Amaryllidaceae, Lycopodiaceae Alkaloids and Coumarins—A Comparative Assessment of Safety and Pharmacological Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Plant Materials Extraction
2.3. Animals
2.4. Drugs
2.5. Experimental Design
2.6. Behavioral Studies
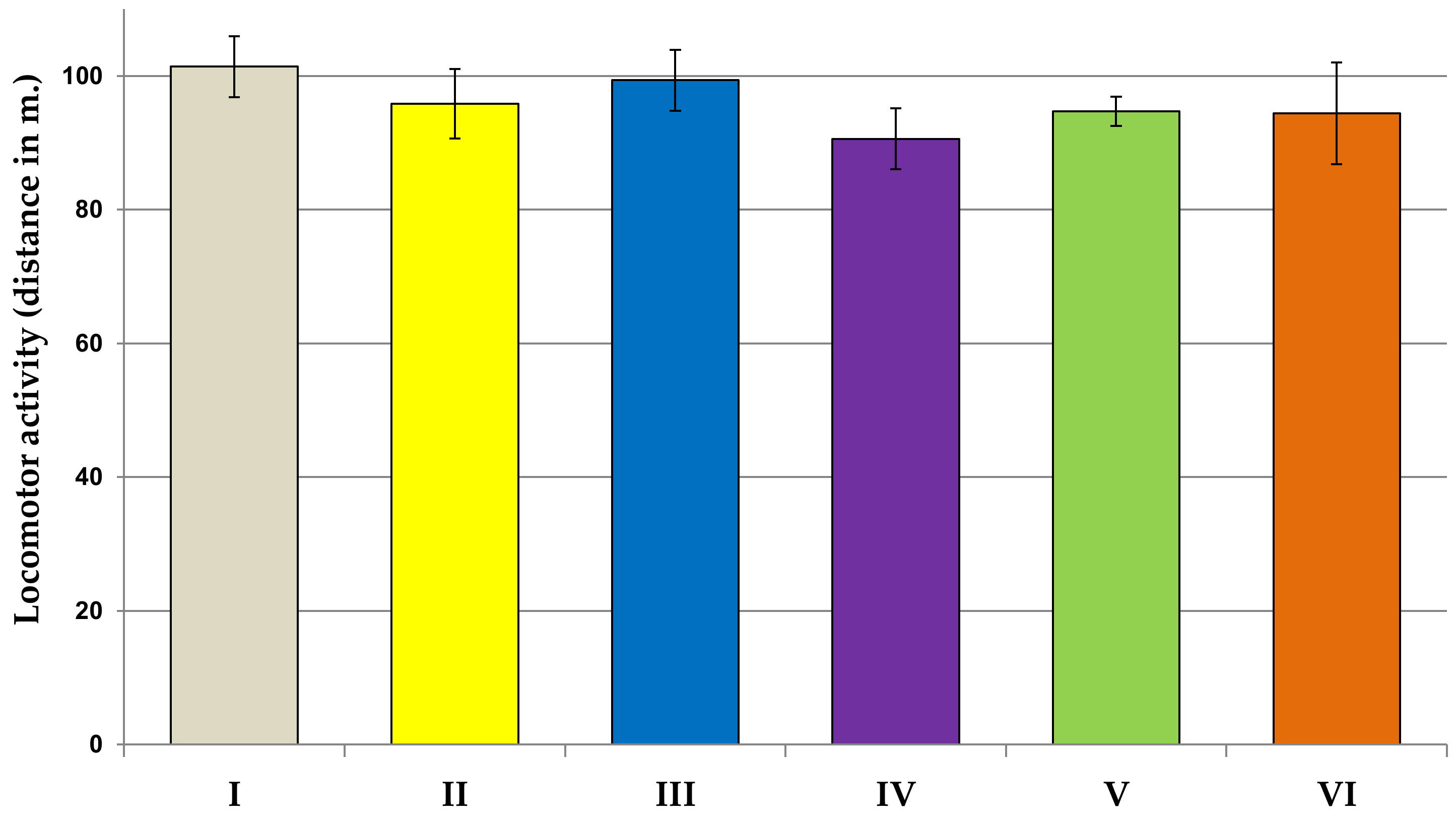
2.6.1. Locomotor Activity Test
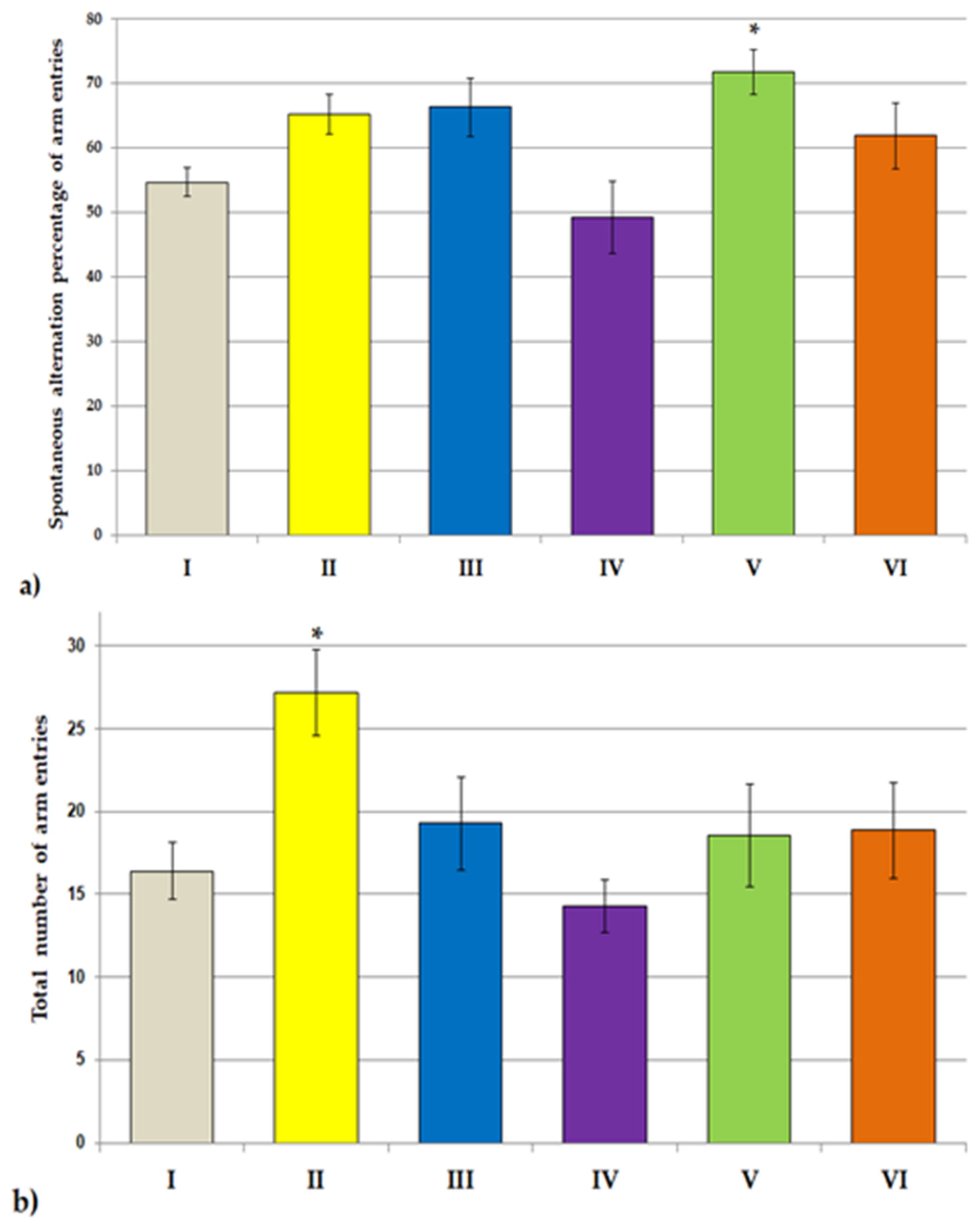
2.6.2. Y Maze Test
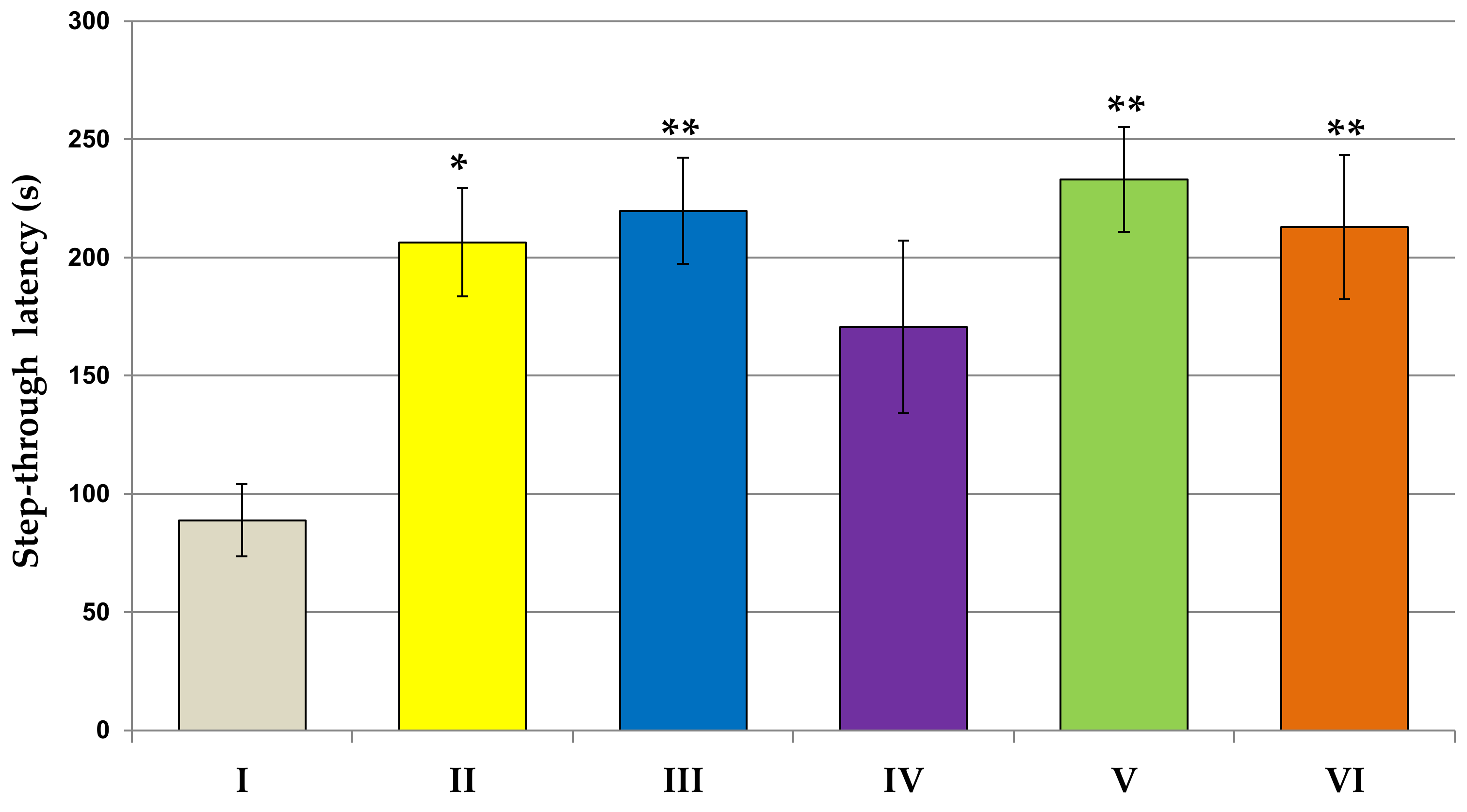
2.6.3. Passive Avoidance Test
2.7. Biochemical Studies
2.7.1. Collection of Blood
2.7.2. Collection of Brains
2.8. Statistical Analysis
3. Results
3.1. Body Weight of Rats
3.2. Behavioral Studies
3.3. Biochemical Studies
3.3.1. AST, ALT, GGT Activity; Urea, Creatinine Concentrations
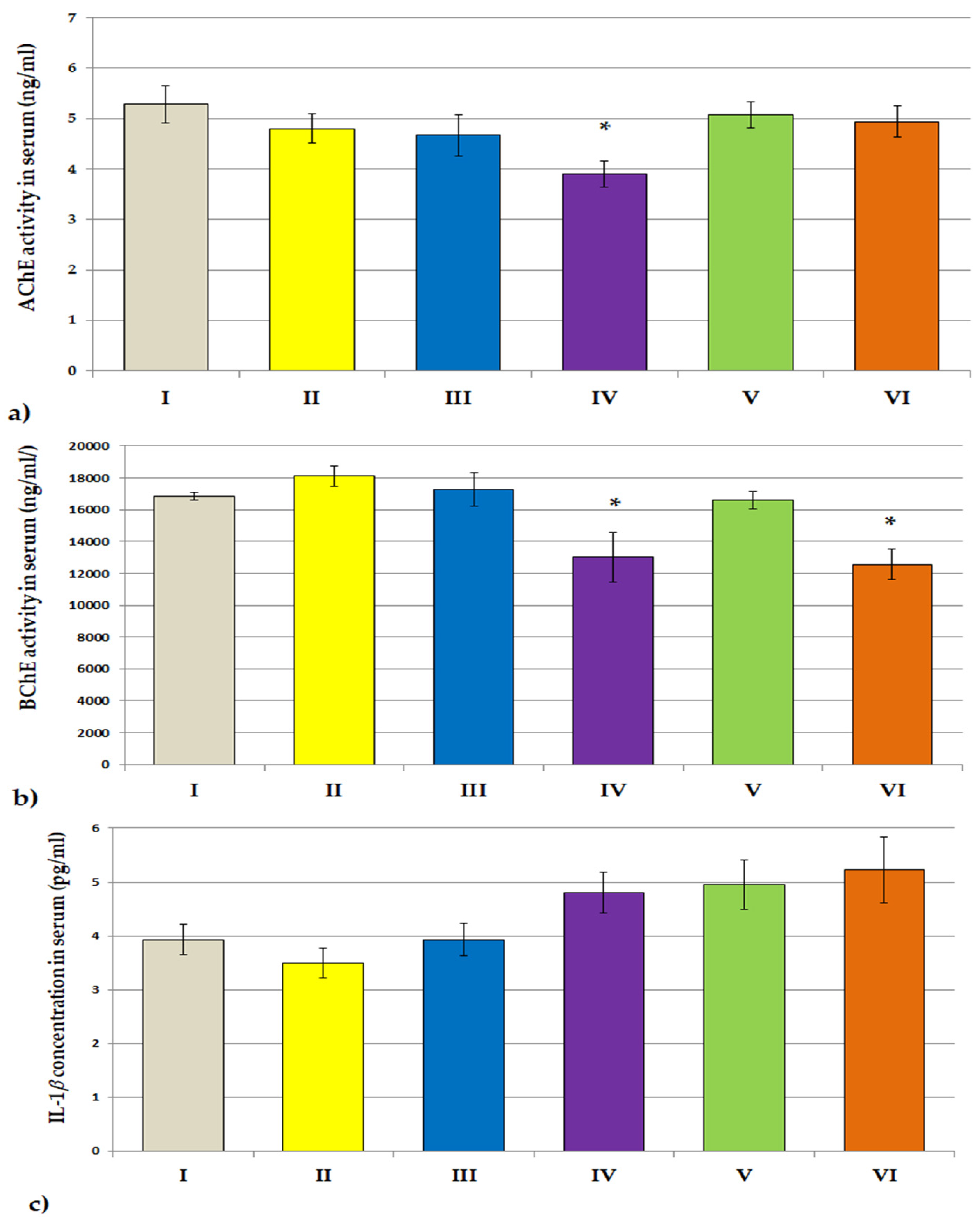
3.3.2. ACHE, BCHE Activity; IL-1β and IL-6 Concentrations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mat Nuri, T.H.; Hong, Y.H.; Ming, L.C.; Mohd Joffry, S.; Othman, M.F.; Neoh, C.F. Knowledge on Alzheimer’s disease among public hospitals and health clinics pharmacists in the state of Selangor, Malaysia. Front. Pharmacol. 2017, 8, 739. [Google Scholar] [CrossRef] [PubMed]
- Bartus, R.T.; Dean, R.L.; Beer, B.; Lippa, A.S. The cholinergic hypothesis of geriatric memory dysfunction. Science 1982, 217, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Kim, H.-G.; Lee, H.-W.; Han, J.-M.; Lee, S.-K.; Kim, D.-W.; Saravanakumar, A.; Son, C.-G. Hippocampal memory enhancing activity of pine needle extract against scopolamine-induced amnesia in a mouse model. Sci. Rep. 2015, 5, 9651. [Google Scholar] [CrossRef] [PubMed]
- Hasselmo, M.E.; Anderson, B.P.; Bower, J.M. Cholinergic modulation of cortical associative memory function. J. Neurophysiol. 1992, 67, 1230–1246. [Google Scholar] [CrossRef] [PubMed]
- Fine, A.; Hoyle, C.; Maclean, C.J.; Levatte, T.L.; Baker, H.F.; Ridley, R.M. Learning Impairments following injection of a selective cholinergic immunotoxin, ME20.4 IgG-Saporin, into the basal nucleus of Meynert in monkeys. Neuroscience 1997, 81, 331–343. [Google Scholar] [CrossRef]
- Sarter, M.; Bruno, J.P. Cognitive functions of cortical acetylcholine: Toward a unifying hypothesis. Brain Res. Rev. 1997, 23, 28–46. [Google Scholar] [CrossRef]
- Miranda, M.I.; Bermúdez-Rattoni, F. Reversible inactivation of the nucleus basalis magnocellularis induces disruption of cortical acetylcholine release and acquisition, but not retrieval, of aversive memories. Proc. Natl. Acad. Sci. USA 1999, 96, 6478–6482. [Google Scholar] [CrossRef]
- Haam, J.; Yakel, J.L. Cholinergic modulation of the hippocampal region and memory function. J. Neurochem. 2017, 142 (Suppl. S2), 111–121. [Google Scholar] [CrossRef]
- Du, X.; Wang, X.; Geng, M. Alzheimer’s disease hypothesis and related therapies. Transl. Neurodegener. 2018, 7, 2. [Google Scholar] [CrossRef]
