Gastric Cancer Screening in Japan: A Narrative Review
Abstract
:1. Introduction
2. Gastric Cancer in Japan
2.1. Epidemiology of Gastric Cancer
2.2. H. pylori and Gastric Cancer
3. Gastric Cancer Screening Methods Used in Japan
3.1. Current Status and Problems of Upper Gastrointestinal Series
3.2. Current Status and Problems of Upper Gastrointestinal Endoscopy
4. Risk Stratification for Gastric Cancer Screening
4.1. Risk Factors for Gastric Cancer
4.2. Tests Used for Risk Stratification
4.3. Gastric Cancer Screening Tests Performed at Hoki-cho, Tottori Prefecture
5. Future Directions for Gastric Cancer Screening
5.1. Optimal Age and Intervals for Screening
5.2. AI as a New Screening Method
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Schistosomes, liver flukes and Helicobacter pylori. International Agency for Research on Cancer monographs on the evaluation of carcinogenesis risks to humans. IARC Monogr. Eval. Carcinog. Risks Hum. 1994, 61, 208–220.
- Uemura, N.; Okamoto, S.; Yamamoto, S.; Matsumura, N.; Yamaguchi, S.; Yamakido, M.; Taniyama, K.; Sasaki, N.; Schlemper, R.J. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 2001, 345, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Katanoda, K.; Hori, M.; Saito, E.; Shibata, A.; Ito, Y.; Minami, T.; Ikeda, S.; Suzuki, T.; Matsuda, T. Updated trends in cancer in Japan: Incidence in 1985–2015 and mortality in 1958–2018—A sign of decrease in cancer incidence. J. Epidemiol. 2021, 31, 426–450. [Google Scholar] [CrossRef]
- Kobayashi, T.; Kikuchi, S.; Lin, Y.; Yagyu, K.; Obata, Y.; Ogihara, A.; Hasegawa, A.; Miki, K.; Kaneko, E.; Mizukoshi, H.; et al. Trends in the incidence of gastric cancer in Japan and their associations with Helicobacter pylori infection and gastric mucosal atrophy. Gastric Cancer 2004, 7, 233–239. [Google Scholar] [CrossRef] [Green Version]
- Ueda, J.; Gosho, M.; Inui, Y.; Matsuda, T.; Sakakibara, M.; Mabe, K.; Nakajima, S.; Shimoyama, T.; Yasuda, M.; Kawai, T.; et al. Prevalence of Helicobacter pylori infection by birth year and geographic area in Japan. Helicobacter 2014, 19, 105–110. [Google Scholar] [CrossRef]
- Kamada, T.; Haruma, K.; Ito, M.; Inoue, K.; Manabe, N.; Matsumoto, H.; Kusunoki, H.; Hata, J.; Yoshihara, M.; Sumii, K.; et al. Time trends in Helicobacter pylori infection and atrophic gastritis over 40 years in Japan. Helicobacter 2015, 20, 192–198. [Google Scholar] [CrossRef]
- Sugano, K. Screening of gastric cancer in Asia. Best Pract. Res. Clin. Gastroenterol. 2015, 29, 895–905. [Google Scholar] [CrossRef]
- Wang, C.; Nishiyama, T.; Kikuchi, S.; Inoue, M.; Sawada, N.; Tsugane, S.; Lin, Y. Changing trends in the prevalence of H. pylori infection in Japan (1908–2003): A systematic review and meta-regression analysis of 170,752 individuals. Sci. Rep. 2017, 7, 15491. [Google Scholar] [CrossRef]
- Lin, Y.; Kawai, S.; Sasakabe, T.; Nagata, C.; Naito, M.; Tanaka, K.; Sugawara, Y.; Mizoue, T.; Sawada, N.; Matsuo, K. Effects of Helicobacter pylori eradication on gastric cancer incidence in the Japanese population: A systematic evidence review. Jpn. J. Clin. Oncol. 2021, 51, 1158–1170. [Google Scholar] [CrossRef]
- National Cancer Center. Center for Cancer Control and Information Services. 2021. Available online: https://ganjoho.jp/public/index.html (accessed on 1 January 2022).
