1. [Google Scholar] [CrossRef]
- Irving, P.M.; Iqbal, T.; Nwokolo, C.; Subramanian, S.; Bloom, S.; Prasad, N.; Hart, A.; Murray, C.; Lindsay, J.O.; Taylor, A.; et al. A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Pilot Study of Cannabidiol-Rich Botanical Extract in the Symptomatic Treatment of Ulcerative Colitis. Inflamm. Bowel Dis. 2018, 24, 714–724. [Google Scholar] [CrossRef]
- Stero Biotechs Ltd. A Phase 2a Study to Evaluate the Safety, Tolerability and Efficacy of Cannabidiol as a Steroid-Sparing Therapy in Steroid-Dependent Crohn’s Disease Patients. Available online: https://ClinicalTrials.gov/show/NCT04056442 (accessed on 12 July 2022).
- Gibson, P.R. Use of the Low-FODMAP Diet in Inflammatory Bowel Disease. J. Gastroenterol. Hepatol. 2017, 32, 40–42. [Google Scholar] [CrossRef] [Green Version]
- McCormick, M.; Reed-Knight, B.; Lewis, J.D.; Gold, B.D.; Blount, R.L. Coping Skills for Reducing Pain and Somatic Symptoms in Adolescents with IBD. Inflamm. Bowel Dis. 2010, 16, 2148–2157. [Google Scholar] [CrossRef]
- Keefer, L.; Taft, T.H.; Kiebles, J.L.; Martinovich, Z.; Barrett, T.A.; Palsson, O.S. Gut-Directed Hypnotherapy Significantly Augments Clinical Remission in Quiescent Ulcerative Colitis. Aliment. Pharmacol. Ther. 2013, 38, 761–771. [Google Scholar] [CrossRef]
- García-Vega, E.; Fernandez-Rodriguez, C. A Stress Management Programme for Crohn’s Disease. Behav. Res. Ther. 2004, 42, 367–383. [Google Scholar] [CrossRef]
- Camilleri, M.; Boeckxstaens, G. Dietary and Pharmacological Treatment of Abdominal Pain in IBS. Gut 2017, 66, 966–974. [Google Scholar] [CrossRef]
- Barrett, J.S.; Irving, P.M.; Shepherd, S.J.; Muir, J.G.; Gibson, P.R. Comparison of the Prevalence of Fructose and Lactose Malabsorption across Chronic Intestinal Disorders. Aliment. Pharmacol. Ther. 2009, 30, 165–174. [Google Scholar] [CrossRef]
- Cox, S.R.; Prince, A.C.; Myers, C.E.; Irving, P.M.; Lindsay, J.O.; Lomer, M.C.; Whelan, K. Fermentable Carbohydrates [FODMAPs] Exacerbate Functional Gastrointestinal Symptoms in Patients with Inflammatory Bowel Disease: A Randomised, Double-Blind, Placebo-Controlled, Cross-over, Re-Challenge Trial. J. Crohns Colitis 2017, 11, 1420–1429. [Google Scholar] [CrossRef] [Green Version]
- Szigethy, E.; Bujoreanu, S.I.; Youk, A.O.; Weisz, J.; Benhayon, D.; Fairclough, D.; Ducharme, P.; Gonzalez-Heydrich, J.; Keljo, D.; Srinath, A.; et al. Randomized Efficacy Trial of Two Psychotherapies for Depression in Youth with Inflammatory Bowel Disease. J. Am. Acad. Child Adolesc. Psychiatry 2014, 53, 726–735. [Google Scholar] [CrossRef] [Green Version]
- Gerbarg, P.L.; Jacob, V.E.; Stevens, L.; Bosworth, B.P.; Chabouni, F.; DeFilippis, E.M.; Warren, R.; Trivellas, M.; Patel, P.V.; Webb, C.D.; et al. The Effect of Breathing, Movement, and Meditation on Psychological and Physical Symptoms and Inflammatory Biomarkers in Inflammatory Bowel Disease: A Randomized Controlled Trial. Inflamm. Bowel Dis. 2015, 21, 2886–2896. [Google Scholar] [CrossRef]
- Mikocka-Walus, A.; Bampton, P.; Hetzel, D.; Hughes, P.; Esterman, A.; Andrews, J.M. Cognitive-Behavioural Therapy for Inflammatory Bowel Disease: 24-Month Data from a Randomised Controlled Trial. Int. J. Behav. Med. 2017, 24, 127–135. [Google Scholar] [CrossRef]
- Maunder, R.G.; Levenstein, S. The Role of Stress in the Development and Clinical Course of Inflammatory Bowel Disease: Epidemiological Evidence. Curr. Mol. Med. 2008, 8, 247–252. [Google Scholar] [CrossRef] [Green Version]
- Berger, A.A.; Liu, Y.; Jin, K.; Kaneb, A.; Welschmeyer, A.; Cornett, E.M.; Kaye, A.D.; Imani, F.; Khademi, S.-H.; Varrassi, G.; et al. Efficacy of Acupuncture in the Treatment of Chronic Abdominal Pain. Anesth. Pain Med. 2021, 11, e113027. [Google Scholar] [CrossRef]