- Walczak-Nowicka, Ł.J.; Herbet, M. Acetylcholinesterase inhibitors in the treatment of neurodegenerative diseases and the role of acetylcholinesterase in their pathogenesis. Int. J. Mol. Sci. 2021, 22, 9290. [Google Scholar] [CrossRef]
- Liu, P.P.; Xie, Y.; Meng, X.Y.; Kang, J.S. History and progress of hypotheses and clinical trials for Alzheimer’s disease. Signal Transduct. Target. Ther. 2019, 4, e17023. [Google Scholar] [CrossRef]
- Hampel, H.; Mesulam, M.M.; Cuello, A.C.; Khachaturian, A.S.; Vergallo, A.; Farlow, M.R.; Snyder, P.J.; Giacobini, E.; Khachaturian, Z.S. Revisiting the cholinergic hypothesis in Alzheimer’s disease: Emerging evidence from translational and clinical research. J. Prev. Alzheimer’s Dis. 2019, 6, 2–15. [Google Scholar] [CrossRef]
- Mufson, E.J.; Counts, S.E.; Perez, S.E.; Ginsberg, S.D. Cholinergic system during the progression of Alzheimer’s disease: Therapeutic implications. Exp. Rev. Neurother. 2008, 8, 1703–1718. [Google Scholar] [CrossRef]
- Zhang, X.-J.; Greenberg, D.S. Acetylcholinesterase involvement in apoptosis. Front. Mol. Neurosci. 2012, 5, 40. [Google Scholar] [CrossRef]
- Toiber, D.; Berson, A.; Greenberg, D.; Melamed-Book, N.; Diamant, S.; Soreq, H. N-acetylcholinesterase-induced apoptosis in Alzheimer’s disease. PLoS ONE 2008, 3, e3108. [Google Scholar] [CrossRef]
- Zhang, B.; Gaiteri, C.; Bodea, L.-G.; Wang, Z.; McElwee, J.; Podtelezhnikov, A.A.; Zhang, C.; Xie, T.; Tran, L.; Dobrin, R.; et al. Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer’s disease. Cell 2013, 153, 707–720. [Google Scholar] [CrossRef]
- Guerreiro, R.; Wojtas, A.; Bras, J.; Carrasquillo, M.; Rogaeva, E.; Majounie, E.; Cruchaga, C.; Sassi, C.; Kauwe, J.S.K.; Younkin, S.; et al. TREM2 variants in Alzheimer’s disease. N. Engl. J. Med. 2013, 368, 117–127. [Google Scholar] [CrossRef]
- Song, W.; Hooli, B.; Mullin, K.; Jin, S.C.; Cella, M.; Ulland, T.K.; Wang, Y.; Tanzi, R.; Colonna, M. Alzheimer’s disease-associated TREM2 variants exhibit either decreased or increased ligand-dependent activation. Alzheimer’s Dement. 2017, 13, 381–387. [Google Scholar] [CrossRef]
- Colonna, M.; Wang, Y. TREM2 variants: New keys to decipher Alzheimer disease pathogenesis. Nat. Rev. Neurosci. 2016, 17, 201–207. [Google Scholar] [CrossRef]
- Bolós, M.; Perea, J.R.; Avila, J. Alzheimer’s disease as an inflammatory disease. Biomol. Concepts 2017, 8, 37–43. [Google Scholar] [CrossRef]
- Bliss, T.V.P.; Collingridge, G.L.; Morris, R.G.M. Synaptic plasticity in health and disease: Introduction and overview. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130129. [Google Scholar] [CrossRef]
- Fu, H.; Liu, B.; Frost, J.L.; Hong, S.; Jin, M.; Ostaszewski, B.; Shankar, G.M.; Costantino, I.M.; Carroll, M.C.; Mayadas, T.N.; et al. Complement component C3 and complement receptor type 3 contribute to the phagocytosis and clearance of fibrillar Aβ by microglia. Glia 2012, 60, 993–1003. [Google Scholar] [CrossRef]
