1. Introduction
Glaucoma is a progressive optic neuropathy affecting the visual function of 80 million people worldwide [
1]. Affected individuals are burdened with frequent clinical visits to monitor their disease progression [
2] and to receive tailored medical intervention to reduce the risk of irreversible vision loss. The need for frequent reviews places strain on the healthcare system, resulting in lengthy wait times [
3,
4]. Furthermore, a proportion of patients with glaucoma experience vision loss due to the fact of delayed follow up [
5].
Telemedicine (TM) of visual function is a novel management approach that could potentially reduce some of the shortcomings of standard in-clinic testing with Ganzfeld bowl-based computerised perimetry by providing doctors with supplementary information of a patient’s visual status in-between scheduled clinical reviews. The modelling suggests that clinical progression can be detected earlier with more frequent TM compared to the standard 6 month clinic-based visits [
6]. Several options for visual field telemedicine (VFTM) of glaucoma are available using virtual-reality-based headsets [
7,
8], but these require special purpose equipment for home use. One promising alternative technology is tablet-based perimetry such as the Melbourne Rapid Fields glaucoma iPad application (MRF, Glance Optical Pty., Ltd., Melbourne, Australia) [
9,
10] and the Eyecatcher [
11,
12,
13]. Tablet technology has the benefit that it can provide a more familiar environment to patients than virtual reality goggles given the ubiquity of smart devices among the population and the fact that many patients may own such devices or be able to borrow them from relatives or friends.
Our previous studies have shown that VFTM with the MRF has excellent uptake and compliance by participants with glaucoma in the short term (6 weeks) [
14]. We reported 72% compliance to our request for weekly VFTM and 87% retention to home testing (active testing within 28 days). Despite a large proportion (44%) of home tests having low reliability (FP > 25%; FL > 25%) compared to in-clinic assays with the Humphrey Field Analyzer (HFA, 18%), a strong correlation was found between the mean deviation (MD) of the MRF test and the HFA (r = 0.85) over the 6 weeks of this short-term study [
14]. This finding indicates that a large number of tests returned from the weekly VFTMs act to buffer any high levels of intra-test variability [
6] to yield reliable outcomes. This is evident in the mean absolute error (MAE) calculation that we reported in that paper as well as by the lower coefficient of repeatability found for the MRF (4.3 dB) compared with the HFA (6.2 dB) [
14]. Other research groups have shown excellent test compliance and participant retention to VFTM (98% retention to monthly testing) over a 6 month period [
11].
Given these promising preliminary findings we report on a home monitoring trial with telemedicine over the long term (12 months). In this study, we recruited participants who had stable, treated glaucoma at their routine glaucoma review and asked them to perform a weekly VFTM with MRF from home (MRFh) using a loaned iPad tablet and broadband access. In this manuscript, we considered the reliability of tests returned from home and the compliance of participants to the request for weekly testing over a 12 month period. Given the nature of our study population (all had stable glaucoma), in a small number of cases we also consider the capacity of VFTM to; 1. detect early visual field change in comparison to the HFA GPA, and 2. identify progression in comparison to a clinician-based diagnosis derived using standard clinical assays.
2. Methods
Ethics approval for this long-term VFTM trial was obtained from the Royal Victorian Eye and Ear Hospital ethics committee (HREC: 12/1220H, approved on 19 May 2020). All experiments were conducted in accordance with the tenets of the Declaration of Helsinki. Informed consent was obtained from all participants prior to enrolment.
2.1. Participants
Participants were recruited for this study from the glaucoma clinic of the Royal Victorian Eye and Ear Hospital, Melbourne, Australia, during a routine clinical review. Inclusion criteria were a diagnosis of stable glaucoma in the study eye. The clinical diagnosis was made by a glaucoma specialist who considered results from funduscopy, HFA perimetry, intraocular pressure and optical coherence tomography during routine 6 month clinical reviews, and stability was established by finding no change over the past 2 review periods. The other eye may have been normal; however, if both eyes were diagnosed with stable glaucoma, the eye with the better HFA MD at baseline was selected as the study eye. Additionally, there was a requirement for test subjects to have previously performed at least 2 reliable visual field examinations (24-2 SITA standard and HFA) before entry to our study in order to achieve an adequate number of tests for guided progression analysis (GPA). During the clinical trial, participants undertook a further 3 HFA tests at 6 month intervals to yield the 5 HFA examinations needed for the GPA. This enabled us to generate a slope for the mean deviation (MD, HFA) and establish stability or progression in our participants to compare to MRFh outcomes. During the trial, participants were asked to adhere to their prescribed glaucoma treatment schedule as per the advice from their ophthalmologist. Clinical reviews were conducted at baseline, 6 and 12 months. Exclusion criteria were unstable glaucoma or glaucoma surgery within the past 6 months, a recent change in glaucoma medications, visual acuity worse than 6/12, or the inability to understand English instructions provided by the audio of the iPad application.
