Shortcomings of Administrative Data to Derive Preventive Strategies for Inhospital Drug-Induced Acute Kidney Failure—Insights from Patient Record Analysis
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Setting
2.2. Validity of ICD-10 Codes for Inhospital Renal Failure
2.3. Causality Assessment and Conceivable Prevention Strategies
2.4. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Validity of ICD-10 Codes for Inhospital AKI
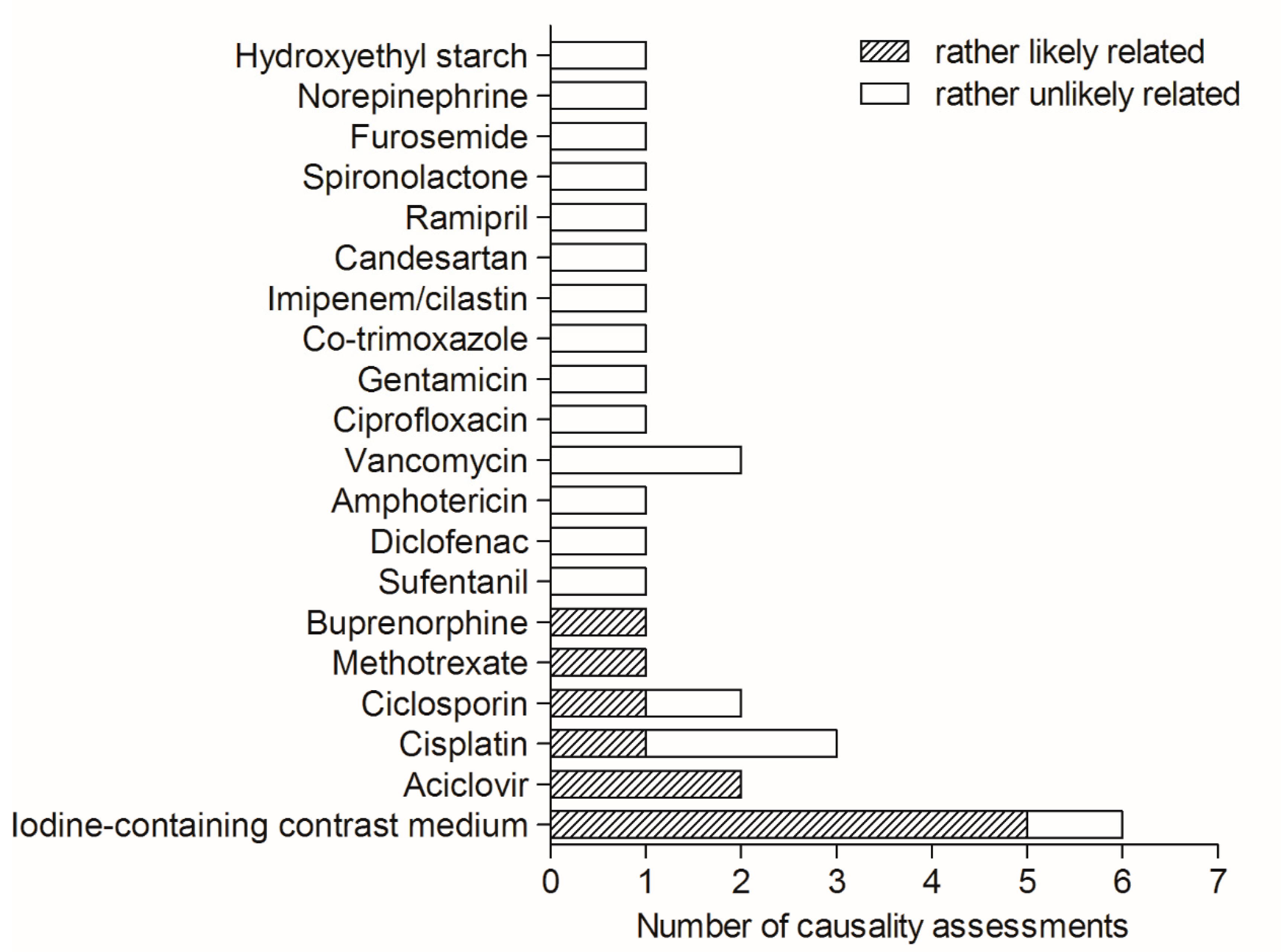
3.3. Causality Assessment and Preventive Strategies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADE | adverse drug events |
| CAD | clinical administrative data |
| ICD-10 | International Classification of Diseases-10th revision |
| AE | adverse events |
| AKI | acute kidney injury |
| SCr | serum creatinine |
| SD | standard deviation |
| ppv | positive predictive value |
References
- Cox, A.; Anton, C.; Goh, C.H.F.; Easter, M.; Langford, N.J.; Ferner, R. Adverse drug reactions in patients admitted to hospital identified by discharge ICD-10 codes and by spontaneous reports. Br. J. Clin. Pharmacol. 2001, 52, 337–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stausberg, J.; Hasford, J. Drug-related admissions and hospital-acquired adverse drug events in Germany: A longitudinal analysis from 2003 to 2007 of ICD-10-coded routine data. BMC Health Serv. Res. 2011, 11, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hohl, C.M.; Kuramoto, L.; Yu, E.; Rogula, B.; Stausberg, J.; Sobolev, B. Evaluating adverse drug event reporting in administrative data from emergency departments: A validation study. BMC Health Serv. Res. 2013, 13, 473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellis, J.R.; Kirkham, J.J.; Nunn, A.J.; Pirmohamed, M. Clinical coding of prospectively identified paediatric adverse drug reactions--a retrospective review of patient records. BMC Pharmacol. Toxicol. 2014, 15, 72. [Google Scholar] [CrossRef] [Green Version]
- Walter, S.R.; Day, R.O.; Gallego, B.; Westbrook, J.I. The impact of serious adverse drug reactions: A population-based study of a decade of hospital admissions in New South Wales, Australia. Br. J. Clin. Pharmacol. 2017, 83, 416–426. [Google Scholar] [CrossRef] [Green Version]
