Ulva pertusa, a Marine Green Alga, Attenuates DNBS-Induced Colitis Damage via NF-κB/Nrf2/SIRT1 Signaling Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Induction of Experimental Colitis
2.4. Experimental Groups
2.5. Histological Evaluation
2.6. Toluidine Blue Staining
2.7. Immunohistochemical Analysis of iNOS, COX-2 and Nitrotyrosine
2.8. Myeloperoxidase (MPO) Activity
2.9. Malondialdehyde (MDA) Assay
2.10. Western Blot Analysis of iNOS, COX-2, IκB-α, NF-κB, MnSOD, Nrf2, HO-1, SIRT1, p53, Bcl-2, Bax, p-IκB-α and p-NF-κB
2.11. Enzyme-Linked Immunosorbent Assay (ELISA) Kits
2.12. Nitric Oxide (NO) Measurements
2.13. Statical Analysis
3. Results
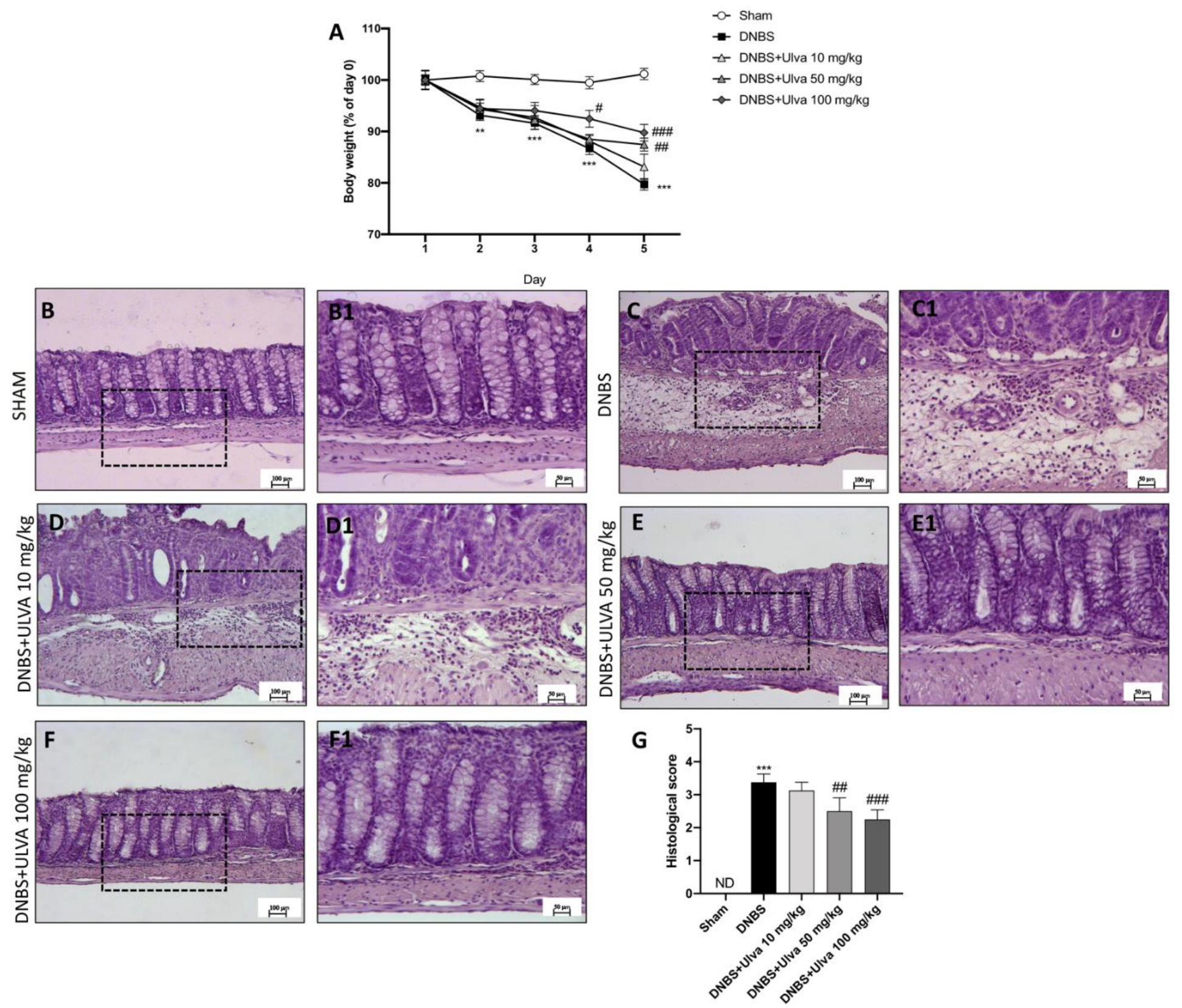
3.1. Ulva pertusa Administration Reduced Weight Loss and Histological Damage following DNBS-Induced Colitis
3.2. Ulva Treatments Reduced Mast Cells Degranulation/Number and MPO Activity
3.3. Ulva pertusa Treatments Attenuated Inflammation Driven by the NF-κB Pathway and Modulated Pro-Inflammatory Interleukins Production
3.4. Ulva pertusa Treatments Reduced Pro-Inflammatory Mediators Release of iNOS, COX-2 and Attenuated Nitrosative Stress
3.5. Ulva Treatment Blockade Apoptosis Pathway Exacerbated after DNBS-Injection
3.6. Oxidative Stress Attenuation by Ulva in DNBS-Induced Colitis by Nrf2/SIRT1 Pathway Modulation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mentella, M.C.; Scaldaferri, F.; Pizzoferrato, M.; Gasbarrini, A.; Miggiano, G.A.D. Nutrition, IBD and Gut Microbiota: A Review. Nutrients 2020, 12, 944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sturm, A.; Maaser, C.; Mendall, M.; Karagiannis, D.; Karatzas, P.; Ipenburg, N.; Sebastian, S.; Rizzello, F.; Limdi, J.; Katsanos, K.; et al. European Crohn’s and Colitis Organisation Topical Review on IBD in the Elderly. J. Crohns Colitis 2017, 11, 263–273. [Google Scholar] [CrossRef] [Green Version]
- Rosen, M.J.; Dhawan, A.; Saeed, S.A. Inflammatory Bowel Disease in Children and Adolescents. JAMA Pediatr. 2015, 169, 1053–1060. [Google Scholar] [CrossRef] [Green Version]
- Sairenji, T.; Collins, K.L.; Evans, D.V. An Update on Inflammatory Bowel Disease. Prim. Care 2017, 44, 673–692. [Google Scholar] [CrossRef] [PubMed]
- Giuffrida, P.; Cococcia, S.; Delliponti, M.; Lenti, M.V.; Di Sabatino, A. Controlling Gut Inflammation by Restoring Anti-Inflammatory Pathways in Inflammatory Bowel Disease. Cells 2019, 8, 397. [Google Scholar] [CrossRef] [Green Version]
- Balmus, I.M.; Ciobica, A.; Trifan, A.; Stanciu, C. The implications of oxidative stress and antioxidant therapies in Inflammatory Bowel Disease: Clinical aspects and animal models. Saudi J. Gastroenterol. 2016, 22, 3–17. [Google Scholar] [CrossRef]