- Mastrangelo, M.A.; Sudol, K.L.; Narrow, W.C.; Bowers, W.J. Interferon-γ differentially affects Alzheimer’s disease pathologies and induces neurogenesis in triple transgenic-AD mice. Am. J. Pathol. 2009, 175, 2076–2088. [Google Scholar] [CrossRef]
- Tobinick, E. Perispinal etanercept for neuroinflammatory disorders. Drug Discov. Today 2009, 14, 168–177. [Google Scholar] [CrossRef]
- O’Bryant, S.E.; Waring, S.C.; Hobson, V.; Hall, J.R.; Moore, C.B.; Bottiglieri, T.; Massman, P.; Diaz-Arrastia, R. Decreased C-reactive protein levels in Alzheimer disease. J. Geriatr. Psychiatry Neurol. 2010, 23, 49–53. [Google Scholar] [CrossRef]
- Das, P.; Golde, T. Dysfunction of TGF-β signaling in Alzheimer’s disease. J. Clin. Investig. 2006, 116, 2855–2857. [Google Scholar] [CrossRef]
- Weisman, D.; Hakimian, E.; Ho, G.J. Interleukins, inflammation, and mechanisms of Alzheimer’s disease. Vitam. Horm. 2006, 74, 505–530. [Google Scholar] [CrossRef] [PubMed]
- Lleó, A. Current therapeutic options for Alzheimer’s disease. Curr. Genom. 2007, 8, 550–558. [Google Scholar] [CrossRef]
- Ali, M.M.; Ghouri, R.G.; Ans, A.H.; Akbar, A.; Toheed, A. Recommendations for anti-inflammatory treatments in Alzheimer’s disease: A comprehensive review of the literature. Cureus 2019, 11, e4620. [Google Scholar] [CrossRef]
- Gupta, P.P.; Pandey, R.D.; Jha, D.; Shrivastav, V.; Kumar, S. Role of traditional nonsteroidal anti-inflammatory drugs in Alzheimer’s disease: A meta-analysis of randomized clinical trials. Am. J. Alzheimers Dis. Other Demen. 2015, 30, 178–182. [Google Scholar] [CrossRef]
- Azam, F.; Alabdullah, N.H.; Ehmedat, H.M.; Abulifa, A.R.; Taban, I.; Upadhyayula, S. NSAIDs as potential treatment option for preventing amyloid β toxicity in Alzheimer’s disease: An investigation by docking, molecular dynamics, and DFT studies. J. Biomol. Struct. Dyn. 2018, 36, 2099–2117. [Google Scholar] [CrossRef]
- López, S.; Bastida, J.; Viladomat, F.; Codina, C. Acetylcholinesterase inhibitory activity of some amaryllidaceae alkaloids and narcissus extracts. Life Sci. 2002, 71, 2521–2529. [Google Scholar] [CrossRef]
- Mroczek, T. Highly efficient, selective and sensitive molecular screening of acetylcholinesterase inhibitors of natural origin by solid-phase extraction-liquid chromatography/electrospray ionisation-octopole-orthogonal acceleration time-of-flight-mass spectrometry and novel thin-layer chromatography-based bioautography. J. Chromatogr. A 2009, 1216, 2519–2528. [Google Scholar] [CrossRef] [PubMed]
- Dymek, A.; Widelski, J.; Wojtanowski, K.K.; Vivcharenko, V.; Przekora, A.; Mroczek, T. Fractionation of lycopodiaceae alkaloids and evaluation of their anticholinesterase and cytotoxic activities. Molecules 2021, 26, 6379. [Google Scholar] [CrossRef]
- Bai, Y.; Li, D.; Zhou, T.; Qin, N.; Li, Z.; Yu, Z.; Hua, H. Coumarins from the roots of Angelica dahurica with antioxidant and antiproliferative activities. J. Funct. Foods 2016, 20, 453–462. [Google Scholar] [CrossRef]
