Establishing a Learning Model for Correct Hand Hygiene Technique in a NICU
Abstract
:1. Introduction
Problem Statement and Hypothesis
- (1)
- The main institutional level risk is that imperfect HH is dangerous for the patients and the staff; and with ABHR, the correct technique needs to be learned: the goal is to achieve perfect HH, and thus reduce the risk of infection;
- (2)
- Hospitals invest a lot of energy in staff training, but we do not know how effective it is (typically, their shortcomings can be observed easily): the goal is to know and apply effective training methods;
- (3)
- There is a large deviation in ABHR consumption: while 1 mL is a straight precursor for inadequate hygiene, 8 mL is waste—therefore the hospital’s goal is to reach an optimal level and establish the range for the staff that guarantees complete HH.
2. Materials and Methods
2.1. Measurements
2.2. Statistical Analysis
2.3. Learning Modelling
3. Results
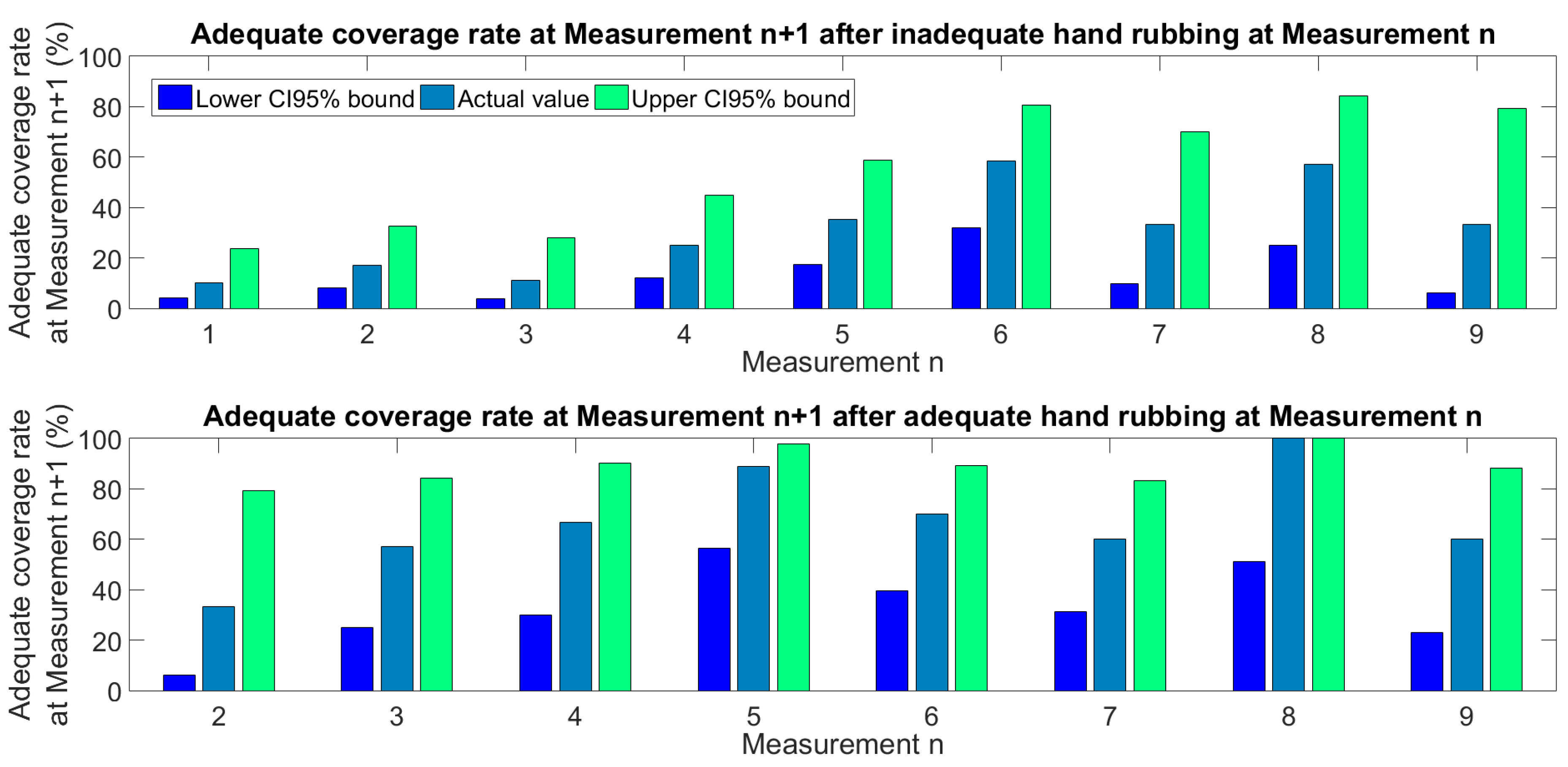
4. Discussion
- -
- Low ABHR volume correlates with transmission risk, therefore monitoring ABHR application volume per HH event can be a proxy for quality outcome;
- -
- With 1.5 mL ABHR only, the learning curve is slower, which imposes a patient risk at a NICUr
- -
- The faster competence acquisition including 3 mL ABHR mean a saving on training time;
- -
- Complete hand coverage is far from trivial even with 3 mL ABRH, therefore, regular skill training is required;
- -
- It was observed that the quality of HH strongly correlated with the size of the hands, therefore larger hands are supposed to receive larger amounts of ABHR.
5. Limitations and Future Work
- (a)
- It reduces the number of events—this means an increase in quality of life and a decrease in costs;
- (b)
- Speeds up learning—this saves on personnel costs (but depends on staff turnover, etc.);
- (c)
- Can increase disinfectant loss if too low—it is not known if it reduces wastage (it depends on the pattern of disinfectant loss).