- Yin, Y.; Wu, X.; Peng, B.; Zou, H.; Li, S.; Wang, J.; Cao, J. Curcumin improves necrotising microscopic colitis and cell pyroptosis by activating SIRT1/NRF2 and inhibiting the TLR4 signalling pathway in newborn rats. Innate Immun. 2020, 26, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Zhang, Q.; Zhao, T.; Chen, R.; Zhang, H.; Niu, X.; Li, Z. Antioxidant activity of different sulfate content derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta) in vitro. Int. J. Biol. Macromol. 2005, 37, 195–199. [Google Scholar] [CrossRef]
- Chi, Y.; Zhang, M.; Wang, X.; Fu, X.; Guan, H.; Wang, P. Ulvan lyase assisted structural characterization of ulvan from Ulva pertusa and its antiviral activity against vesicular stomatitis virus. Int. J. Biol. Macromol. 2020, 157, 75–82. [Google Scholar] [CrossRef]
- Li, Y.; Ye, H.; Wang, T.; Wang, P.; Liu, R.; Li, Y.; Tian, Y.; Zhang, J. Characterization of Low Molecular Weight Sulfate Ulva Polysaccharide and its Protective Effect against IBD in Mice. Mar. Drugs 2020, 18, 499. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, H.; Qiao, L.; Du, C.; Wei, Z.; Wang, T.; Wang, J.; Liu, R.; Wang, P. Intestinal Anti-Inflammatory Effects of Selenized Ulva pertusa Polysaccharides in a Dextran Sulfate Sodium-Induced Inflammatory Bowel Disease Model. J. Med. Food 2021, 24, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.; Chen, X.; Xu, R.; Dong, H.; Yang, F.; Wang, Y.; Zhang, Z.; Ju, J. Polysaccharides from natural resources exhibit great potential in the treatment of ulcerative colitis: A review. Carbohydr. Polym. 2021, 254, 117189. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Hu, Y.; Li, J.; Cai, J.; Qiu, Y.; Dong, C. Anti-inflammatory Effect of a Novel Pectin Polysaccharide From Rubus chingii Hu on Colitis Mice. Front. Nutr. 2022, 9, 868657. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, T.; Onizawa, M.; Saito, C.; Hikichi, R.; Yamada, D.; Minamidate, A.; Mochimaru, T.; Asahara, S.I.; Kido, Y.; Oshima, S.; et al. Oral administration of D-serine prevents the onset and progression of colitis in mice. J. Gastroenterol. 2021, 56, 732–745. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, S.; Lee, H.; Ju, S.; Park, S.; Kwon, D.; Yoo, J.W.; Yoon, I.S.; Min, D.S.; Jung, Y.S.; et al. A Colon-Targeted Prodrug, 4-Phenylbutyric Acid-Glutamic Acid Conjugate, Ameliorates 2,4-Dinitrobenzenesulfonic Acid-Induced Colitis in Rats. Pharmaceutics 2020, 12, 843. [Google Scholar] [CrossRef]
- Charpentier, C.; Chan, R.; Salameh, E.; Mbodji, K.; Ueno, A.; Coeffier, M.; Guerin, C.; Ghosh, S.; Savoye, G.; Marion-Letellier, R. Dietary n-3 PUFA May Attenuate Experimental Colitis. Mediators Inflamm. 2018, 2018, 8430614. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Omega-3 fatty acids in inflammation and autoimmune diseases. J. Am. Coll. Nutr. 2002, 21, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Casili, G.; Cordaro, M.; Impellizzeri, D.; Bruschetta, G.; Paterniti, I.; Cuzzocrea, S.; Esposito, E. Dimethyl Fumarate Reduces Inflammatory Responses in Experimental Colitis. J. Crohns Colitis 2016, 10, 472–483. [Google Scholar] [CrossRef]