- Udo, R.; Der Zee, A.H.M.-V.; Egberts, T.C.G.; Breeijen, J.H.D.; Leufkens, H.G.M.; Van Solinge, W.W.; De Bruin, M.L. Validity of diagnostic codes and laboratory measurements to identify patients with idiopathic acute liver injury in a hospital database. Pharmacoepidemiol. Drug Saf. 2016, 25 (Suppl. 1), 21–28. [Google Scholar] [CrossRef] [Green Version]
- Parikh, S.; Christensen, D.; Stuchbery, P.; Peterson, J.; Hutchinson, A.; Jackson, T. Exploring in-hospital adverse drug events using ICD-10 codes. Aust. Health Rev. 2014, 38, 454–460. [Google Scholar] [CrossRef]
- Du, W.; Pearson, S.-A.; Buckley, N.; Day, C.; Banks, E. Diagnosis-based and external cause-based criteria to identify adverse drug reactions in hospital ICD-coded data: Application to an Australia population-based study. Public Health Res. Pract. 2017, 27, 2. [Google Scholar] [CrossRef] [Green Version]
- Rottenkolber, D.; Hasford, J.; Stausberg, J. Costs of Adverse Drug Events in German Hospitals—A Microcosting Study. Value Health 2012, 15, 868–875. [Google Scholar] [CrossRef] [Green Version]
- Amelung, S.; Meid, A.D.; Nafe, M.; Thalheimer, M.; Hoppe-Tichy, T.; Haefeli, W.E.; Seidling, H.M. Association of preventable adverse drug events with inpatients’ length of stay-A propensity-matched cohort study. Int. J. Clin. Pract. 2017, 71, e12990. [Google Scholar] [CrossRef] [Green Version]
- Sousa-Pinto, B.; Marques, B.; Lopes, F.; Freitas, A. Frequency and Impact of Adverse Events in Inpatients: A Nationwide Analysis of Episodes between 2000 and 2015. J. Med Syst. 2018, 42, 48. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Bell, C.M.; Wodchis, W.P. Incidence and economic burden of adverse drug reactions among elderly patients in Ontario emergency departments: A retrospective study. Drug Saf. 2012, 35, 769–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deutsche Kodierrichtlinien-Allgemeine und Spezielle Kodierrichtlinien für die Verschlüsselung von Krankheiten und Prozeduren Version 2012. Institut für das Entgeltsystem im Krankenhaus: Siegburg, Germany, 2011. Available online: https://www.g-drg.de/Media/Files/Kodierrichtlinien/DKR_2012/Deutsche_Kodierrichtlinien_2012_Endversion_A4_PDF_5.0 (accessed on 21 July 2020).