2.2. Melbourne Rapid Fields App
The Melbourne Rapid Fields software comes in several formats and, here, we report findings for the glaucoma version of this application, which has been described elsewhere [
9,
10,
14]. In short, MRF is an application available for the Apple iPad (Apple, Cupertino, CA, USA) that can be used by participants distant to the clinic in a self-monitoring mode. It has both a polar test grid and a 24-2 test grid and, in this study, we report on the use of the 66 point polar test grid consistent with past publications. Four changes of fixation to each of the corners of the device are required to examine the full extent of visual field. Human visual fields are efficiently thresholded using a three-step Bayesian protocol based on a probability density function determined from approximately 40,000 normal and diseased data points [
9].
To facilitate self-monitoring, the MRF has audio prompts in many languages that guide patients through the examination, including requesting fixation changes to test peripheral regions of the visual field as well as in the submission of results. In this trial, we used the English set of prompts and required participants to understand the English language.
The MRF checks for fixation stability when testing during the central fixation phase using a blind spot monitor. This presents stimuli to the blind spot to estimate fixation accuracy. During the peripheral fixation phases, MRF provides audio commands that remind participants to maintain fixation to the red fixation cross.
Testing conditions at home are standardised by asking the patient to test in a quiet room free from distractions and reflections off the screen. The desired test distance of 33 cm can be achieved by training the patient to place their elbow at the edge of the iPad and using the palm of this hand to cover the non-tested eye.
Test outputs are stored on cloud portal with Health Insurance Portability and Accountability Act (HIPAA) compliant methods, and clinicians can access these results via the online portal, where a decision can be made to review a patient sooner if disease progression is confirmed. For this trial, this early recall option was not implemented: patients were reviewed on a 6 month cycle, and all clinical decisions were based on the data collected at these 6 month visits including HFA outcomes.
2.3. Testing Procedures
Patients were introduced to the MRF as reported elsewhere [
14,
15] and were given in-clinic supervised training on how to perform the test and to familiarise them to the audio instructions (
Figure 1). The training session served as an opportunity for test subjects to receive feedback on their technique for VFTM testing and results submission, and to clarify any outstanding queries with the study coordinator. A loan iPad (iPad Air 2 or iPad Pro, Apple, Cupertino, CA, USA) with cellular broadband connection was allocated to each participant and an appropriate day and time was mutually agreed upon for a text message reminder to be sent for the need for testing. Although participants might have had their own iPad, all appreciated the loaned unit and complimentary broadband connection which we provided as an inducement for continued participation to our trial. A set of step-by-step written instructions along with contact details of the study coordinator were provided for easy resolution of any technical difficulties.
On the day a home-based examination was due, participants were asked to test in a quiet room free from distractions and light reflections off the tablet screen. A simple technique was demonstrated whereby an elbow could be placed at the edge of the iPad case/keyboard to achieve the correct test distance of 33 cm. The palm of the same hand could be simultaneously used to occlude the non-tested eye. The participant was instructed to wear their habitual near correction for the duration of testing. Test subjects were asked to perform VFTM using MRFh once a week for one year and to ensure that they returned for their 6-monthly clinical reviews (
Figure 1). The compliance to clinical review timing was frustrated by the onset of the COVID-19 pandemic as will be detailed later. All loan equipment was returned at the 12 month visit and the study was ended.
2.4. Data Analysis
Data analysis was conducted on the eye with the better HFA MD at baseline. Data collected from participants with uptake of VFTM (≥1 home examination, see
Figure 2) were used to calculate the mean average error (MAE, see later). The MAE was calculated from the median MD outcome for each participant, as the median is less affected by outliers. In our analysis, we included data with reliable HFA outcomes (5 tests) and compared to reliable MRFh outcomes returned from home.