- Lee, H.H.; Ahn, J.H.; Kwon, A.R.; Lee, E.S.; Kwak, J.H.; Min, Y.H. Chemical composition and antimicrobial activity of the essential oil of apricot seed. Phytother. Res. 2014, 28, 1867–1872. [Google Scholar] [CrossRef]
- Pengzhan, Y.; Ning, L.; Xiguang, L.; Gefei, Z.; Quanbin, Z.; Pengcheng, L. Antihyperlipidemic effects of different molecular weight sulfated polysaccharides from Ulva pertusa (Chlorophyta). Pharmacol. Res. 2003, 48, 543–549. [Google Scholar] [CrossRef]
- Lanza, M.; Casili, G.; Torre, G.L.; Giuffrida, D.; Rotondo, A.; Esposito, E.; Ardizzone, A.; Rando, R.; Bartolomeo, G.; Albergamo, A.; et al. Properties of a New Food Supplement Containing Actinia equina Extract. Antioxidants 2020, 9, 945. [Google Scholar] [CrossRef]
- Filippone, A.; Casili, G.; Ardizzone, A.; Lanza, M.; Mannino, D.; Paterniti, I.; Esposito, E.; Campolo, M. Inhibition of Prolyl Oligopeptidase Prevents Consequences of Reperfusion following Intestinal Ischemia. Biomedicines 2021, 9, 1354. [Google Scholar] [CrossRef] [PubMed]
- Colombo, G.; Clemente, N.; Zito, A.; Bracci, C.; Colombo, F.S.; Sangaletti, S.; Jachetti, E.; Ribaldone, D.G.; Caviglia, G.P.; Pastorelli, L.; et al. Neutralization of extracellular NAMPT (nicotinamide phosphoribosyltransferase) ameliorates experimental murine colitis. J. Mol. Med. 2020, 98, 595–612. [Google Scholar] [CrossRef] [PubMed]
- Ardizzone, A.; Fusco, R.; Casili, G.; Lanza, M.; Impellizzeri, D.; Esposito, E.; Cuzzocrea, S. Effect of Ultra-Micronized-Palmitoylethanolamide and Acetyl-l-Carnitine on Experimental Model of Inflammatory Pain. Int. J. Mol. Sci. 2021, 22, 1967. [Google Scholar] [CrossRef]
- Campolo, M.; Crupi, R.; Cordaro, M.; Cardali, S.M.; Ardizzone, A.; Casili, G.; Scuderi, S.A.; Siracusa, R.; Esposito, E.; Conti, A.; et al. Co-Ultra PEALut Enhances Endogenous Repair Response Following Moderate Traumatic Brain Injury. Int. J. Mol. Sci. 2021, 22, 8717. [Google Scholar] [CrossRef]
- Lanza, M.; Campolo, M.; Casili, G.; Filippone, A.; Paterniti, I.; Cuzzocrea, S.; Esposito, E. Sodium Butyrate Exerts Neuroprotective Effects in Spinal Cord Injury. Mol. Neurobiol. 2019, 56, 3937–3947. [Google Scholar] [CrossRef]
- Mullane, K.M.; Kraemer, R.; Smith, B. Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. J. Pharmacol. Methods 1985, 14, 157–167. [Google Scholar] [CrossRef]
- Scuderi, S.A.; Casili, G.; Lanza, M.; Filippone, A.; Paterniti, I.; Esposito, E.; Campolo, M. Modulation of NLRP3 Inflammasome Attenuated Inflammatory Response Associated to Diarrhea-Predominant Irritable Bowel Syndrome. Biomedicines 2020, 8, 519. [Google Scholar] [CrossRef]