- Kellum, J.A.; Aspelin, P.; Barsoum, R.S.; Burdmann, E.A.; Goldstein, S.L.; Herzog, C.A. Section 2: AKI Definition. Kidney Int. Suppl. 2012, 2, 19–36. [Google Scholar] [CrossRef] [Green Version]
- G-DRG German Diagnosis Related Groups Version 2012 Definitionshandbuch Kompaktversion Band 1. Institut für das Entgeltsystem im Krankenhaus: Siegburg, Germany, 2011. Available online: https://www.g-drg.de/Media/Files/Archiv/DRG_Systemjahr_2012_Datenjahr_2010/Definitionshandbuch/Definitionshandbuch_2012_Kompaktversion/Definitionshandbuch_2012_Kompaktversion_Band_1 (accessed on 21 July 2020).
- Arimone, Y.; Bidault, I.; Dutertre, J.-P.; Gérardin, M.; Guy, C.; Haramburu, F.; Hillaire-Buys, D.; Meglio, C.; Penfornis, C.; Théophile, H.; et al. Updating the French Method for the Causality Assessment of Adverse Drug Reactions. Therapies 2013, 68, 69–76. [Google Scholar] [CrossRef]
- Brochard, L.; Abroug, F.; Brenner, M.; Broccard, A.F.; Danner, R.L.; Ferrer, M.; Laghi, F.; Magder, S.; Papazian, L.; Pelosi, P.; et al. An Official ATS/ERS/ESICM/SCCM/SRLF Statement: Prevention and Management of Acute Renal Failure in the ICU Patient: An international consensus conference in intensive care medicine. Am. J. Respir. Crit. Care Med. 2010, 181, 1128–1155. [Google Scholar] [CrossRef]
- Pruchnicki, M.C.; Dasta, J.F. Acute Renal Failure in Hospitalized Patients: Part I. Ann. Pharmacother. 2002, 36, 1261–1267. [Google Scholar] [CrossRef]
- Singri, N.; Ahya, S.N.; Levin, M.L. Acute Renal Failure. JAMA 2003, 289, 747–751. [Google Scholar] [CrossRef]
- Canadian Incident Analysis Framework. Edmonton, AB: Canadian Patient Safety Institute; 2012 [Incident Analysis Collaborating Parties]. Available online: http://www.patientsafetyinstitute.ca/en/toolsResources/IncidentAnalysis/Documents/Canadian%20Incident%20Analysis%20Framework.PDF (accessed on 21 July 2020).