Compliance to weekly testing was classified as successful if returning a test within 7 + 1 days of a previous test, as requested on recruitment. MRF test results were considered reliable if the false positive (FP) and fixation loss (FL) rates were below 33% [
16].
The MD raw data and time dependent trends generated over the 12 month window of VFTM were calculated individually. MD values were compared to an individual’s median MD over the 12 months by computing the 95th percentile for each MD severity level based on the baseline HFA exam: normal (MD ≥ −2.1 dB, 0.73 dB), mild (−6 dB < MD < −2.1 dB, 3.34 dB), moderate (−12 dB < MD < −6 dB, 4.05 dB) and severe (MD < −12 dB, 3.83 dB). ‘Fluctuation events’ were recorded as greater than the expected variability returned by our test cohorts 95th percentile for their given MD severity level. We classified MD trend as ‘stable’ if there was ≤1 fluctuation event or fluctuating if there was >1 fluctuation event post learning phase (test 10 onwards, see mean absolute error,
Figure 3). Fluctuation events could be subdivided into the ‘learning phase’ (tests 1–9, see MAE,
Figure 3) and the ‘post learning phase’ (test 10 onwards).
Outcomes from supervised visual field testing in-clinic (HFA and MRFc) were compared to unsupervised testing from home (MRFh) by performing a t-test on the mean deviation and the pattern standard deviation (or pattern defect for the MRF). Concordance between the HFA and MRFh MD are given by Spearman rank-order correlations (rs) after Kolmogorov–Smirnov testing established that the data were non-Gaussian (GraphPad Prism 8.4, San Diego, CA, USA). Test reliability at-home versus in-clinic were compared in a 2 × 2 table with a Fisher’s exact test. The average coefficient of repeatability (CoR) was calculated for each method and significance was tested with an F-ratio.
Concordance of home monitoring with in-clinic HFA outcomes was determined by Bland–Altman analysis comparing the MD slopes for MRFh and the HFA guided progression analysis (GPA, MD slope). The MRFh trend was derived from all data collected over the study window (12 months, 20 participants), whereas GPA data were spread out over a period of 36 months that encompassed the VFTM period. MRFh progression was defined as a linear trend that was ≤−1.25 dB/yr [
17], which was calculated post hoc. Two participants were identified as showing progression by a glaucoma specialist who considered results via funduscopy, HFA perimetry, intraocular pressure, and optical coherence tomography during the 6 month clinical reviews and was blinded to the results of the VFTM.
4. Discussion
The VFTM with a tablet device is a potentially useful method for identifying progression in glaucoma in-between scheduled hospital/clinical reviews. Testing can be completed at a time that is convenient for the patient using equipment that is relatively inexpensive and readily available. In this study, we report on the compliance and reliability of home test results from a cohort of glaucoma patients and the concordance of these results to in-clinic observations made by a glaucoma specialist during routine 6-monthly reviews and outcomes returned by HFA GPA from testing performed at these reviews.
At the conclusion of 12 months of VFTM, we found a compliance rate to weekly testing of 75% in our analysed group, similar to what we reported in our short-term study (72%) [
14]. It should be noted, however, that only 32% of enrolled subjects (15/47) made it to the end of the trial. As detailed in a previous publication, the lack of compliance arises due to the fact of time constraints, IT logistical reasons or lack of motivation [
14], and the common rate of compliance between the 6 week and 12 month studies suggests that these factors manifest early during the home monitoring cycle. Moreover, we found that 25% of our cases showed a learning effect that we believe reflects an inability to cope with the technology and that this effect created artifactual improvements in their outcomes. It is our opinion that training and close monitoring of these people might result in better outcomes earlier. Given this finding, close supervision early during home monitoring and training would help improve test reliability.