- Lili, W.; Yehong, S.; Qi, Y.; Yan, H.; Jinhui, Z.; Yan, L.; Cheng, G. In vitro permeability analysis, pharmacokinetic and brain distribution study in mice of imperatorin, isoimperatorin and cnidilin in radix Angelicae dahuricae. Fitoterapia 2013, 85, 144–153. [Google Scholar] [CrossRef]
- Dymek, A.; Widelski, J.; Wojtanowski, K.K.; Płoszaj, P.; Zhuravchak, R.; Mroczek, T. Optimization of pressurized liquid extraction of Lycopodiaceae alkaloids obtained from two Lycopodium species. Molecules 2021, 26, 1626. [Google Scholar] [CrossRef]
- Mroczek, T.; Dymek, A.; Widelski, J.; Wojtanowski, K.K. The bioassay-guided fractionation and identification of potent acetylcholinesterase inhibitors from narcissus c.v. ‘Hawera’ using optimized vacuum liquid chromatography, high resolution mass spectrometry and bioautography. Metabolites 2020, 10, 395. [Google Scholar] [CrossRef]
- Ahmed, F.; Ghalib, R.M.; Sasikala, P.; Ahmed, K.K.M. Cholinesterase inhibitors from botanicals. Pharmacogn. Rev. 2013, 7, 121–130. [Google Scholar] [CrossRef]
- Konrath, E.L.; Neves, B.M.; Passos, C.D.S.; Lunardi, P.S.; Ortega, M.G.; Cabrera, J.L.; Gonçalves, C.A.; Henriques, A.T. Huperzia quadrifariata and Huperzia reflexa alkaloids inhibit acetylcholinesterase activity in vivo in mice brain. Phytomedicine 2012, 19, 1321–1324. [Google Scholar] [CrossRef][Green Version]
- Mohammed, B.; Mahmood, O. Evaluating the genotoxicity enhancement of the antileukemic drug 6-mercaptopurin when combined with Iraqi Nerium oleander and Narcissus tazetta extracts in vivo. J. Biotechnol. Res. Center 2018, 12, 92–102. [Google Scholar] [CrossRef]
- Liao, W.-J.; Fan, M.; Yang, Y.-H.; Yang, W.-T.; Li, L.-Y.; Liu, M.-L. Effects of Angelica sinensis injection on the neuronal metabolites and blood flow speed within reperfusion following the ischemic cerebral injury in rats. Chin. J. Appl. Physiol. 2003, 19, 209–212. [Google Scholar]
- Lee, B.; Sur, B.; Shim, I.; Lee, H.; Hahm, D.-H. Angelica gigas ameliorate depression-like symptoms in rats following chronic corticosterone injection. BMC Complement. Altern. Med. 2015, 15, 210. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Y.; Zheng, X.; Fang, T.; Yang, X.; Luo, X.; Guo, A.; Newell, K.A.; Huang, X.-F.; Yu, Y. Galantamine improves cognition, hippocampal inflammation, and synaptic plasticity impairments induced by lipopolysaccharide in mice. J. Neuroinflamm. 2018, 15, 112. [Google Scholar] [CrossRef]
- Hager, K.; Baseman, A.S.; Nye, J.S.; Brashear, H.R.; Han, J.; Sano, M.; Davis, B.; Richards, H.M. Effects of galantamine in a 2-year, randomized, placebo-controlled study in Alzheimer’s disease. Neuropsychiatr. Dis. Treat. 2014, 10, 391–401. [Google Scholar] [CrossRef]