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khamis, A.; Meng, J.; Wang, J.; Azar, A.T.; Prestes, E.; Li, H.; Hameed, I.A.; Takács, Á.; Rudas, I.J.; Haidegger, T. Robotics and Intelligent Systems Against a Pandemic. Acta Polytech. Hung. 2021, 18, 13–35. [Google Scholar] [CrossRef]
- Anand, S.; Sandlas, G.; Nabar, N.; Joshi, P.; Terdal, M.; Suratkal, S. Operating Within the Neonatal Intensive Care Unit: A Retrospective Analysis from a Tertiary Care Center. Cureus 2021, 13, e16077. [Google Scholar] [CrossRef] [PubMed]
- Pantvaidya, G.; Joshi, S.; Nayak, P.; Kannan, S.; DeSouza, A.; Poddar, P.; Prakash, G.; Vijaykumaran, P.; Nair, D.; Vaish, R.; et al. Surgical Site Infections in patients undergoing major oncological surgery during the COVID-19 paNdemic (SCION): A propensity-matched analysis. J. Surg. Oncol. 2021, 125, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.A.; Sands, K.E.; Huang, S.S.; Kleinman, K.; Septimus, E.J.; Varma, N.; Blanchard, J.; Poland, R.E.; Coady, M.H.; Yokoe, D.S.; et al. The Impact of COVID-19 on Healthcare-Associated Infections. In Open Forum Infectious Diseases; Oxford University Press: Oxford, UK, 2021; Volume 8, pp. S102–S103. [Google Scholar]
- Despotovic, A.; Milosevic, B.; Cirkovic, A.; Vujovic, A.; Cucanic, K.; Cucanic, T.; Stevanovic, G. The Impact of COVID-19 on the Profile of Hospital-Acquired Infections in Adult Intensive Care Units. Antibiotics 2021, 10, 1146. [Google Scholar] [CrossRef] [PubMed]
- Jehle, K.; Jarrett, N.; Matthews, S. Clean and green: Saving water in the operating theatre. Ann. R. Coll. Surg. Engl. 2008, 90, 22–24. [Google Scholar] [CrossRef] [Green Version]
- Scheithauer, S.; Haefner, H.; Schwanz, T.; Lopez-Gonzalez, L.; Bank, C.; Schulze-Röbbecke, R.; Lemmen, S.W. Hand hygiene in medical students: Performance, education and knowledge. Int. J. Hyg. Environ. Health 2012, 215, 536–539. [Google Scholar] [CrossRef]
- OEK National Nosocomial Surveillance Report (NSSR) 2017; OEK: Budapest, Hungary, 2018.