- Oshima, A. A critical review of cancer screening programs in Japan. Int. J. Technol. Assess. Health Care 1994, 10, 346–358. [Google Scholar] [CrossRef]
- Hamashima, C. Systematic review group and guideline development group for gastric cancer screening guidelines. Update version of the Japanese Guidelines for Gastric Cancer Screening. Jpn. J. Clin. Oncol. 2018, 48, 673–683. [Google Scholar] [CrossRef] [Green Version]
- Hamashima, C. Cancer screening guidelines and policy making: 15 years of experience in cancer screening guideline development in Japan. Jpn. J. Clin. Oncol. 2018, 48, 278–286. [Google Scholar] [CrossRef]
- Hamashima, C.; Goto, R. Potential capacity of endoscopic screening for gastric cancer in Japan. Cancer Sci. 2017, 108, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Hamashima, C. Overdiagnosis of gastric cancer by endoscopic screening. World J. Gastrointest. Endosc. 2017, 9, 55–60. [Google Scholar] [CrossRef]
- Hamashima, C.; Yoshimura, K.; Fukao, A. A study protocol for expanding the screening interval of endoscopic screening for gastric cancer based on individual risks: Prospective cohort study of gastric cancer screening. Ann. Transl. Med. 2020, 8, 1604. [Google Scholar] [CrossRef]
- Mabe, K.; Inoue, K.; Kamada, T.; Kato, K.; Kato, M.; Haruma, K. Endoscopic screening for gastric cancer in Japan: Current status and future perspectives. Dig. Endosc. 2022, 34, 412–419. [Google Scholar] [CrossRef]
- Matsuo, T.; Ito, M.; Takata, S.; Tanaka, S.; Yoshihara, M.; Chayama, K. Low prevalence of Helicobacter pylori-negative gastric cancer among Japanese. Helicobacter 2011, 16, 415–419. [Google Scholar] [CrossRef]
- Ono, S.; Kato, M.; Suzuki, M.; Ishigaki, S.; Takahashi, M.; Haneda, M.; Mabe, K.; Shimizu, Y. Frequency of Helicobacter pylori-negative gastric cancer and gastric mucosal atrophy in a Japanese endoscopic submucosal dissection series including histological, endoscopic and serological atrophy. Digestion 2012, 86, 59–65. [Google Scholar] [CrossRef]
- Mizota, Y.; Yamamoto, S. How long should we continue gastric cancer screening? From an epidemiological point of view. Gastric Cancer 2019, 22, 456–462. [Google Scholar] [CrossRef] [Green Version]
- Kishikawa, H. The clinical benefits, limitations, and perspectives of the ABC method. Intern. Med. 2020, 59, 1471–1472. [Google Scholar] [CrossRef] [Green Version]
- Yao, K.; Uedo, T.; Kamada, T.; Hirasawa, T.; Nagahama, T.; Yoshinaga, S.; Oka, M.; Inoue, K.; Mabe, K.; Yao, T.; et al. Guidelines for endoscopic diagnosis of early gastric cancer. Dig. Endosc. 2020, 32, 663–698. [Google Scholar] [CrossRef]
- Hirasawa, T.; Aoyama, K.; Tanimoto, T.; Ishihara, S.; Shichijo, S.; Ozawa, T.; Ohnishi, T.; Fujishiro, M.; Matsuo, K.; Fujisaki, J.; et al. Application of artificial intelligence using a convolutional neural network for detecting gastric cancer in endoscopic images. Gastric Cancer 2018, 21, 653–660. [Google Scholar] [CrossRef] [Green Version]
- Ishioka, M.; Hirasawa, T.; Tada, T. Detecting gastric cancer from video images using convolutional neural networks. Dig. Endosc. 2019, 31, e34–e35. [Google Scholar] [CrossRef] [Green Version]
- Ikenoyama, Y.; Hirasawa, T.; Ishioka, M.; Namikawa, K.; Yoshimizu, S.; Horiuchi, Y.; Ishiyama, A.; Yoshio, T.; Tsuchida, T.; Takeuchi, Y.; et al. Detecting early gastric cancer: Comparison between the diagnostic ability of convolutional neural networks and endoscopists. Dig. Endosc. 2021, 33, 141–150. [Google Scholar] [CrossRef]
- Park, J.Y.; Greenberg, E.R.; Parsonnnet, J.; Wild, C.P.; Forman, D.; Herrero, R. Summary of IARC Working Group Meeting on Helicobacter pylori Eradication as a Strategy for Preventing Gastric Cancer; IARC Working Group Report 8; International Agency for Research on Cancer: Lyon, France, 2014; pp. 1–4. [Google Scholar]
- Greenberg, E.R.; Park, J.Y. Effectiveness of Helicobacter pylori eradication. In Helicobacter pylori Eradication as a Strategy for Preventing Gastric Cancer; IARC Working Group Report 8; International Agency for Research on Cancer: Lyon, France, 2014; pp. 64–71. [Google Scholar]