- Valu, M.-V.; Ducu, C.; Moga, S.; Negrea, D.; Hritcu, L.; Boiangiu, R.S.; Vamanu, E.; Balseanu, T.A.; Carradori, S.; Soare, L.C. Effects of the hydroethanolic extract of Lycopodium selago L. on scopolamine-induced memory deficits in zebrafish. Pharmaceuticals 2021, 14, 568. [Google Scholar] [CrossRef]
- Ma, X.; Tan, C.; Zhu, D.; Gang, D.R.; Xiao, P. Huperzine A from Huperzia species—An ethnopharmacolgical review. J. Ethnopharmacol. 2007, 113, 15–34. [Google Scholar] [CrossRef]
- Raves, M.L.; Harel, M.; Pang, Y.P.; Silman, I.; Kozikowski, A.P.; Sussman, J.L. Structure of acetylcholinesterase complexed with the nootropic alkaloid, (-)-huperzine A. Nat. Struct. Biol. 1997, 4, 57–63. [Google Scholar] [CrossRef]
- Wang, H.; Wu, M.; Diao, J.; Li, J.; Sun, Y.; Xiao, X. Huperzine A ameliorates obesity-related cognitive performance impairments involving neuronal insulin signaling pathway in mice. Acta Pharmacol. Sin. 2020, 41, 145–153. [Google Scholar] [CrossRef]
- Du, Q.; Zhu, X.; Si, J. Angelica polysaccharide ameliorates memory impairment in Alzheimer’s disease rat through activating BDNF/TrkB/CREB pathway. Exp. Biol. Med. 2020, 245, 1–10. [Google Scholar] [CrossRef]
- Kim, M.; Song, M.; Oh, H.-J.; Hui, J.; Bae, W.; Shin, J.; Ji, S.-D.; Koh, Y.H.; Suh, J.W.; Park, H.; et al. Evaluating the memory enhancing effects of angelica gigas in mouse models of mild cognitive impairments. Nutrients 2019, 12, 97. [Google Scholar] [CrossRef]
- Zhu, W.-L.; Zheng, J.-Y.; Cai, W.-W.; Dai, Z.; Li, B.-Y.; Xu, T.-T.; Liu, H.-F.; Liu, X.-Q.; Wei, S.-F.; Luo, Y.; et al. Ligustilide improves aging-induced memory deficit by regulating mitochondrial related inflammation in SAMP8 Mice. Aging 2020, 12, 3175–3189. [Google Scholar] [CrossRef]
- Xu, Y.-J.; Mei, Y.; Qu, Z.-L.; Zhang, S.-J.; Zhao, W.; Fang, J.-S.; Wu, J.; Yang, C.; Liu, S.-J.; Fang, Y.-Q.; et al. Ligustilide ameliorates memory deficiency in APP/PS1 transgenic mice via restoring mitochondrial dysfunction. BioMed Res. Int. 2018, 2018, e4606752. [Google Scholar] [CrossRef]
- Berkov, S.; Ivanov, I.; Georgiev, V.; Codina, C.; Pavlov, A. Galanthamine biosynthesis in plant in vitro systems. Eng. Life Sci. 2014, 14, 643–650. [Google Scholar] [CrossRef]
- Russo, P.; Frustaci, A.; Del Bufalo, A.; Fini, M.; Cesario, A. From traditional european medicine to discovery of new drug candidates for the treatment of dementia and Alzheimer’s disease: Acetylcholinesterase inhibitors. Curr. Med. Chem. 2013, 20, 976–983. [Google Scholar] [CrossRef]
- Yao, L.; Jin, Z.; Mao, C.; Zhang, Y.; Luo, Y. Traditional Chinese Medicine clinical records classification with BERT and domain specific corpora. J. Am. Med. Inform. Assoc. 2019, 26, 1632–1636. [Google Scholar] [CrossRef]
- Fu, K.-L.; Li, X.; Ye, J.; Lu, L.; Xu, X.-K.; Li, H.-L.; Zhang, W.-D.; Shen, Y.-H. Chemical constituents of Narcissus tazetta Var. Chinensis and their antioxidant activities. Fitoterapia 2016, 113, 110–116. [Google Scholar] [CrossRef]