- Vincent, C.; Taylor-Adams, S.; Chapman, E.J.; Hewett, D.; Prior, S.; Strange, P.; Tizzard, A. How to investigate and analyse clinical incidents: Clinical Risk Unit and Association of Litigation and Risk Management protocol. BMJ 2000, 320, 777–781. [Google Scholar] [CrossRef] [Green Version]
- Altman, D.G. Practical Statistics for Medical Research; Taylor & Francis: Abingdon, UK, 1990. [Google Scholar]
- Nash, K.; Hafeez, A.; Hou, S. Hospital-acquired renal insufficiency. Am. J. Kidney Dis. 2002, 39, 930–936. [Google Scholar] [CrossRef]
- Brown, J.R.; Solomon, R.J.; Sarnak, M.J.; McCullough, P.A.; Splaine, M.E.; Davies, L.; Ross, C.S.; Dauerman, H.L.; Stender, J.L.; Conley, S.M.; et al. Reducing Contrast-Induced Acute Kidney Injury Using a Regional Multicenter Quality Improvement Intervention. Circ. Cardiovasc. Qual. Outcomes 2014, 7, 693–700. [Google Scholar] [CrossRef] [Green Version]
- Eng, J.; Subramaniam, R.M.; Wilson, R.F.; Turban, S.; Choi, M.J.; Zhang, A.; Suarez-Cuervo, C.; Sherrod, C.; Hutfless, S.; Iyoha, E.E.; et al. AHRQ Comparative Effectiveness Reviews. In Contrast-Induced Nephropathy: Comparative Effects of Different Contrast Media; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2015. [Google Scholar]
- Sadat, U.; Usman, A.; Boyle, J.R.; Hayes, P.D.; Solomon, R.J. Contrast Medium-Induced Acute Kidney Injury. Cardiorenal Med. 2015, 5, 219–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Francesco, M.; Ronco, C.; Wacinski, P.; Wessely, R.; Hernández, F.; LaMotte, M. Economic impact of contrast-induced acute kidney injury associated with invasive cardiology: Role of iso-osmolar contrast media in Germany, Italy, Poland, and Spain. J. Med. Econ. 2016, 19, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Kuklik, N.; Stausberg, J.; Amiri, M.; Jöckel, K.-H. Improving drug safety in hospitals: A retrospective study on the potential of adverse drug events coded in routine data. BMC Health Serv. Res. 2019, 19, 555. [Google Scholar] [CrossRef] [Green Version]
- Kuklik, N.; Stausberg, J.; Jockel, K.-H. Adverse drug events in German hospital routine data: A validation of International Classification of Diseases, 10th revision (ICD-10) diagnostic codes. PLoS ONE 2017, 12, e0187510. [Google Scholar] [CrossRef] [Green Version]
- Iavecchia, L.; Cereza Garcia, G.; Sabate Gallego, M.; Vidal Guitart, X.; Ramos Terrades, N.; de la Torre, J.; Medrano, A.S.; Escasany, A.A. Drug-related acute renal failure in hospitalised patients. Nefrologia 2015, 35, 523–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, N.P.; Chalmers, L.; Peterson, G.M.; Bereznicki, B.J.; Curtain, C.M.; Bereznicki, L.R. Prospective identification versus administrative coding of adverse drug reaction-related hospitalizations in the elderly: A comparative analysis. Pharmacoepidemiol. Drug Saf. 2018, 27, 1281–1285. [Google Scholar] [CrossRef] [PubMed]
- Jannot, A.-S.; Burgun, A.; Thervet, E.; Pallet, N. The Diagnosis-Wide Landscape of Hospital-Acquired AKI. Clin. J. Am. Soc. Nephrol. 2017, 12, 874–884. [Google Scholar] [CrossRef]
- Grams, M.E.; Waikar, S.S.; MacMahon, B.; Whelton, S.; Ballew, S.; Coresh, J. Performance and Limitations of Administrative Data in the Identification of AKI. Clin. J. Am. Soc. Nephrol. 2014, 9, 682–689. [Google Scholar] [CrossRef] [Green Version]