- Carter, B.; Collins, J.T.; Barlow-Pay, F.; Rickard, F.; Bruce, E.; Verduri, A.; Quinn, T.J.; Mitchell, E.; Price, A.; Vilches-Moraga, A.; et al. Nosocomial COVID-19 infection: Examining the risk of mortality. The COPE-Nosocomial Study (COVID in Older PEople). J. Hosp. Infect. 2020, 106, 376–384. [Google Scholar] [CrossRef]
- Szilágyi, L.; Haidegger, T.; Lehotsky, Á.; Nagy, M.; Csonka, E.A.; Sun, X.Y.; Ooi, K.L.; Fisher, D. A large-scale assessment of hand hygiene quality and the effectiveness of the “WHO 6-steps”. BMC Infect. Dis. 2013, 13, 249. [Google Scholar] [CrossRef] [Green Version]
- Pires, D.; Soule, H.; Bellissimo-Rodrigues, F.; Gayet-Ageron, A.; Pittet, D. Hand hygiene with alcohol-based hand rub: How long is long enough? Infect. Control. Hosp. Epidemiol. 2017, 38, 547–552. [Google Scholar] [CrossRef]
- Larson, E.L.; Eke, P.I.; Wilder, M.P.; Laughon, B.E. Quantity of soap as a variable in handwashing. Infect. Control. Hosp. Epidemiol. 1987, 8, 371–375. [Google Scholar] [CrossRef]
- Caini, S.; Hajdu, A.; Kurcz, A.; Böröcz, K. Hospital-Acquired Infections due to Multidrug-Resistant Organisms in Hungary, 2005–2010. 2011. Available online: http://www.eurosurveillance.org/images/dynamic/EE/V18N02/art20352.pdf (accessed on 15 June 2022).
- Ferenci, T. Different approaches to quantify years of life lost from COVID-19. Eur. J. Epidemiol. 2021, 36, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Lakoh, S.; Firima, E.; Williams, C.E.E.; Conteh, S.K.; Jalloh, M.B.; Sheku, M.G.; Adekanmbi, O.; Sevalie, S.; Kamara, S.A.; Kamara, M.A.S.; et al. An Intra-COVID-19 Assessment of Hand Hygiene Facility, Policy and Staff Compliance in Two Hospitals in Sierra Leone: Is There a Difference between Regional and Capital City Hospitals? Trop. Med. Infect. Dis. 2021, 6, 204. [Google Scholar] [CrossRef] [PubMed]
- McLean, K.A.; Mountain, K.E.; Shaw, C.A.; Drake, T.M.; Pius, R.; Knight, S.R.; Fairfield, C.J.; Sgrò, A.; Bouamrane, M.; Cambridge, W.A.; et al. Remote diagnosis of surgical-site infection using a mobile digital intervention: A randomised controlled trial in emergency surgery patients. NPJ Digit. Med. 2021, 4, 160. [Google Scholar] [CrossRef] [PubMed]
- Hermann, Z.; Péntek, M.; Gulácsi, L.; Kopcsóné Németh, I.A.; Zrubka, Z. Measuring the acceptability of EQ-5D-3L health states for different ages: A new adaptive survey methodology. Eur. J. Health Econ. 2022; in press. [Google Scholar] [CrossRef] [PubMed]
- Pittet, D.; Allegranzi, B.; Boyce, J.; World Health Organization World Alliance for Patient Safety First Global Patient Safety Challenge Core Group of Experts. The World Health Organization guidelines on hand hygiene in health care and their consensus recommendations. Infect. Control Hosp. Epidemiol. 2009, 30, 611–622. [Google Scholar] [CrossRef]