- Bergendorff, O.; Dekermendjian, K.; Nielsen, M.; Shan, R.; Witt, R.; Ai, J.; Sterner, O. Furanocoumarins with affinity to brain benzodiazepine receptors in vitro. Phytochemistry 1997, 44, 1121–1124. [Google Scholar] [CrossRef]
- Park, A.Y.; Park, S.-Y.; Lee, J.; Jung, M.; Kim, J.; Kang, S.S.; Youm, J.-R.; Han, S.B. Simultaneous Determination of five coumarins in Angelicae dahuricae radix by HPLC/UV and LC-ESI-MS/MS. Biom. Chromatogr. 2009, 23, 1034–1043. [Google Scholar] [CrossRef]
- Kim, D.K.; Lim, J.P.; Yang, J.H.; Eom, D.O.; Eun, J.S.; Leem, K.H. Acetylcholinesterase inhibitors from the roots of Angelica dahurica. Arch. Pharm. Res. 2002, 25, 856–859. [Google Scholar] [CrossRef]
- Choi, S.Y.; Ahn, E.-M.; Song, M.-C.; Kim, D.W.; Kang, J.H.; Kwon, O.-S.; Kang, T.-C.; Baek, N.-I. In vitro GABA-transaminase inhibitory compounds from the root of Angelica dahurica. Phytother. Res. 2005, 19, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Chinese Pharmacopoeia Commission. Chinese Pharmacopoeia; China Medical Science and Technology Press: Beijing, China, 2010; Volume 3. [Google Scholar]
- Fan, G.; Deng, R.; Zhou, L.; Meng, X.; Kuang, T.; Lai, X.; Zhang, J.; Zhang, Y. Development of a rapid resolution liquid chromatographic method combined with chemometrics for quality control of Angelicae dahuricae radix. Phytochem. Anal. 2012, 23, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Chikhi, N.; Holic, N.; Guellaen, G.; Laperche, Y. Gamma-glutamyl transpeptidase gene organization and expression: A comparative analysis in rat, mouse, pig and human species. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1999, 122, 367–380. [Google Scholar] [CrossRef]
- Hanigan, M.H. Gamma-glutamyl transpeptidase: Redox regulation and drug resistance. Adv. Cancer Res. 2014, 122, 103–141. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Evans, J.C.; Robins, S.J.; Wilson, P.W.; Albano, I.; Fox, C.S.; Wang, T.J.; Benjamin, E.J.; D’Agostino, R.B.; Vasan, R.S. Gamma Glutamyl transferase and metabolic syndrome, cardiovascular disease, and mortality risk: The framingham heart study. Arter. Thromb. Vasc. Biol. 2007, 27, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Van Hemelrijck, M.; Jassem, W.; Walldius, G.; Fentiman, I.S.; Hammar, N.; Lambe, M.; Garmo, H.; Jungner, I.; Holmberg, L. Gamma-glutamyltransferase and risk of cancer in a cohort of 545,460 persons—The Swedish AMORIS study. Eur. J. Cancer 2011, 47, 2033–2041. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Laukkanen, J.A. Gamma glutamyltransferase and risk of future dementia in middle-aged to older finnish men: A new prospective cohort study. Alzheimers Dement. 2016, 12, 931–941. [Google Scholar] [CrossRef]
- The Liver in Normal Pregnancy—Madame Curie Bioscience Database—NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK6005/ (accessed on 27 January 2022).
- Ozer, J.; Ratner, M.; Shaw, M.; Bailey, W.; Schomaker, S. The current state of serum biomarkers of hepatotoxicity. Toxicology 2008, 245, 194–205. [Google Scholar] [CrossRef]