- Li, W.Q.; Ma, J.L.; Zhang, L.; Brown, L.M.; Li, J.Y.; Shen, L.; Pan, K.F.; Liu, W.D.; Hu, Y.; Han, Z.X.; et al. Effects of Helicobacter pylori treatment on gastric cancer incidence and mortality in subgroups. J. Natl. Cancer Inst. 2014, 106, dju116. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.C.; Chiang, T.H.; Chou, C.K.; Tu, Y.K.; Liao, W.C.; Wu, M.S.; Graham, D.Y. Association between Helicobacter pylori eradication and gastric cancer incidence: A systematic review and meta-analysis. Gastroenterology 2016, 150, 1113–1124.e5. [Google Scholar] [CrossRef] [Green Version]
- Sugano, K. Effect of helicobacter pylori eradication on the incidence of gastric cancer: A systematic review and meta-analysis. Gastric Cancer 2019, 22, 435–445. [Google Scholar] [CrossRef] [Green Version]
- Ford, A.C.; Yuan, Y.; Moayyedi, P. Helicobacter pylori eradication therapy to prevent gastric cancer: Systematic review and metaanalysis. Gut 2020, 69, 2113–2121. [Google Scholar] [CrossRef]
- Fukase, K.; Kato, M.; Kikuchi, S.; Inoue, K.; Uemura, N.; Okamoto, S.; Terao, S.; Amagai, K.; Hayashi, S.; Asaka, M.; et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: An openlabel, randomised controlled trial. Lancet 2008, 372, 392–397. [Google Scholar] [CrossRef] [Green Version]
- Li, W.Q.; Zhang, J.Y.; Ma, J.L.; Li, Z.X.; Zhang, L.; Zhang, Y.; Guo, Y.; Zhou, T.; Li, J.Y.; Shen, L.; et al. Effects of helicobacter pylori treatment and vitamin and garlic supplementation on gastric cancer incidence and mortality: Follow-up of a randomized intervention trial. BMJ 2019, 366, l5016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, I.J.; Kim, C.G.; Lee, J.Y.; Kim, Y.I.; Kook, M.C.; Park, B.; Joo, J. Family History of Gastric Cancer and Helicobacter pylori Treatment. N. Engl. J. Med. 2020, 382, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Chiang, T.; Chang, W.; Chen, S.L.; Yen, A.M.; Fann, J.C.; Chiu, S.Y.; Chen, Y.R.; Chuang, S.L.; Shieh, C.F.; Liu, C.Y.; et al. Mass eradication of Helicobacter pylori to reduce gastric cancer incidence and mortality: A long-term cohort study on Matsu Islands. Gut 2021, 70, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Take, S.; Mizuno, M.; Ishiki, K.; Kusumoto, C.; Imada, T.; Hamada, F.; Yoshida, T.; Yokota, K.; Mitsuhashi, T.; Okada, H. Risk of gastric cancer in the second decade of follow-up after helicobacter pylori eradication. J. Gastroenterol. 2020, 55, 281–288. [Google Scholar] [CrossRef] [Green Version]
- Satomi, S.; Yamakawa, A.; Matsunaga, S.; Masaki, R.; Inagaki, T.; Okuda, T.; Suto, H.; Ito, Y.; Yamazaki, Y.; Kuriyama, M.; et al. Relationship between the diversity of the cagA gene of Helicobacter pylori and gastric cancer in Okinawa, Japan. J. Gastroenterol. 2006, 41, 668–673. [Google Scholar] [CrossRef]
- Yamaoka, Y.; Kato, M.; Asaka, M. Geographic differences in gastric cancer incidence can be explained by differences between Helicobacter pylori strains. Intern. Med. 2008, 47, 1077–1083. [Google Scholar] [CrossRef] [Green Version]
- Kpoghomou, M.A.; Wang, J.; Wang, T.; Jin, G. Association of Helicobacter pylori babA2 gene and gastric cancer risk: A meta-analysis. BMC Cancer 2020, 20, 465. [Google Scholar] [CrossRef]
- Asaka, M.; Mabe, K. Strategies for eliminating death from gastric cancer in Japan. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2014, 90, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Kawai, S.; Wang, C.; Lin, Y.; Sasakabe, T.; Okuda, M.; Kikuchi, S. Lifetime incidence risk for gastric cancer in the Helicobacter pylori-infected and uninfected population in Japan: A Monte Carlo simulation study. Int. J. Cancer 2022, 150, 18–27. [Google Scholar] [CrossRef]
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma-3rd English edition. Gastric Cancer 2011, 14, 101–112. [Google Scholar] [CrossRef] [Green Version]