- Filippone, A.; Lanza, M.; Campolo, M.; Casili, G.; Paterniti, I.; Cuzzocrea, S.; Esposito, E. Protective effect of sodium propionate in Abeta1-42 -induced neurotoxicity and spinal cord trauma. Neuropharmacology 2020, 166, 107977. [Google Scholar] [CrossRef]
- Filippone, A.; Lanza, M.; Campolo, M.; Casili, G.; Paterniti, I.; Cuzzocrea, S.; Esposito, E. The Anti-Inflammatory and Antioxidant Effects of Sodium Propionate. Int. J. Mol. Sci. 2020, 21, 3026. [Google Scholar] [CrossRef] [PubMed]
- Lanza, M.; Filippone, A.; Ardizzone, A.; Casili, G.; Paterniti, I.; Esposito, E.; Campolo, M. SCFA Treatment Alleviates Pathological Signs of Migraine and Related Intestinal Alterations in a Mouse Model of NTG-Induced Migraine. Cells 2021, 10, 2756. [Google Scholar] [CrossRef] [PubMed]
- Lanza, M.; Casili, G.; Filippone, A.; Campolo, M.; Paterniti, I.; Cuzzocrea, S.; Esposito, E. Evaluating the Protective Properties of a Xyloglucan-Based Nasal Spray in a Mouse Model of Allergic Rhinitis. Int. J. Mol. Sci. 2021, 22, 10472. [Google Scholar] [CrossRef]
- Rajasankar, S.; Manivasagam, T.; Surendran, S. Ashwagandha leaf extract: A potential agent in treating oxidative damage and physiological abnormalities seen in a mouse model of Parkinson’s disease. Neurosci. Lett. 2009, 454, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Elsherif, Y.; Alexakis, C.; Mendall, M. Determinants of Weight Loss prior to Diagnosis in Inflammatory Bowel Disease: A Retrospective Observational Study. Gastroenterol. Res. Pract. 2014, 2014, 762191. [Google Scholar] [CrossRef] [Green Version]
- Ghishan, F.K.; Kiela, P.R. Vitamins and Minerals in Inflammatory Bowel Disease. Gastroenterol. Clin. N. Am. 2017, 46, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.; Chuang, L.S.; Giri, M.; Villaverde, N.; Hsu, N.Y.; Sabic, K.; Joshowitz, S.; Gettler, K.; Nayar, S.; Chai, Z.; et al. Inflamed Ulcerative Colitis Regions Associated With MRGPRX2-Mediated Mast Cell Degranulation and Cell Activation Modules, Defining a New Therapeutic Target. Gastroenterology 2021, 160, 1709–1724. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Xie, F.; Ma, S.; Deng, G.; Li, Y.; Nie, Y.; Wang, F.; Yu, G.; Gao, Z.; Chen, K.; et al. Caveolin-1 protects against DSS-induced colitis through inhibiting intestinal nitrosative stress and mucosal barrier damage in mice. Biochem. Pharmacol. 2020, 180, 114153. [Google Scholar] [CrossRef] [PubMed]