Although our data found modest compliance to weekly testing, another study that considered tablet-based home monitoring of visual field in glaucoma patients found an adherence rate of 98% to monthly testing [
11]. Post hoc analysis of our data found a 97% adherence rate when analysed on a monthly cycle consistent with this past analysis. With a weekly compliance rate of 75%, on average, we can expect to receive approximately 40 reliable examinations from our patients each year when weekly testing is requested. The MAE analysis suggests that a slow progressor (−0.8 dB/yr) [
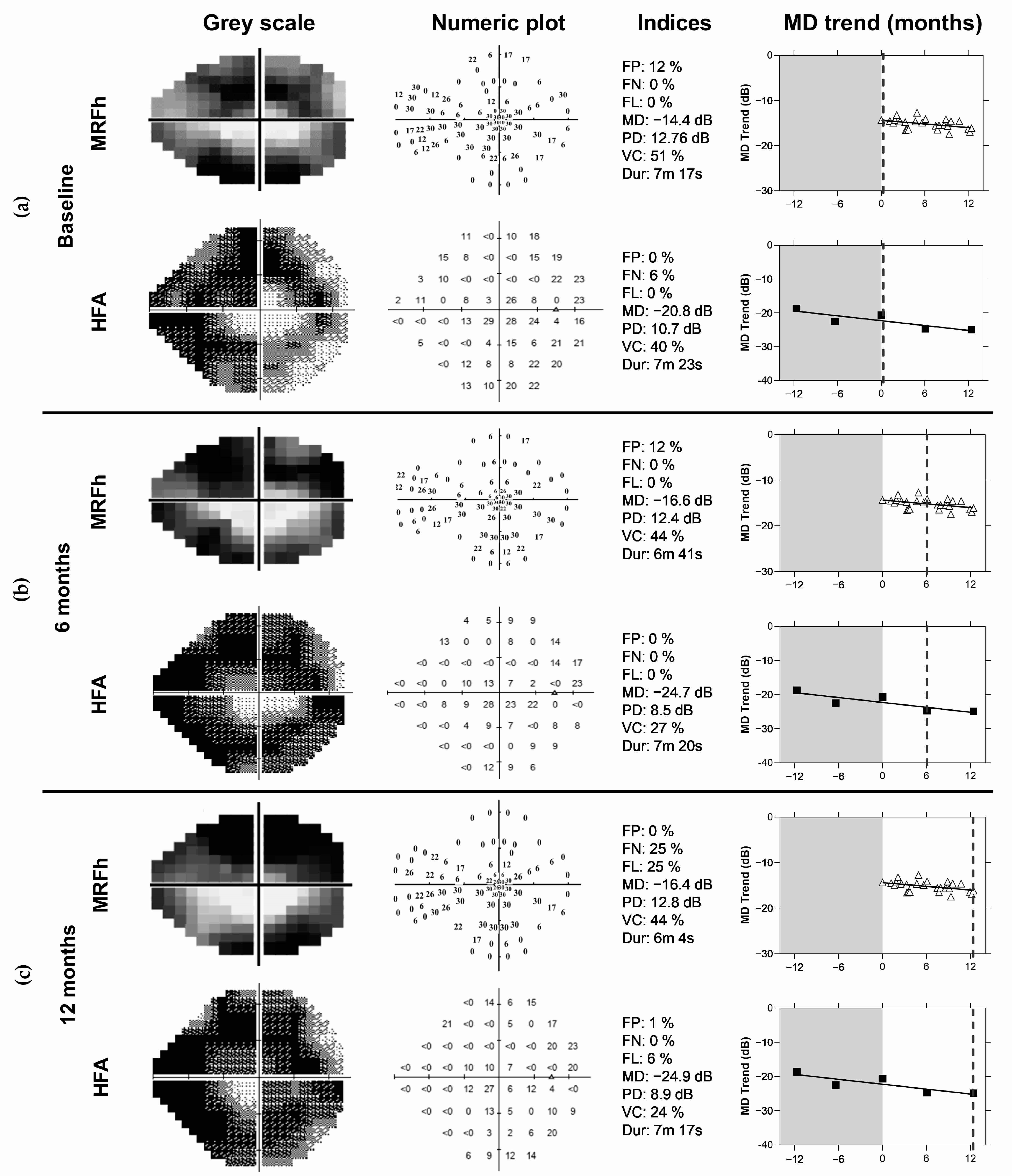
17] can be detected after 10 home tests given that the MAE reduces. This would help doctors identify progression early (as evident by the dotted lines in
Figure 9a,b), prompting an unscheduled clinical review, and resulting in better visual outcomes for patients. Conversely, patients with stable fields and no progression may have their clinical reviews extended beyond 6 months, freeing up valuable hospital and clinical resources. It should be noted that variability was reduced after 10 VFTM examinations. This suggests that prior to 10 examinations, only large changes in MD can be detected, whereas after 10 examinations, smaller changes in MD can be exposed. We chose to analyse concordance by correlating the MD trend of the MRFh to the MD trend generated by the HFA GPA. A moderate correlation is observed (R = 0.05, 95% LoA: −1.1 to 1.2 dB/yr,
Figure 8), which is likely due to the differences in spot size, spot location and background luminance of the MRFh. Elsewhere, we showed that larger spots produce higher thresholds and reduce threshold variability [
19]. Furthermore, other research groups have reported that larger size V spots performed as well as size III spots for longitudinal glaucoma progression analysis likely due to the reduced variability [
20]. Our present data indicate a similar level of variability (
Table 2, CoR) which might arise from the smaller sample size of our current study as we have shown that this is the case in past clinical trials based on larger samples (see ref. [
14]).