- Molnar, A.O.; Van Walraven, C.; McArthur, E.; Fergusson, D.; Garg, A.X.; Knoll, G. Validation of Administrative Database Codes for Acute Kidney Injury in Kidney Transplant Recipients. Can. J. Kidney Health Dis. 2016, 3, 18. [Google Scholar] [CrossRef] [Green Version]
- van Mourik, M.S.M.; Van Duijn, P.J.; Moons, K.G.M.; Bonten, M.J.M.; Lee, G.M. Accuracy of administrative data for surveillance of healthcare-associated infections: A systematic review. BMJ Open 2015, 5, e008424. [Google Scholar] [CrossRef]
- Juurlink, D.; Preyra, C.; Croxford, R.; Chong, A.; Austin, P.; Tu, J.; Laupacis, A. Canadian Institute for Health Information Discharge Abstract Database: A Validation Study; Institute for Clinical Evaluative Sciences: Toronto, ON, Canada, 2006. [Google Scholar]
- Glance, L.G.; Osler, T.M.; Mukamel, D.B.; Dick, A.W. Impact of the present-on-admission indicator on hospital quality measurement: Experience with the Agency for Healthcare Research and Quality (AHRQ) Inpatient Quality Indicators. Med. Care 2008, 46, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Agbabiaka, T.B.; Savovic, J.; Ernst, E. Methods for causality assessment of adverse drug reactions: A systematic review. Drug Saf. 2008, 31, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Théophile, H.; André, M.; Miremont-Salamé, G.; Arimone, Y.; Begaud, B. Comparison of Three Methods (An Updated Logistic Probabilistic Method, the Naranjo and Liverpool Algorithms) for the Evaluation of Routine Pharmacovigilance Case Reports Using Consensual Expert Judgement as Reference. Drug Saf. 2013, 36, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Macedo, A.F.; Marques, F.B.; Ribeiro, C.F. Can decisional algorithms replace global introspection in the individual causality assessment of spontaneously reported ADRs? Drug Saf. 2006, 29, 697–702. [Google Scholar] [CrossRef]
- Thiessard, F.; Roux, E.; Miremont-Salamé, G.; Fourrier-Réglat, A.; Haramburu, F.; Tubert-Bitter, P.; Bégaud, B. Trends in Spontaneous Adverse Drug Reaction Reports to the French Pharmacovigilance System (1986–2001). Drug Saf. 2005, 28, 731–740. [Google Scholar] [CrossRef]
- Schmiedl, S.; Rottenkolber, M.; Hasford, J.; Rottenkolber, D.; Farker, K.; Drewelow, B.; Hippius, M.; Saljé, K.; Thürmann, P. Self-Medication with Over-the-Counter and Prescribed Drugs Causing Adverse-Drug-Reaction-Related Hospital Admissions: Results of a Prospective, Long-Term Multi-Centre Study. Drug Saf. 2014, 37, 225–235. [Google Scholar] [CrossRef]
| ICD-10 Code | Code Description | Patient Cases (n) | N with an Inhospital Event | Ppv for an Inhospital Event * | N = 10 excluded because of stem cell transplantation | N | N with an Inhospital, Drug-Related Event | Ppv for an Inhospital, Drug-Induced Event * | N with an Inhospital, Drug-Related, Potentially Preventable Event | Ppv for an Inhospital, Drug-Induced and Potentially Preventable Event * |
| N14.1 | Nephropathy induced by other drugs, medicaments and biological substances | 21 | 11 | 0.52 | 18 | 4 | 0.22 | 4 | 0.22 | |
| N14.2 | Nephropathy induced by unspecified drug, medicament, or biological substance | 23 | 10 | 0.43 | 18 | 3 | 0.17 | 0 | 0.00 | |
| N99.0 | Postprocedural renal failure | 25 | 20 | 0.80 | 23 | 4 | 0.17 | 2 | 0.09 | |
| Total | 69 | 41 | 0.59 | 59 | 11 | 0.19 | 6 | 0.10 | ||
| Patient Characteristic | N (%) or Mean ± SD [Min-Median-Max] |