- Feng, G.; Jun, H.; Elaine, G.; Haitao, S. Powdered Activated Charcoal Tracing in Hand Hygiene Training and Compliance Assessment During the COVID-19 Pandemic. Risk Manag. Health Policy 2021, 14, 675–683. [Google Scholar] [CrossRef]
- Kopcsone Nemeth, I. Economic Implications of the Institution-Level Implementation of Evidence-Based Infection Control. Ph.D. Thesis, Corvinus University, Budapest, Hungary, 2021. [Google Scholar]
- Lehotsky, Á.; Szilágyi, L.; Ferenci, T.; Kovács, L.; Pethes, R.; Wéber, G.; Haidegger, T. Quantitative impact of direct, personal feedback on hand hygiene technique. J. Hosp. Infect. 2015, 91, 81–84. [Google Scholar] [CrossRef]
- Jansson, M.M.; Syrjälä, H.P.; Ohtonen, P.P.; Meriläinen, M.H.; Kyngäs, H.A.; Ala-Kokko, T.I. Simulation education as a single intervention does not improve hand hygiene practices: A randomized controlled follow-up study. Am. J. Infect. Control 2016, 44, 625–630. [Google Scholar] [CrossRef]
- Lacey, G.; Showstark, M.; Van Rhee, J. Training to Proficiency in the WHO Hand Hygiene Technique. J. Med. Educ. Curric. Dev. 2019, 6, 2382120519867681. [Google Scholar] [CrossRef] [Green Version]
- Duvivier, R.J.; van Dalen, J.; Muijtjens, A.M.; Moulaert, V.R.; van der Vleuten, C.P.; Scherpbier, A.J. The role of deliberate practice in the acquisition of clinical skills. BMC Med. Educ. 2011, 11, 101. [Google Scholar] [CrossRef] [Green Version]
- Goetz, T. Harnessing the Power of Feedback Loops, Wired. 2011. Available online: http://www.wired.com/magazine/2011/06/ff_feedbackloop/all/1 (accessed on 15 June 2022).
- Fish, L.; Bopp, D.; Gregory, D.; Kerley, K.D.; Gakhar, S.; Lavigne, M.C.; Boyd, F. Hand hygiene feedback impacts compliance. Am. J. Infect. Control. 2021, 49, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Bánsághi, S.; Soule, H.; Guitart, C.; Pittet, D.; Haidegger, T. Critical reliability issues of common type alcohol-based handrub dispensers. Antimicrob. Resist. Infect. Control 2020, 9, 90. [Google Scholar] [CrossRef] [PubMed]
- Bánsághi, S.; Sári, V.; Szerémy, P.; Lehotsky, Á.; Takács, B.; Tóth, B.K.; Haidegger, T. Evidence-based Hand Hygiene–Can You Trust the Fluorescent-based Assessment Methods? Acta Polytech. Hung. 2021, 18, 269–283. [Google Scholar] [CrossRef]
- Lehotsky, Á.; Szilágyi, L.; Bánsághi, S.; Szerémy, P.; Wéber, G.; Haidegger, T. Towards objective hand hygiene technique—Validation of the UV dye based hand rubbing quality assessment procedure. J. Hosp. Infect. 2017, 97, 26–29. [Google Scholar] [CrossRef]
- Kopcsóné Németh, I.; Péntek, M.; Zrubka, Z. Costs of Infection Control and Special Challenges during the Covid-19 Pandemic: Experiences in a Military Hospital. Acad. Appl. Res. Mil. Public Manag. Sci. 2021, 20, 95–110. [Google Scholar] [CrossRef]
- Tompkins, P.; Lawley, J. Feedback Loops, The Developing Group 4 June 2005. Available online: https://cleanlanguage.co.uk/articles/articles/227/1/Feedback-loops/Page1.html (accessed on 28 August 2019).