- Rafter, I.; Gråberg, T.; Kotronen, A.; Strömmer, L.; Mattson, C.M.; Kim, R.W.; Ehrenborg, E.; Andersson, H.B.; Yki-Järvinen, H.; Schuppe-Koistinen, I.; et al. Isoform-specific alanine aminotransferase measurement can distinguish hepatic from extrahepatic injury in humans. Int. J. Mol. Med. 2012, 30, 1241–1249. [Google Scholar] [CrossRef]
- Patocka, J.; Kuca, K.; Jun, D. Acetylcholinesterase and butyrylcholinesterase—Important enzymes of human body. Acta Med. 2004, 47, 215–228. [Google Scholar] [CrossRef]
- Mushtaq, G.; Greig, N.H.; Khan, J.A.; Kamal, M.A. Status of acetylcholinesterase and butyrylcholinesterase in Alzheimer’s disease and type 2 diabetes mellitus. CNS Neurol. Disord. Drug Targets 2014, 13, 1432–1439. [Google Scholar] [CrossRef]
- Jope, R.S.; Walter-Ryan, W.G.; Alarcon, R.D.; Lally, K.M. Cholinergic processes in blood samples from patients with major psychiatric disorders. Biol. Psychiatry 1985, 20, 1258–1266. [Google Scholar] [CrossRef]
- Kaplay, S.S. Acetylcholinesterase and butyrylcholinesterase of developing human brain. Biol. Neonat. 1976, 28, 65–73. [Google Scholar] [CrossRef]
- Silver, A. The Biology of Cholinesterases; Elsevier: Amsterdam, The Netherlands; Agricultural Research Council Institute: New York, NY, USA, 1974; pp. 426–447. [Google Scholar]
- Darvesh, S.; Hopkins, D.A.; Geula, C. Neurobiology of butyrylcholinesterase. Nat. Rev. Neurosci. 2003, 4, 131–138. [Google Scholar] [CrossRef]
- Perry, E.K. The cholinergic system in old age and Alzheimer’s disease. Age Ageing 1980, 9, 1–8. [Google Scholar] [CrossRef]
- Atack, J.R.; Perry, E.K.; Bonham, J.R.; Candy, J.M.; Perry, R.H. Molecular forms of acetylcholinesterase and butyrylcholinesterase in the aged human central nervous system. J. Neurochem. 1986, 47, 263–277. [Google Scholar] [CrossRef]
- Rosenberg, P.B. Clinical aspects of inflammation in Alzheimer’s disease. Int. Rev. Psychiatry 2005, 17, 503–514. [Google Scholar] [CrossRef]
- Bellinger, F.P.; Madamba, S.G.; Campbell, I.L.; Siggins, G.R. Reduced long-term potentiation in the dentate gyrus of transgenic mice with cerebral overexpression of interleukin-6. Neurosci. Lett. 1995, 198, 95–98. [Google Scholar] [CrossRef]
- Rosas-Ballina, M.; Tracey, K.J. Cholinergic control of inflammation. J. Intern. Med. 2009, 265, 663–679. [Google Scholar] [CrossRef]
- Das, U.N. Acetylcholinesterase and butyrylcholinesterase as possible markers of low-grade systemic inflammation. Med. Sci. Monit. 2007, 13, RA214–RA221. [Google Scholar]
- Chen, J.; Sun, Z.; Jin, M.; Tu, Y.; Wang, S.; Yang, X.; Chen, Q.; Zhang, X.; Han, Y.; Pi, R. Inhibition of AGEs/RAGE/Rho/ROCK pathway suppresses non-specific neuroinflammation by regulating BV2 microglial M1/M2 polarization through the NF-ΚB pathway. J. Neuroimmunol. 2017, 305, 108–114. [Google Scholar] [CrossRef]