- Matisz, C.E.; Faz-Lopez, B.; Thomson, E.; Al Rajabi, A.; Lopes, F.; Terrazas, L.I.; Wang, A.; Sharkey, K.A.; McKay, D.M. Suppression of colitis by adoptive transfer of helminth antigen-treated dendritic cells requires interleukin-4 receptor-alpha signaling. Sci. Rep. 2017, 7, 40631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zizzo, M.G.; Caldara, G.; Bellanca, A.; Nuzzo, D.; Di Carlo, M.; Serio, R. Preventive effects of guanosine on intestinal inflammation in 2, 4-dinitrobenzene sulfonic acid (DNBS)-induced colitis in rats. Inflammopharmacology 2019, 27, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Mazzon, E.; Esposito, E.; Crisafulli, C.; Riccardi, L.; Muia, C.; Di Bella, P.; Meli, R.; Cuzzocrea, S. Melatonin modulates signal transduction pathways and apoptosis in experimental colitis. J. Pineal Res. 2006, 41, 363–373. [Google Scholar] [CrossRef]
- Guan, G.; Lan, S. Implications of Antioxidant Systems in Inflammatory Bowel Disease. Biomed. Res. Int. 2018, 2018, 1290179. [Google Scholar] [CrossRef]
- Piotrowska, M.; Swierczynski, M.; Fichna, J.; Piechota-Polanczyk, A. The Nrf2 in the pathophysiology of the intestine: Molecular mechanisms and therapeutic implications for inflammatory bowel diseases. Pharmacol. Res. 2021, 163, 105243. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; He, J.; Yin, X.; Shi, Y.; Wan, J.; Tian, Z. The protective effect of lithocholic acid on the intestinal epithelial barrier is mediated by the vitamin D receptor via a SIRT1/Nrf2 and NF-kappaB dependent mechanism in Caco-2 cells. Toxicol. Lett. 2019, 316, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Mohammad Jafari, R.; Shayesteh, S.; Ala, M.; Yousefi-Manesh, H.; Rashidian, A.; Hashemian, S.M.; Sorouri, M.; Dehpour, A.R. Dapsone Ameliorates Colitis through TLR4/NF-kB Pathway in TNBS Induced Colitis Model in Rat. Arch Med. Res. 2021, 52, 595–602. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Fusco, R.; Genovese, T.; Cordaro, M.; D'Amico, R.; Trovato Salinaro, A.; Ontario, M.L.; Modafferi, S.; Cuzzocrea, S.; Di Paola, R.; et al. Coriolus Versicolor Downregulates TLR4/NF-kappaB Signaling Cascade in Dinitrobenzenesulfonic Acid-Treated Mice: A Possible Mechanism for the Anti-Colitis Effect. Antioxidants 2022, 11, 406. [Google Scholar] [CrossRef] [PubMed]
- Dziabowska-Grabias, K.; Sztanke, M.; Zajac, P.; Celejewski, M.; Kurek, K.; Szkutnicki, S.; Korga, P.; Bulikowski, W.; Sztanke, K. Antioxidant Therapy in Inflammatory Bowel Diseases. Antioxidants 2021, 10, 412. [Google Scholar] [CrossRef]
- Hambardikar, V.R.; Mandlik, D.S. Protective effect of naringin ameliorates TNBS-induced colitis in rats via improving antioxidant status and pro-inflammatory cytokines. Immunopharmacol. Immunotoxicol. 2022, 44, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Cheng, J.; Zhu, S.; Zhao, J.; Ye, Q.; Xu, Y.; Dong, H.; Zheng, X. Regulating effect of baicalin on IKK/IKB/NF-kB signaling pathway and apoptosis-related proteins in rats with ulcerative colitis. Int. Immunopharmacol. 2019, 73, 193–200. [Google Scholar] [CrossRef] [PubMed]






| Experimental Groups | Experimental Procedure | N |