- Schlemper, R.J.; Riddell, R.H.; Kato, Y.; Borchard, F.; Cooper, H.S.; Dawsey, S.M.; Dixon, M.F.; Fenoglio-Preiser, C.M.; Fléjou, J.F.; Geboes, K.; et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut 2000, 47, 251–255. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, Y.; Fujisaki, J.; Omae, M.; Hirasawa, T.; Igarashi, M. Helicobacter pylori-negative gastric cancer: Characteristics and endoscopic findings. Dig. Endosc. 2015, 27, 551–561. [Google Scholar] [CrossRef]
- Imamura, Y.; Watanabe, M.; Oki, E.; Morita, M.; Baba, H. Esophagogastric junction adenocarcinoma shares characteristics with gastric adenocarcinoma: Literature review and retrospective multicenter cohort study. Ann. Gastroenterol. Surg. 2020, 5, 46–59. [Google Scholar] [CrossRef]
- Crew, K.D.; Neugut, A.I. Epidemiology of gastric cancer. World J. Gastroenterol. 2006, 12, 354–362. [Google Scholar] [CrossRef]
- Ohshima, A.; Hirata, N.; Ubukata, T.; Umeda, K.; Fujimoto, I. Evaluation of a mass screening program for stomach with a case control study design. Int. J. Cancer 1986, 38, 829–833. [Google Scholar] [CrossRef]
- Fukao, A.; Tsubono, Y.; Tsuji, I.; Hisamichi, S.; Sugahara, N.; Takano, A. The evaluation of screening for gastric cancer in Miyagi Prefecture, Japan: A population-based case-control study. Int. J. Cancer 1995, 60, 45–48. [Google Scholar] [CrossRef]
- Hamashima, C.; Shabana, M.; Okada, K.; Okamoto, M.; Osaki, Y. Mortality reduction from gastric cancer by endoscopic and radiographic screening. Cancer Sci. 2015, 106, 1744–1749. [Google Scholar] [CrossRef] [Green Version]
- Jun, J.K.; Choi, K.S.; Lee, H.Y.; Suh, M.; Park, B.; Song, S.H.; Jung, K.W.; Lee, C.W.; Choi, I.J.; Park, E.C.; et al. Effectiveness of the Korean National Cancer Screening Program in Reducing Gastric Cancer Mortality. Gastroenterology 2017, 152, 1319–1328.e7. [Google Scholar] [CrossRef]
- The Japanese Society of Gastrointestinal Cancer Screening. New Guidelines of Radiography for Gastric Cancer Screening. 2011. Available online: https://www.jsgcs.or.jp (accessed on 14 December 2020).
- The Japanese Society of Gastrointestinal Cancer Screening. Annual Report of Gastrointestinal Cancer Screening 2014. Available online: https://www.jsgcs.or.jp (accessed on 14 December 2020).
- Togo, R.; Yamamichi, N.; Mabe, K.; Takahashi, Y.; Takeuchi, C.; Kato, M.; Sakamoto, N.; Ishihara, K.; Ogawa, T.; Haseyama, M. Detection of gastritis by a deep convolutional neural network from double-contrast upper gastrointestinal barium x-ray radiography. J. Gastroenterol. 2019, 54, 321–329. [Google Scholar] [CrossRef] [Green Version]
- Choi, K.S.; Jun, J.K.; Suh, M.; Park, B.; Noh, D.K.; Song, S.H.; Jung, K.W.; Lee, H.Y.; Choi, I.J.; Park, E.C. Effect of endoscopy screening on stage at gastric cancer diagnosis: Results of the National Cancer Screening Programme in Korea. Br. J. Cancer 2015, 112, 608–612. [Google Scholar] [CrossRef] [Green Version]
- Choi, K.S.; Jun, J.K.; Park, E.C.; Park, S.; Jung, K.W.; Han, M.A.; Choi, I.J.; Lee, H.Y. Performance of different gastric cancer screening methods in Korea: A population-based study. PLoS ONE 2012, 7, e50041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, S.; Yoshida, Y. Efficacy of endoscopic screening in an isolated island: A case-control study. Indian J. Gastroenterol. 2014, 33, 46–49. [Google Scholar] [CrossRef]
- Hamashima, C.; Ogoshi, K.; Okamoto, M.; Shabana, M.; Kishimoto, T.; Fukao, A. A community-based, case-control study evaluating mortality reduction from gastric cancer by endoscopic screening in Japan. PLoS ONE 2013, 8, e79088. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Li, M.; Chen, S.; Hu, J.; Guo, Q.; Liu, R.; Zheng, H.; Jin, Z.; Yuan, Y.; Xi, Y.; et al. Endoscopic Screening in Asian Countries Is Associated With Reduced Gastric Cancer Mortality: A Meta-analysis and Systematic Review. Gastroenterology 2018, 155, 347–354.e9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Japanese Society of Gastrointestinal Cancer Screening. Quality Assurance Manual of Endoscopic Screening for Gastric Cancer in Japanese Communities. Jpn. J. Clin. Oncol. 2016, 46, 1053–1061. (In Japanese) [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosokawa, O.; Shinbo, T.; Matsuda, K.; Miyanaga, T. Impact of opportunistic endoscopic screening on the decrease of mortality from gastric cancer. J. Gatsroenterol. Cancer Screen 2011, 49, 401–409. (In Japanese) [Google Scholar]