- Kamal, M.; Bellante, V.; Greig, N. Butyrylcholinesterase inhibitors modulate cytokines production in peripheral blood mononuclear cells. Alzheimer’s Dement. 2011, 7, S671–S672. [Google Scholar] [CrossRef]
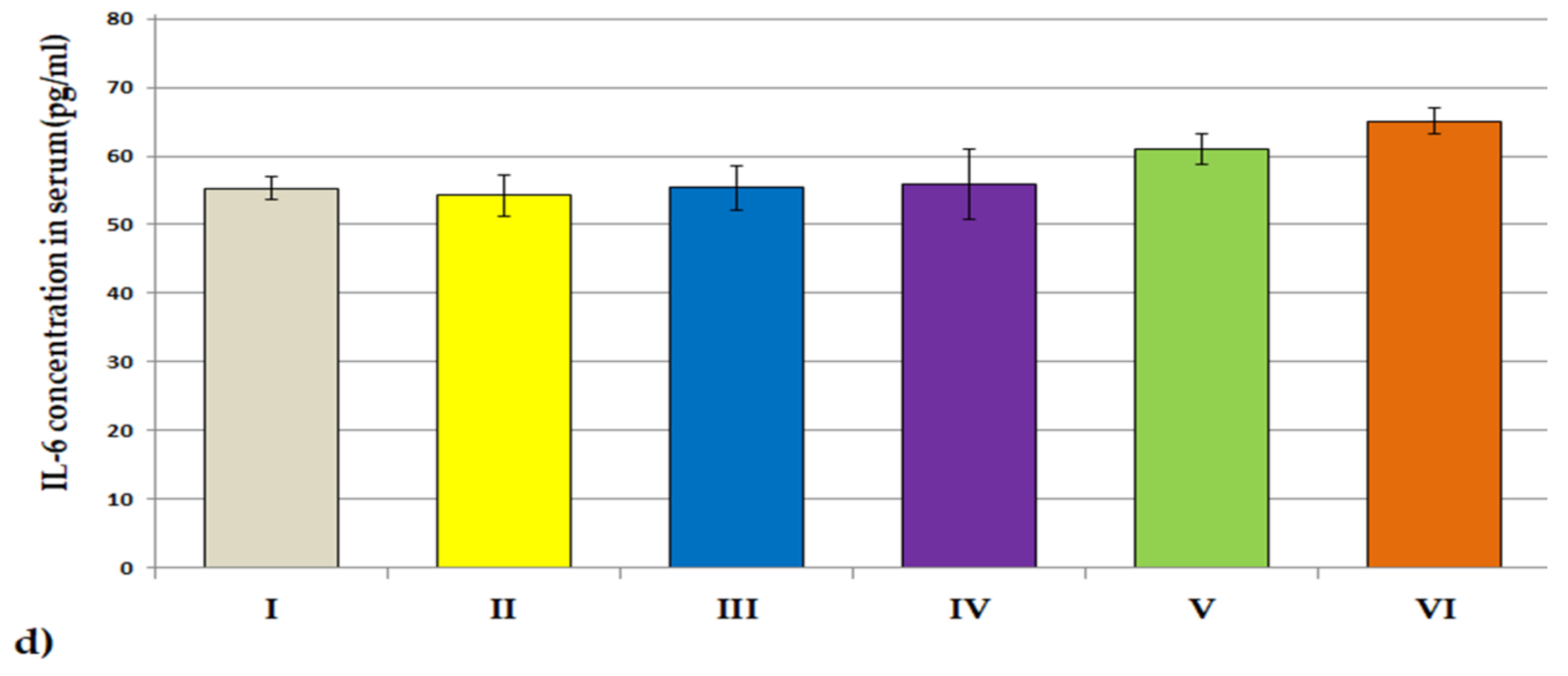

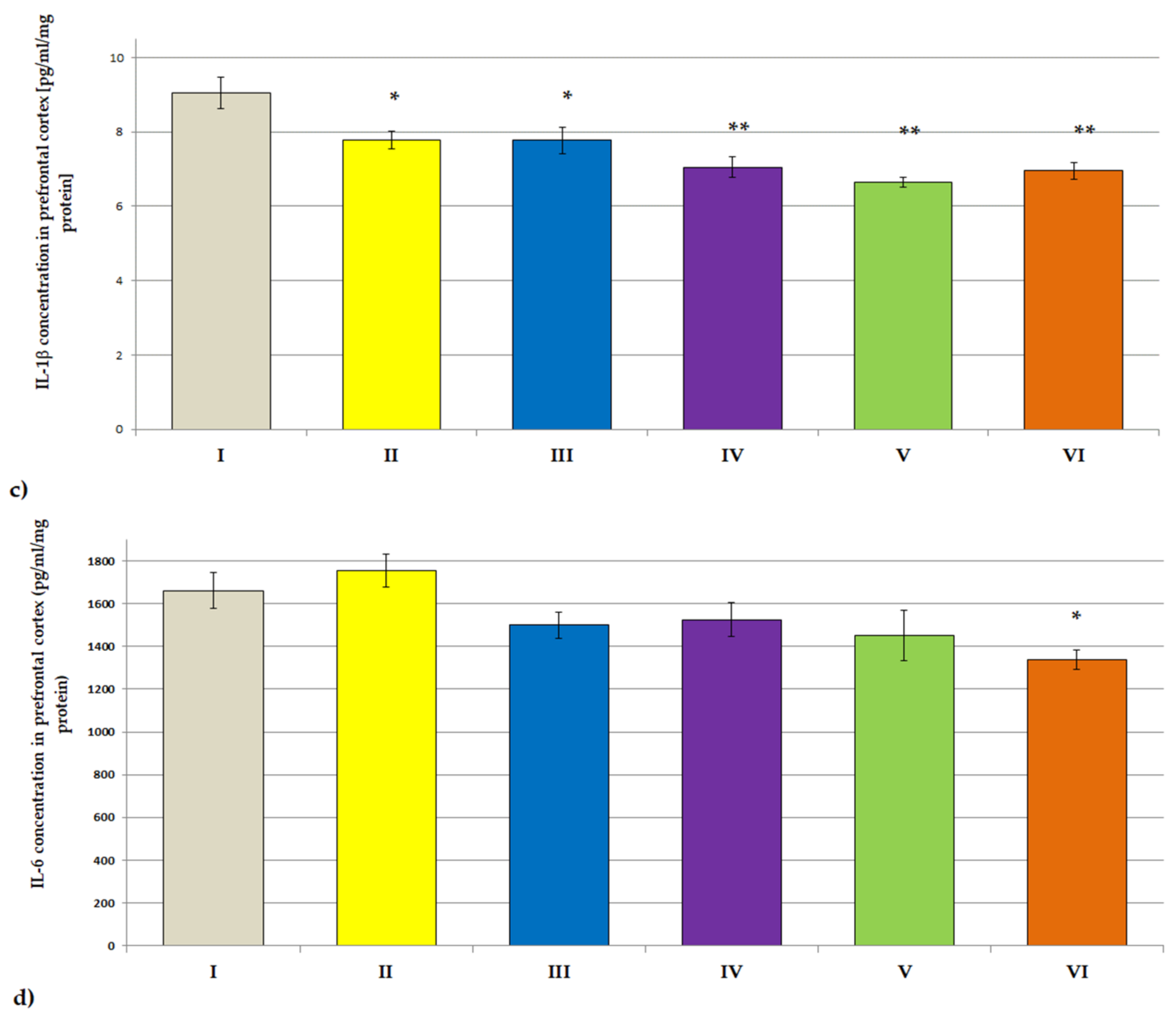
| Treatment | Day 1 | Day 7 | Day 14 | Day 21 | Day 28 |
|---|---|---|---|---|---|
| Control group | 257.5 ± 5.26 | 281.1 ± 6.5 | 299.4 ± 6.9 | 316.2 ± 5.8 | 333.4 ± 5.9 |
| Narcissus triandrus L. | 283.7 ± 13.5 | 291.8 ± 9.3 | 303.1 ± 24.4 | 333.0 ± 10.8 | 346.4 ± 11 |
| Lycopodium clavatum L. | 265.5 ± 10.6 | 283.7 ± 11.6 | 296.1 ± 12.9 | 306.57 ± 12.5 | 319.2 ± 12 |
| Lycopodium annotinum L. | 257.1 ± 4.5 | 279.7 ± 4.4 | 291.4 ± 4.4 | 302.8 ± 4.4 | 302.7 ± 13 |
| Huperzia selago L. | 276.0 ± 6.2 | 295.0 ± 7.6 | 313.0 ± 9.4 | 325.2 ± 9.9 | 334.1 ± 9.6 |
| Angelica dahurica (sp.) | 255.0 ± 8.1 | 277.7 ± 10.9 | 294.1 ± 13.1 | 305.7 ± 13 | 321 ± 12.5 |
| Treatment | AST [U/L] | ALT [U/L] | GGT [U/L] | UREA [mg/dL] | Creatinine [mg/dL] |
|---|---|---|---|---|---|
| Control group | 77.57 ± 2.30 | 29.43 ± 2.5 | 5.39 ± 0.95 | 37.93 ± 8.8 | 0.32 ± 0.05 |
| Narcissus triandrus L. | 63.52 ± 5.98 | 24.51 ± 1.5 | 3.90 ± 0.29 | 33.80 ± 4.3 | 0.34 ± 0.037 |
| Lycopodium clavatum L. | 62.2 ± 1.89 | 25.6 ± 0.98 | 2.77 ± 0.23 ** | 44.7 ± 2.7 | 0.30 ± 0.02 |
| Lycopodium annotinum L. | 65.03 ± 8.5 | 21.75 ± 0.97 * | 1.88 ± 0.37 ** | 36.96 ± 1.6 | 0.28 ± 0.01 |
| Huperzia selago L. | 60.45 ± 3.9 | 24.46 ± 1.1 * | 2.51 ± 0.36 ** | 41.09 ± 4.2 | 0.29 ± 0.01 |
| Angelica dahurica (sp.) | 61.11 ± 3.47 | 26.33 ± 3.05 | 3.96 ± 0.22 | 26.75 ± 2.7 | 0.28 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herbet, M.; Widelski, J.; Piątkowska-Chmiel, I.; Pawłowski, K.; Dymek, A.; Mroczek, T. Amaryllidaceae, Lycopodiaceae Alkaloids and Coumarins—A Comparative Assessment of Safety and Pharmacological Activity. J. Clin. Med. 2022, 11, 4291. https://doi.org/10.3390/jcm11154291
Herbet M, Widelski J, Piątkowska-Chmiel I, Pawłowski K, Dymek A, Mroczek T. Amaryllidaceae, Lycopodiaceae Alkaloids and Coumarins—A Comparative Assessment of Safety and Pharmacological Activity. Journal of Clinical Medicine. 2022; 11(15):4291. https://doi.org/10.3390/jcm11154291
Chicago/Turabian StyleHerbet, Mariola, Jarosław Widelski, Iwona Piątkowska-Chmiel, Kamil Pawłowski, Aleksandra Dymek, and Tomasz Mroczek. 2022. "Amaryllidaceae, Lycopodiaceae Alkaloids and Coumarins—A Comparative Assessment of Safety and Pharmacological Activity" Journal of Clinical Medicine 11, no. 15: 4291. https://doi.org/10.3390/jcm11154291
APA StyleHerbet, M., Widelski, J., Piątkowska-Chmiel, I., Pawłowski, K., Dymek, A., & Mroczek, T. (2022). Amaryllidaceae, Lycopodiaceae Alkaloids and Coumarins—A Comparative Assessment of Safety and Pharmacological Activity. Journal of Clinical Medicine, 11(15), 4291. https://doi.org/10.3390/jcm11154291