- Bansaghi, S.; Haidegger, T. Towards Objective Hand Size Assessment and a Standardized Measurement Technique. In Proceedings of the 2020 IEEE 20th International Symposium on Computational Intelligence and Informatics (CINTI), Budapest, Hungary, 5–7 November 2020; pp. 139–144. [Google Scholar]
- Macinga, D.R.; Shumaker, D.J.; Werner, H.P.; Edmonds, S.L.; Leslie, R.A.; Parker, A.E.; Arbogast, J.W. The relative influences of product volume, delivery format and alcohol concentration on dry-time and efficacy of alcohol-based hand rubs. BMC Infect. Dis. 2014, 14, 511. [Google Scholar] [CrossRef] [Green Version]
- Kampf, G.; Ruselack, S.; Eggerstedt, S.; Nowak, N.; Bashir, M. Less and less—Influence of volume on hand coverage and bactericidal efficacy in hand disinfection. BMC Infect. Dis. 2013, 13, 472. [Google Scholar] [CrossRef] [Green Version]
- Zingg, W.; Haidegger, T.; Pittet, D. Hand coverage by alcohol-based handrub varies: Volume and hand size matter. Am. J. Infect. Control 2016, 44, 1689–1691. [Google Scholar] [CrossRef]
- Bellissimo-Rodrigues, F.; Soule, H.; Gayet-Ageron, A.; Martin, Y.; Pittet, D. Should alcohol-based handrub use be customized to healthcare workers’ hand size? Infect. Control Hosp. Epidemiol. 2016, 37, 219–221. [Google Scholar] [CrossRef]