- Zielińska, M.; Jarmuż, A.; Wasilewski, A.; Sałaga, M.; Fichna, J. Role of Transient Receptor Potential Channels in Intestinal Inflammation and Visceral Pain: Novel Targets in Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2015, 21, 419–427. [Google Scholar] [CrossRef]
- Agostini, S.; Petrella, C. The Endogenous Nociceptin/Orphanin FQ-NOP Receptor System as a Potential Therapeutic Target for Intestinal Disorders. Neurogastroenterol. Motil. 2014, 26, 1519–1526. [Google Scholar] [CrossRef]
- Gavioli, E.C.; Romão, P.R.T. NOP Receptor Ligands as Potential Agents for Inflammatory and Autoimmune Diseases. J. Amino Acids 2011, 2011, 836569. [Google Scholar] [CrossRef] [Green Version]
- Fichna, J.; Sobczak, M.; Mokrowiecka, A.; Cygankiewicz, A.I.; Zakrzewski, P.K.; Cenac, N.; Sałaga, M.; Timmermans, J.-P.; Vergnolle, N.; Małecka-Panas, E.; et al. Activation of the Endogenous Nociceptin System by Selective Nociceptin Receptor Agonist SCH 221510 Produces Antitransit and Antinociceptive Effect: A Novel Strategy for Treatment of Diarrhea-Predominant IBS. Neurogastroenterol. Motil. 2014, 26, 1539–1550. [Google Scholar] [CrossRef]
- Sobczak, M.; Mokrowiecka, A.; Cygankiewicz, A.I.; Zakrzewski, P.K.; Sałaga, M.; Storr, M.; Kordek, R.; Małecka-Panas, E.; Krajewska, W.M.; Fichna, J. Anti-Inflammatory and Antinociceptive Action of an Orally Available Nociceptin Receptor Agonist SCH 221510 in a Mouse Model of Inflammatory Bowel Diseases. J. Pharmacol. Exp. Ther. 2014, 348, 401–409. [Google Scholar] [CrossRef] [Green Version]
- Fichna, J.; Mokrowiecka, A.; Cygankiewicz, A.I.; Zakrzewski, P.K.; Małecka-Panas, E.; Janecka, A.; Krajewska, W.M.; Storr, M.A. Transient Receptor Potential Vanilloid 4 Blockade Protects against Experimental Colitis in Mice: A New Strategy for Inflammatory Bowel Diseases Treatment? Neurogastroenterol. Motil. 2012, 24, e557–e560. [Google Scholar] [CrossRef] [PubMed]
- Volz, M.S.; Farmer, A.; Siegmund, B. Reduction of Chronic Abdominal Pain in Patients with Inflammatory Bowel Disease through Transcranial Direct Current Stimulation: A Randomized Controlled Trial. Pain 2016, 157, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Hurtado-Lorenzo, A.; Honig, G.; Weaver, S.A.; Larkin, P.B.; Heller, C. Chronic Abdominal Pain in IBD Research Initiative: Unraveling Biological Mechanisms and Patient Heterogeneity to Personalize Treatment and Improve Clinical Outcomes. Crohn’s Colitis 360 2021, 3, otab034. [Google Scholar] [CrossRef]

| Treatment | Study Design | Study Intervention | Age (Year) Sex F | Number of Patients | Abdominal Pain Outcome |
|---|---|---|---|---|---|
| Pharmacological treatment | |||||
| tricyclic antidepressants (TCA) [70] (nortriptyline, amitriptyline, desipramine, doxepin) | Retrospective cohort study | IBD patients with inactive or mildly active disease and persistent gastrointestinal symptoms (median TCA dose: 25 mg (10–150 mg)) | 41.3 69% | 58 CD/23 UC | TCA improved gastrointestinal symptoms in 59.3% of IBD patients (Likert score ≥ 2) Response was better in UC than in CD patients (1.86 ± 0.13 vs. 1.26 ± 0.11, respectively, p = 0.003) |
| Antibiotics: metronidazole or ciprofloxacin [73] | RCT | CD patients with small intestinal bacterial overgrowth (confirmed by hydrogen/methane breath and glucose tests) receiving metronidazole 250 mg t.d.s (group A) or ciprofloxacin 500 mg b.d (group B) for 10 days | 39 41% | 29 CD | Improvement of abdominal pain in 50% (group A) and 43% (group B) of cases |