- Ogoshi, K.; Narisawa, R.; Kato, T.; Saito, S.; Funagoshi, K.; Kinameri, K. Evaluation of endoscopic screening for gastric cancer in Niigata City: The reduction of the mortality rate. J. Gatsroenterol. Cancer Screen 2009, 47, 531–541. (In Japanese) [Google Scholar]
- Shichijo, S.; Uedo, N.; Michida, T. Detection of Early Gastric Cancer after Helicobacter pylori Eradication. Digestion 2021, 2, 54–61. [Google Scholar] [CrossRef]
- Kurumi, H.; Kanda, T.; Ikebuchi, Y.; Yoshida, A.; Kawaguchi, K.; Yashima, K.; Isomoto, H. Current Status of Photodynamic Diagnosis for Gastric Tumors. Diagnostics 2021, 11, 1967. [Google Scholar] [CrossRef]
- Ministry of Health, Labour and Welfare. The Report of Health Promotion and Community Health 2013. Available online: http://www.e-stat.go.jp/SG1/estat/GL08020101.do?_-toGL08020101_&tstatCode=000001030884&requestSender=dsearch (accessed on 1 September 2015).
- Shabana, M.; Hamashima, C.; Nishida, M.; Miura, K.; Kishimoto, T. Current status and evaluation of endoscopic screening for gastric cancer. Jpn. J. Cancer Det. Diagn. 2010, 17, 229–235. (In Japanese) [Google Scholar]
- Kitagawa, S.; Miyagawa, K.; Iriguchi, Y. The report of gastroenterological screening in 2012. J. Gastroenterol. Cancer Screen 2015, 53, 60–86. (In Japanese) [Google Scholar]
- Suzuki, A.; Katoh, H.; Komura, D.; Kakiuchi, M.; Tagashira, A.; Yamamoto, S.; Tatsuno, K.; Ueda, H.; Nagae, G.; Fukuda, S.; et al. Defined lifestyle and germline factors predispose Asian populations to gastric cancer. Sci. Adv. 2020, 6, eaav9778. [Google Scholar] [CrossRef]
- Sugano, K.; Tack, J.; Kuipers, E.J.; Graham, D.Y.; El-Omar, E.M.; Miura, S.; Haruma, K.; Asaka, M.; Uemura, N.; Malfertheiner, P. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015, 64, 1353–1367. [Google Scholar] [CrossRef] [Green Version]
- Haruma, K.; Kato, M.; Inoue, K.; Murakami, K.; Kamada, T. Kyoto Classification of Gastritis; Nihon Medical Center: Tokyo, Japan, 2017. [Google Scholar]
- Yoshii, S.; Mabe, K.; Watano, K.; Ohno, M.; Matsumoto, M.; Ono, S.; Kudo, T.; Nojima, M.; Kato, M.; Sakamoto, N. Validity of endoscopic features for the diagnosis of helicobacter pylori infection status based on the Kyoto classification of gastritis. Dig. Endosc. 2020, 32, 74–83. [Google Scholar] [CrossRef] [Green Version]
- Isomoto, H.; Mizuta, Y.; Inoue, K.; Matsuo, T.; Hayakawa, T.; Miyazaki, M.; Onita, K.; Takeshima, F.; Murase, K.; Shimokawa, I.; et al. A close relationship between Helicobacter pylori infection and gastric xanthoma. Scand J. Gastroenterol. 1999, 34, 346–352. [Google Scholar] [CrossRef]
- Sekikawa, A.; Fukui, H.; Sada, R.; Fukuhara, M.; Marui, S.; Tanke, G.; Endo, M.; Ohara, Y.; Matsuda, F.; Nakajima, J.; et al. Gastric atrophy and xanthelasma are markers for predicting the development of early gastric cancer. J. Gastroenterol. 2016, 51, 35–42. [Google Scholar] [CrossRef]
- Hirai, R.; Hirai, M.; Shimodate, Y.; Minami, M.; Ishikawa, S.; Kanadani, T.; Takezawa, R.; Doi, A.; Nishimura, N.; Mouri, H.; et al. Feasibility of endoscopic evaluation of Helicobacter pylori infection status by using the Kyoto classification of gastritis in the population-based gastric cancer screening program: A prospective cohort study. Health Sci. Rep. 2021, 4, e325. [Google Scholar] [CrossRef]
- Overview of the 2019 Basic Survey on National Life. Available online: https://www.mhlw.go.jp/toukei/saikin/hw/k-tyosa/k-tyosa19/index.html (accessed on 27 June 2022).