- Voniatis, C.; Bánsághi, S.; Ferencz, A.; Haidegger, T. A large-scale investigation of alcohol-based handrub (ABHR) volume: Hand coverage correlations utilizing an innovative quantitative evaluation system. Antimicrob. Resist. Infect. Control 2021, 10, 49. [Google Scholar] [CrossRef]
- Voniatis, C.; Bánsághi, S.; Szerémy, P.; Veres, D.S.; Ferencz, A.; Jedlovszky-Hajdu, A.; Haidegger, T. Application differences of liquid and gel alcohol based handrubs formulations. In Proceedings of the Chemistry Physics and Biology of Colloids and Interfaces (CPBCI), Eger, Hungary, 6–10 June 2022; p. 94. [Google Scholar]
- Ripabelli, G.; Tamburro, M.; Guerrizio, G.; Fanelli, I.; Agnusdei, C.P.; Sammarco, M.L. A single-arm study to evaluate skin tolerance, effectiveness and adherence to use of an alcohol-based hand rub solution among hospital nurses. J. Infect. Prev. 2019, 20, 224–230. [Google Scholar] [CrossRef]
- Rittenschober-Böhm, J.; Bibl, K.; Schneider, M.; Klasinc, R.; Szerémy, P.; Haidegger, T.; Ferenci, T.; Mayr, M.; Berger, A.; Assadian, O. The association between shift patterns and the quality of hand antisepsis in a neonatal intensive care unit: An observational study. Int. J. Nurs. Stud. 2020, 112, 103686. [Google Scholar] [CrossRef] [PubMed]
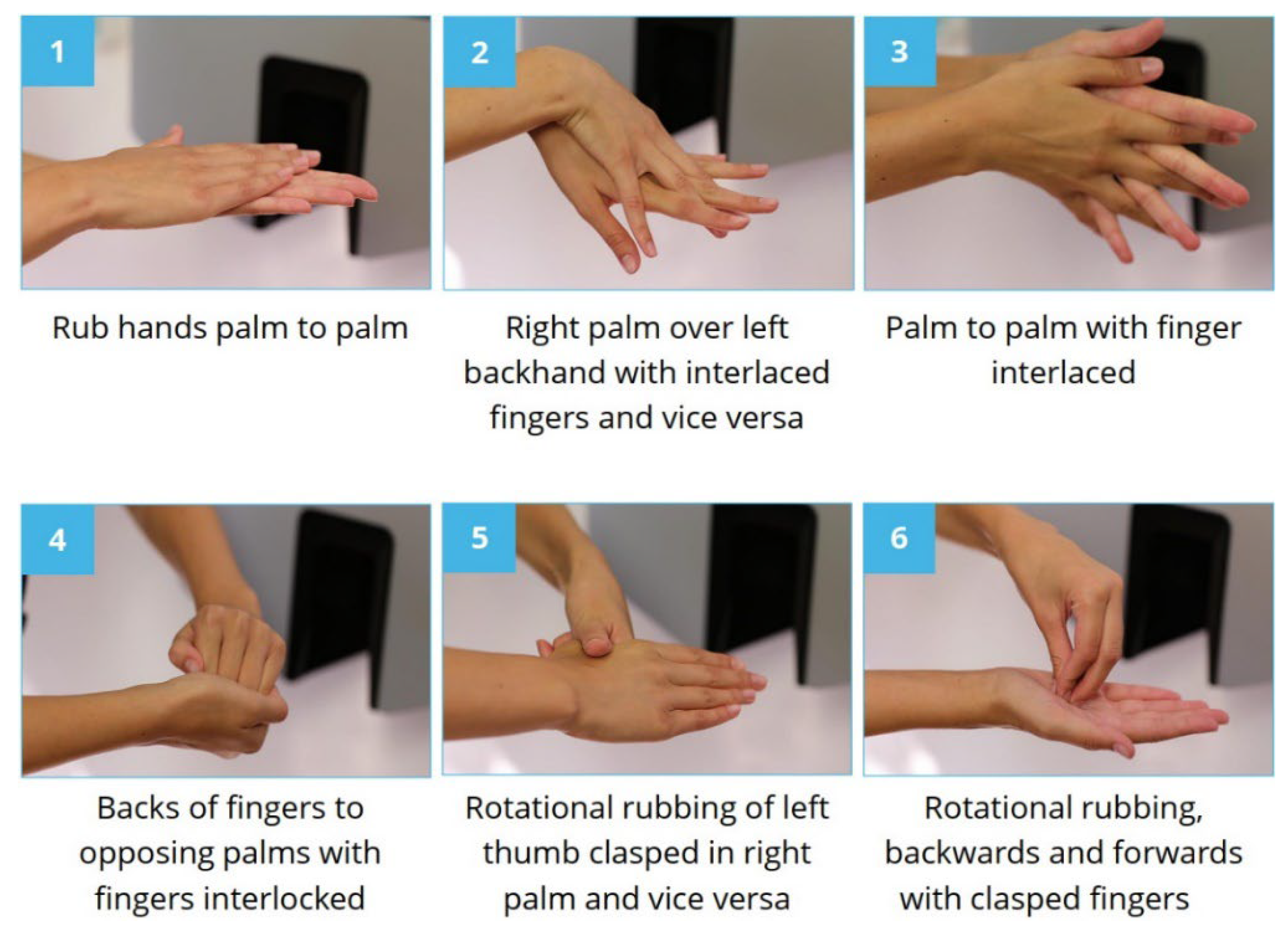




| Classification | Physicians | Other HCWs | |
|---|---|---|---|
| Gender | Male | 3 | 0 |
| Female | 7 | 29 | |
| Dominant hand | Left | 1 | 3 |
| Right | 9 | 26 | |