| Transdermal nicotine patch [77] | Randomized double-bind study | Transdermal nicotine (5 or 15 mg) versus placebo in active UC patients; improvement of abdominal pain was a secondary outcome. | 44 43% | 72 UC | Abdominal pain rate on 0–2 scale at 6 weeks was at 0.3 inthe nicotine group and at 0.6 in the placebo group (p = 0.05) |
| Loperamide oxide [78] | Double-blind investigation | Loperamide 1 mg or placebo after passage of each unformed stool for one week | 35 53% | 34 CD | At one week, the investigator’s assessment of the change in abdominal pain was significant for loperamide oxide (p = 0.020) but not for placebo. |
| Cannabis [11] | Monocentric cohort | Consecutive patients with IBD who had used cannabis specifically for the treatment of IBD or its symptoms were compared with those who had not | 36.6 50% (users) | 303 | 17.6% of patients used cannabis to relieve symptoms associated with their IBD. Cannabis improved abdominal pain (83.9%), abdominal cramping (76.8%), joint pain (48.2%), and diarrhea (28.6%), although side effects were frequent. |
| Dietary measures | |||||
| Low-FODMAPs diet [83] | Retrospective telephone survey | IBD patients in remission Improvement of 5 points or more for gastrointestinal symptoms after dietary information on low-FODMAPs diet | 48 39% | 52 CD/20 UC | Approximately 70% of patients were adherent to the low-FODMAPs diet After 3 months, 56% had clinical improvement of abdominal pain (p < 0.02) |
| Psychological approaches | |||||
| Cognitive behavioral therapy [84] | RCT (CBT versus supportive nondirective therapy) | Evaluation of IBD activity (PCDAI and PUCAI) and depression in young patients (after 3-month course of CBT or supportive nondirective therapy | 14.3 46% and 52% | 161 CD and 56 UC | Compared with supportive non-directive therapy, CBT showed a greater reduction in IBD activity (p = 0.04); both psychotherapies decreased rate of depression scale |
| Gut-directed hypnotherapy [85] | RCT hypnotherapy (HPN) versus nondirective discussion | Patients received seven sessions of HPN or nondirective discussion. Evaluation of proportion of participants in each condition that had remained clinically asymptomatic through 52 weeks post treatment | 38 54% | 54 quiescent UC | 68% versus 40% of patients maintaining remission for 1 year (p = 0.04) |
| Stress management program [86] | RCT stress management, self-directed stress management, or conventional medical treatment | CD patients considered in non-active stage of disease under sulfasalazine Evaluation of symptoms post-treatment | 31.7 64% | 45 CD | Significant decrease in abdominal pain in both stress management arms (14.2% and 6.6% versus 48%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wils, P.; Caron, B.; D’Amico, F.; Danese, S.; Peyrin-Biroulet, L. Abdominal Pain in Inflammatory Bowel Diseases: A Clinical Challenge. J. Clin. Med. 2022, 11, 4269. https://doi.org/10.3390/jcm11154269
Wils P, Caron B, D’Amico F, Danese S, Peyrin-Biroulet L. Abdominal Pain in Inflammatory Bowel Diseases: A Clinical Challenge. Journal of Clinical Medicine. 2022; 11(15):4269. https://doi.org/10.3390/jcm11154269
Chicago/Turabian StyleWils, Pauline, Bénédicte Caron, Ferdinando D’Amico, Silvio Danese, and Laurent Peyrin-Biroulet. 2022. "Abdominal Pain in Inflammatory Bowel Diseases: A Clinical Challenge" Journal of Clinical Medicine 11, no. 15: 4269. https://doi.org/10.3390/jcm11154269
APA StyleWils, P., Caron, B., D’Amico, F., Danese, S., & Peyrin-Biroulet, L. (2022). Abdominal Pain in Inflammatory Bowel Diseases: A Clinical Challenge. Journal of Clinical Medicine, 11(15), 4269. https://doi.org/10.3390/jcm11154269