- Miki, K. Gastric cancer screening by combined assay for serum anti-Helicobacter pylori IgG antibody and serum pepsinogen levels “ABC method”. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2011, 87, 405–414. [Google Scholar] [CrossRef] [Green Version]
- Itoh, T.; Saito, M.; Marugami, N.; Hirai, T.; Marugami, A.; Takahama, J.; Tanaka, T.; Kichikawa, K. Correlation between the ABC classification and radiological findings for assessing gastric cancer risk. Jpn. J. Radiol. 2015, 33, 636–644. [Google Scholar] [CrossRef]
- Terasawa, T.; Nishida, H.; Kato, K.; Miyashiro, I.; Yoshikawa, T.; Takaku, R.; Hamashima, C. Prediction of gastric cancer development by serum pepsinogen test and Helicobacter pylori seropositivity in Eastern Asians: A systematic review and meta-analysis. PLoS ONE 2014, 9, e109783. [Google Scholar] [CrossRef]
- Kiso, M.; Yoshihara, M.; Ito, M.; Inoue, K.; Kato, K.; Nakajima, S.; Mabe, K.; Kobayashi, M.; Uemura, N.; Yada, T.; et al. Characteristics of gastric cancer in negative test of serum anti-Helicobacter pylori antibody and pepsinogen test: A multicenter study. Gastric Cancer 2017, 20, 764–771. [Google Scholar] [CrossRef] [Green Version]
- Kishino, T.; Oyama, T.; Tomori, A.; Takahashi, A.; Shinohara, T. Usefulness and limitations of a serum screening system to predict the risk of gastric cancer. Intern. Med. 2020, 59, 1473–1480. [Google Scholar] [CrossRef] [Green Version]
- Masuyama, H.; Yoshitake, N.; Sasai, T.; Nakamura, T.; Masuyama, A.; Zuiki, T.; Kurashina, K.; Mieda, M.; Sunada, K.; Yamamoto, H.; et al. Relationship between the degree of endoscopic atrophy of the gastric mucosa and carcinogenic risk. Digestion 2015, 91, 30–36. [Google Scholar] [CrossRef] [Green Version]
- Spence, A.D.; Cardwell, C.R.; McMenamin, U.C.; Hicks, B.M.; Johnston, B.T.; Murray, L.J.; Coleman, H.G. Adenocarcinoma risk in gastric atrophy and intestinal metaplasia: A systematic review. BMC Gastroenterol. 2017, 17, 157. [Google Scholar] [CrossRef] [Green Version]
- Kotachi, T.; Ito, M.; Yoshihara, M.; Boda, T.; Kiso, M.; Masuda, K.; Matsuo, T.; Tanaka, S.; Chayama, K. Serological evaluation of gastric cancer risk based on pepsinogen and helicobacter pylori antibody: Relationship to endoscopic findings. Digestion 2017, 95, 314–318. [Google Scholar] [CrossRef]