| Age | <25 | 0 | 2 |
| 26–35 | 4 | 0 | |
| 36–45 | 4 | 12 | |
| 46–55 | 2 | 14 | |
| >56 | 0 | 1 | |
| Total participants | 10 | 29 | |
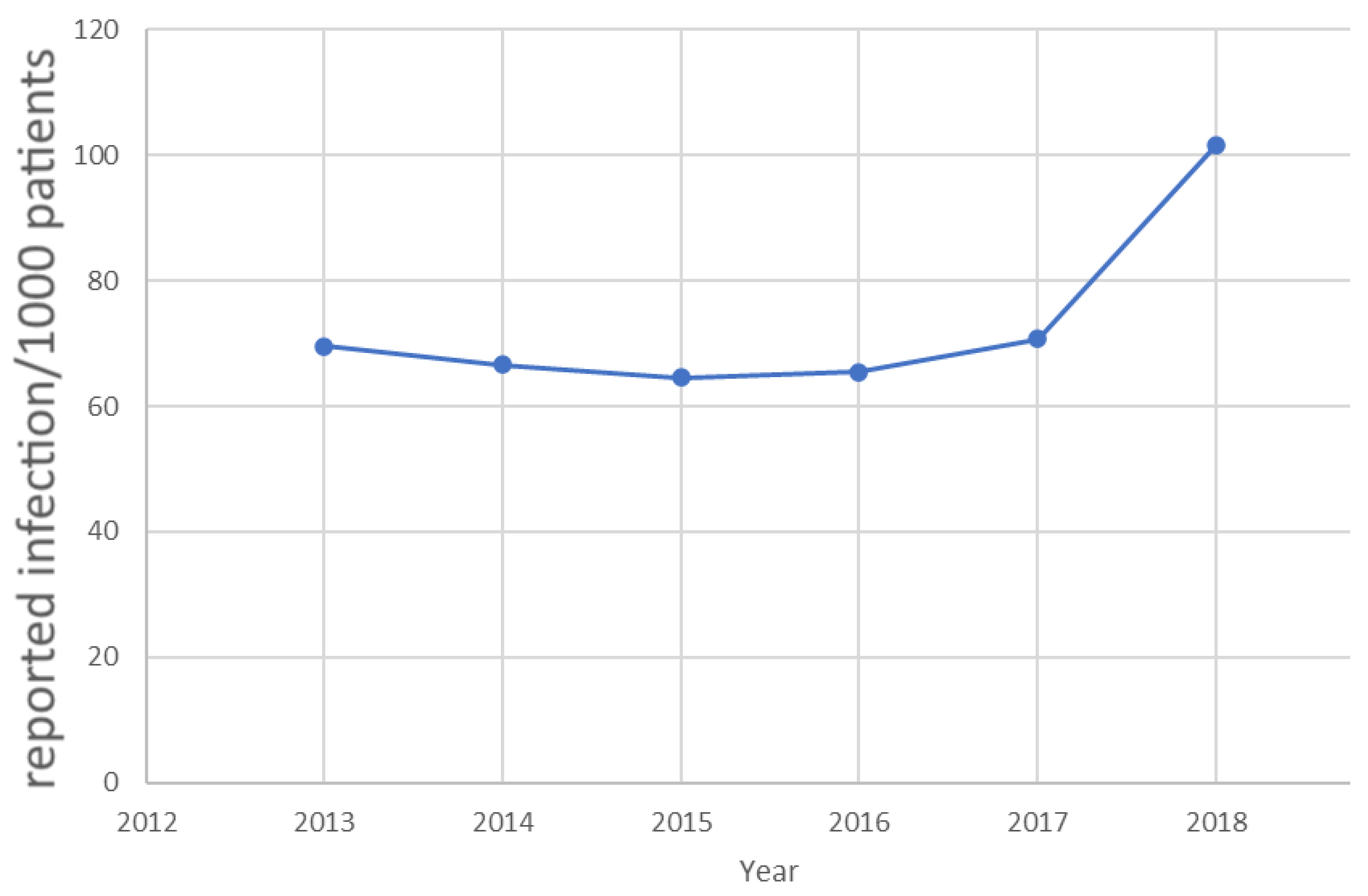
| Year | ABHR Con-Sumption (L) | Patient Days (PD) | L/1000 PD | Patients Tracked w/Microbiology Surveillance | Patients Affected by HAI | Number of Reported HAI | BSI within the HAI |
|---|---|---|---|---|---|---|---|
| 2015 | 591 | 3853.5 | 153.4 | 69 | 9 | 12 | 8 |
| 2016 | 530 | 2203.5 | 240.5 | 43 | 11 | 19 | 13 |
| 2017 | 620 | 4764 | 130.1 | 32 | 15 | 12 | 5 |
| 2018 | 698 | 6524.5 | 106.1 | 59 | 5 | 5 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Németh, I.A.K.; Nádor, C.; Szilágyi, L.; Lehotsky, Á.; Haidegger, T. Establishing a Learning Model for Correct Hand Hygiene Technique in a NICU. J. Clin. Med. 2022, 11, 4276. https://doi.org/10.3390/jcm11154276
Németh IAK, Nádor C, Szilágyi L, Lehotsky Á, Haidegger T. Establishing a Learning Model for Correct Hand Hygiene Technique in a NICU. Journal of Clinical Medicine. 2022; 11(15):4276. https://doi.org/10.3390/jcm11154276
Chicago/Turabian StyleNémeth, Irén A. Kopcsóné, Csaba Nádor, László Szilágyi, Ákos Lehotsky, and Tamás Haidegger. 2022. "Establishing a Learning Model for Correct Hand Hygiene Technique in a NICU" Journal of Clinical Medicine 11, no. 15: 4276. https://doi.org/10.3390/jcm11154276