|---|---|---|
| Group 1: Sham + vehicle | Vehicle solution (saline) was administered by oral gavage for 4 days | 10 |
| Group 2: Sham + Ulva pertusa 10 mg/kg | Ulva pertusa extract 10 mg/kg was administered by oral gavage for 4 days | 10 |
| Group 3: Sham + Ulva pertusa 50 mg/kg | Ulva pertusa extract 50 mg/kg was administered by oral gavage for 4 days | 10 |
| Group 4: Sham + Ulva pertusa 100 mg/kg | Ulva pertusa extract 100 mg/kg was administered by oral gavage for 4 days | 10 |
| Group 5: DNBS + vehicle | Group of mice subjected to DNBS-colitis induction and then administered with vehicle solution (saline) by oral gavage every 24 h, starting from 3 h after the DNBS instillation | 10 |
| Group 6: DNBS + Ulva pertusa 10 mg/kg | Group of mice subjected to DNBS-colitis induction and then administered with Ulva pertusa extract 10 mg/kg by oral gavage every 24 h, starting from 3 h after the DNBS instillation | 10 |
| Group 7: DNBS + Ulva pertusa 50 mg/kg | Group of mice subjected to DNBS-colitis induction and then administered with Ulva pertusa extract 50 mg/kg by oral gavage every 24 h, starting from 3 h after the DNBS instillation | 10 |
| Group 8: DNBS + Ulva pertusa 100 mg/kg | Group of mice subjected to DNBS-colitis induction and then administered with Ulva pertusa extract 100 mg/kg by oral gavage every 24 h, starting from 3 h after the DNBS instillation | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ardizzone, A.; Filippone, A.; Mannino, D.; Scuderi, S.A.; Casili, G.; Lanza, M.; Cucinotta, L.; Campolo, M.; Esposito, E. Ulva pertusa, a Marine Green Alga, Attenuates DNBS-Induced Colitis Damage via NF-κB/Nrf2/SIRT1 Signaling Pathways. J. Clin. Med. 2022, 11, 4301. https://doi.org/10.3390/jcm11154301
Ardizzone A, Filippone A, Mannino D, Scuderi SA, Casili G, Lanza M, Cucinotta L, Campolo M, Esposito E. Ulva pertusa, a Marine Green Alga, Attenuates DNBS-Induced Colitis Damage via NF-κB/Nrf2/SIRT1 Signaling Pathways. Journal of Clinical Medicine. 2022; 11(15):4301. https://doi.org/10.3390/jcm11154301
Chicago/Turabian StyleArdizzone, Alessio, Alessia Filippone, Deborah Mannino, Sarah Adriana Scuderi, Giovanna Casili, Marika Lanza, Laura Cucinotta, Michela Campolo, and Emanuela Esposito. 2022. "Ulva pertusa, a Marine Green Alga, Attenuates DNBS-Induced Colitis Damage via NF-κB/Nrf2/SIRT1 Signaling Pathways" Journal of Clinical Medicine 11, no. 15: 4301. https://doi.org/10.3390/jcm11154301
APA StyleArdizzone, A., Filippone, A., Mannino, D., Scuderi, S. A., Casili, G., Lanza, M., Cucinotta, L., Campolo, M., & Esposito, E. (2022). Ulva pertusa, a Marine Green Alga, Attenuates DNBS-Induced Colitis Damage via NF-κB/Nrf2/SIRT1 Signaling Pathways. Journal of Clinical Medicine, 11(15), 4301. https://doi.org/10.3390/jcm11154301