- Kaji, K.; Hashiba, A.; Uotani, C.; Yamaguchi, Y.; Ueno, T.; Ohno, K.; Takabatake, I.; Wakabayashi, T.; Doyama, H.; Ninomiya, I.; et al. Grading of atrophic gastritis is useful for risk stratification in endoscopic screening for gastric cancer. Am. J. Gastroenterol. 2019, 114, 71–79. [Google Scholar] [CrossRef]
- Kimura, K.; Takemoto, T. An endoscopic recognition for the atrophic border and its significance in chronic gastritis. Endoscopy 1969, 1, 87–97. [Google Scholar] [CrossRef]
- Canakis, A.; Pani, E.; Saumoy, M.; Shah, S.C. Decision model analyses of upper endoscopy for gastric cancer screening and preneoplasia surveillance: A systematic review. Therap. Adv. Gastroenterol. 2020, 13, 1756284820941662. [Google Scholar] [CrossRef] [PubMed]
- Kowada, A. Endoscopy Is Cost-effective for gastric cancer screening after successful Helicobacter pylori eradication. Dig. Dis. Sci. 2021, 66, 4220–4226. [Google Scholar] [CrossRef] [PubMed]
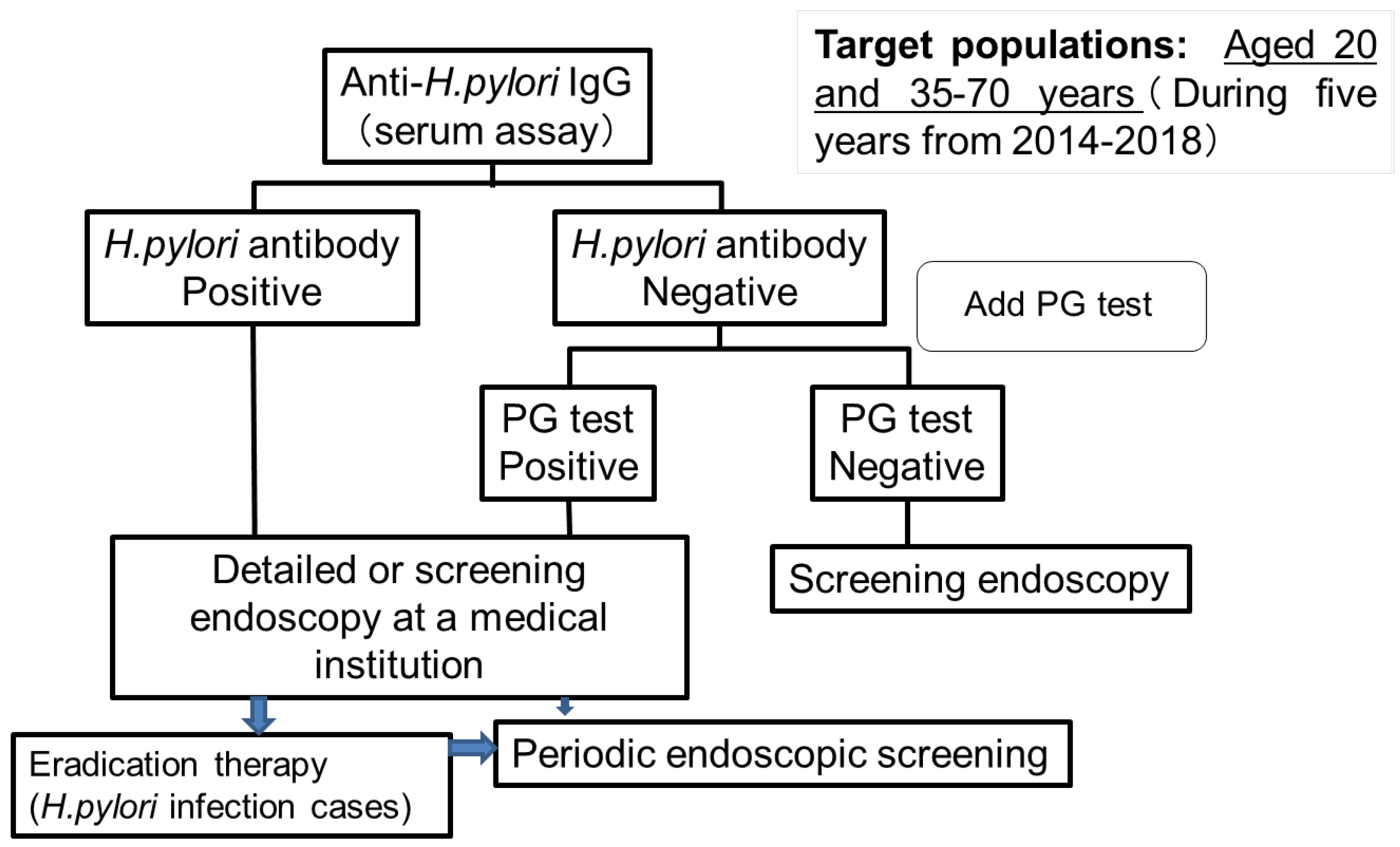
- Yashima, K.; Hasegawa, R.; Shabana, M.; Kawaguchi, G.; Isomoto, H. Mass screening considering Helicobacter pylori infection status for gastric cancer in Hoki-cho, Tottori prefecture. J. Gatsroenterol. Cancer Screen 2019, 57, 561–570. (In Japanese) [Google Scholar]
- Banks, M.; Graham, D.; Jansen, M.; Gotoda, T.; Coda, S.; di Pietro, M.; Uedo, N.; Bhandari, P.; Pritchard, D.M.; Kuipers, E.J.; et al. British Society of Gastroenterology guidelines on the diagnosis and management of patients at risk of gastric adenocarcinoma. Gut 2019, 68, 1545–1575. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.L.; Leung, C.Y.; Saito, E.; Katanoda, K.; Hur, C.; Kong, C.Y.; Nomura, S.; Shibuya, K. Effect and cost-effectiveness of national gastric cancer screening in Japan: A microsimulation modeling study. BMC Med. 2020, 18, 257. [Google Scholar] [CrossRef]
- Dan, Y.Y.; So, J.B.; Yeoh, K.G. Endoscopic screening for gastric cancer. Clin. Gastroenterol. Hepatol. 2006, 4, 709–716. [Google Scholar] [CrossRef]
- Suh, Y.S.; Lee, J.; Woo, H.; Shin, D.; Kong, S.H.; Lee, H.J.; Shin, A.; Yang, H.K. National cancer screening program for gastric cancer in Korea: Nationwide treatment benefit and cost. Cancer 2020, 126, 1929–1939. [Google Scholar] [CrossRef]
- Pimentel-Nunes, P.; Libânio, D.; Marcos-Pinto, R.; Areia, M.; Leja, M.; Esposito, G.; Garrido, M.; Kikuste, I.; Megraud, F.; Matysiak-Budnik, T.; et al. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota96. Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy 2019, 51, 365–388. [Google Scholar] [CrossRef] [Green Version]