|---|---|
| All, n | 69 (100) |
| Men, n | 46 (67) |
| Women, n | 23 (33) |
| Age, y | 62 ± 15.6 [23-63-94] |
| Patients aged ≥ 65 y, n | 32 |
| PCCL | 3 [0-4-4] |
| ICD-10 codes/patient, n | 15 ± 9.2 [2-14-45] |
| Length of stay, d | 22.1 ± 18.3 [1-17-88] |
| Patients exceeding length of stay, n | 10 (14) |
| Exceedance of length of stay, d | 9.7 ± 15.9 [1-52] |
| Case #, Age (y), Sex | Involved Drug | Risk Factors [17,18,19] | Prevention Strategy |
|---|---|---|---|
| #1, 74, female | Intravenous aciclovir | Older age, Diabetes mellitus type 2, Severe underlying malignant disease (diffuse large B cell lymphoma) | Adequate fluid intake, fluid-balancing protocols, slow infusion rate over one hour of intravenous aciclovir [17,23] |
| #2, 83, male | Intravenous aciclovir | Older age, Male gender, Preexisting chronic kidney disease grade II/IIIa, Cardiac disease (mild heart failure), Dehydration | |
| #3, 74, male | Iodine-containing contrast agent (unknown substance) | Older age, Male gender | Adequate fluid intake, fluid-balancing protocols, use of iso-osmolar or low-osmolar preparations in lowest possible doses [24,25,26,27] |
| #4, 81, male | Iodine-containing contrast agent (iomeprol, low-osmolar) | Older age, Male gender, Arterial hypertension, Cardiac disease (NSTEMI, coronary heart disease), Diabetes mellitus type 2, Concomitant infection (urosepsis) | |
| #5, 75, male | Iodine-containing contrast agent (unknown substance) | Older age, Male gender, Preexisting chronic kidney disease grade III, Diabetes mellitus type 2, Concomitant infection (pneumonia) | |
| #6, 94, female | Iodine-containing contrast agent (unknown substance) | Older age, Heart failure, Preexisting chronic kidney disease grade III/IV, Concomitant infection (severe bacterial infection) | |
| #7, 49, male | Iodine-containing contrast agent (unknown substance) | Diabetes mellitus type 2, Male gender |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amelung, S.; Czock, D.; Thalheimer, M.; Hoppe-Tichy, T.; Haefeli, W.E.; Seidling, H.M. Shortcomings of Administrative Data to Derive Preventive Strategies for Inhospital Drug-Induced Acute Kidney Failure—Insights from Patient Record Analysis. J. Clin. Med. 2022, 11, 4285. https://doi.org/10.3390/jcm11154285
Amelung S, Czock D, Thalheimer M, Hoppe-Tichy T, Haefeli WE, Seidling HM. Shortcomings of Administrative Data to Derive Preventive Strategies for Inhospital Drug-Induced Acute Kidney Failure—Insights from Patient Record Analysis. Journal of Clinical Medicine. 2022; 11(15):4285. https://doi.org/10.3390/jcm11154285
Chicago/Turabian StyleAmelung, Stefanie, David Czock, Markus Thalheimer, Torsten Hoppe-Tichy, Walter E. Haefeli, and Hanna M. Seidling. 2022. "Shortcomings of Administrative Data to Derive Preventive Strategies for Inhospital Drug-Induced Acute Kidney Failure—Insights from Patient Record Analysis" Journal of Clinical Medicine 11, no. 15: 4285. https://doi.org/10.3390/jcm11154285
APA StyleAmelung, S., Czock, D., Thalheimer, M., Hoppe-Tichy, T., Haefeli, W. E., & Seidling, H. M. (2022). Shortcomings of Administrative Data to Derive Preventive Strategies for Inhospital Drug-Induced Acute Kidney Failure—Insights from Patient Record Analysis. Journal of Clinical Medicine, 11(15), 4285. https://doi.org/10.3390/jcm11154285