At the end of this VFTM trial, disease progression as defined at routine clinical review by a glaucoma specialist was identified in two eyes of two participants. The diagnoses were made using standard clinical methods which included dilated fundus examination, optical coherence tomography (OCT) and VF examination (HFA, GPA). Home monitoring with the MRFh was able to identify change in both patients after 16 weeks (
Figure 9a,b) which was 10 weeks prior to the next clinical visit. In contrast, the HFA GPA requires 104 weeks to detect change in these same patients assuming 6 month reviews (64 weeks with 3 month reviews). In addition, reliable VF test outcomes at each review and that a stable baseline was established in the first two tests. It should be noted that only 50% of our cohort (20/40 participants with VFTM uptake) returned five reliable HFA tests over the study period enabling GPA analysis, compared to 65% of MRFh tests being available.
Rather than the intensive 12 months of weekly home monitoring that we set out to achieve, our data show that short, intense periods of testing (14–20 weeks) will expose true progressors and retain high levels of interaction (≈75%,
Figure 5b). An alternative monitoring approach would be to adopt a shorter testing interval (<1 week) over a 1 to 2 week period to overcome the initial ‘learning phase’ and reduce the MAE, and then revert to monthly testing thereafter. In the presence of suspected change flag, a shorter testing interval could be adopted again (perhaps the interval may be shortened based on how much the results have deviated from baseline) to either confirm or reject the prospect for change. Both approaches would provide high levels of interaction with the best chance of detecting a progressor. This alternate approach is similar to the ‘wait and see’ concept proposed by Crabb and Garway-Heath [
21].
A limitation of this study was that it was undertaken during the pandemic, which would make it more likely for higher compliance rates to be achieved. Whilst a benefit of the study is that participants were able to continue testing and receiving feedback on their ocular condition during times where hospital reviews were limited, the fear of attending a hospital setting where contracting the virus was high coupled with the free access to the device may have boosted compliance levels. Therefore, our weekly compliance finding of 75% may in fact be lower outside of a pandemic. Despite this, our compliance rates are similar to those reported by other studies [
14,
22], and
Figure 5 shows that our 6 month compliance rate (95%) was similar to that found outside of a pandemic [
11]. Another limitation of this study was the temporary closure of our research department due to the pandemic. This prevented us from recruiting more participants, therefore, limiting our ability to investigate change detection.
One important observation from our study is that even though our research office was closed with limited hospital appointments during the pandemic, participants already enrolled in the study were able to continue home monitor of their vision and submit test results to the study coordinator during periods of lockdown brought on by the pandemic. This demonstrates the value of VFTM as possible with the MRF and when applied to a chronic disease such as glaucoma. Of note for tele-ophthalmology, MRF software is now available as an online browser option (not evaluated in this study) allowing patients to do the testing at home on their own personal computer [
23].
To ensure success of VFTM, we advocate the need for a ‘coordinator’ to actively interact and oversee patient testing at home and to provide the learning and support needed by patients. This could be the same person who would otherwise undertake the visual field testing in the clinic. In this trial, the study coordinator was present at recruitment and performed the MRF training session, where a rapport was developed with the participant. The study coordinator also sent out personalised text messages to each participant the day a test was due and served as a port of call between clinical reviews. Patient feedback was that they liked this personalised attention. One positive outcome of our trial was that numerous participants reported they felt more confident in performing HFA examinations due to the familiarity that they gained on testing with the MRF at home.