- Hosokawa, O.; Tsuda, S.; Kidani, E.; Watanabe, K.; Tanigawa, Y.; Shirasaki, S.; Hayashi, H.; Hinoshita, T. Diagnosis of gastric cancer up to three years after negative upper gastrointestinal endoscopy. Endoscopy 1998, 30, 669–674. [Google Scholar] [CrossRef]
- Pimentaelo, A.R.; Monteirooares, M.; Libanio, D.; Dinisibeiro, M. Missing rate for gastric cancer during upper gastrointestinal endoscopy: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2016, 28, 1041–1049. [Google Scholar] [CrossRef]
- Shichijo, S.; Nomura, S.; Aoyama, K.; Nishikawa, Y.; Miura, M.; Shinagawa, T.; Takiyama, H.; Tanimoto, T.; Ishihara, S.; Matsuo, K.; et al. Application of Convolutional Neural Networks in the Diagnosis of Helicobacter pylori Infection Based on Endoscopic Images. EBioMedicine 2017, 25, 106–111. [Google Scholar] [CrossRef] [Green Version]
- Shichijo, S.; Endo, Y.; Aoyama, K.; Takeuchi, Y.; Ozawa, T.; Takiyama, H.; Matsuo, K.; Fujishiro, M.; Ishihara, S.; Ishihara, R.; et al. Application of convolutional neural networks for evaluating Helicobacter pylori infection status on the basis of endoscopic images. Scand J. Gastroenterol. 2019, 54, 158–163. [Google Scholar] [CrossRef]
- Oura, H.; Matsumura, T.; Fujie, M.; Ishikawa, T.; Nagashima, A.; Shiratori, W.; Tokunaga, M.; Kaneko, T.; Imai, Y.; Oike, T.; et al. Development and evaluation of a double-check support system using artificial intelligence in endoscopic screening for gastric cancer. Gastric Cancer 2022, 25, 392–400. [Google Scholar] [CrossRef]

| Year | 2014 | 2015 | 2016 | 2017, 2018 | Total |
|---|---|---|---|---|---|
| Examinees (n) | 910 | 776 | 311 | 467 | 2464 |
| Cases requiring detailed endoscopy (n) | 323 | 259 | 109 | 121 | 811 |
| Examination required rate (%) | 35.4 | 33.4 | 35.0 | 25.9 | 32.9 |
| Cases undergone screeningendoscopy (n) | 258 | 181 | 61 | 78 | 578 |
| Examination rate (%) | 79.9 | 69.9 | 56.0 | 64.5 | 71.3 |
| Year | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 |
|---|---|---|---|---|---|---|---|
| Target population (n) | 4533 | 4533 | 4533 | 4257 | 4257 | 4257 | 4257 |
| Examinees (n) | 934 | 963 | 1188 | 970 | 986 | 1036 | 1039 |
| Participation rate (%) | 20.6 | 21.2 | 26.2 | 22.8 | 23.2 | 24.3 | 24.4 |
| Proportion of endoscopy among gastric cancer screening tests (%) | 26.1 | 35.3 | 52.9 | 50.7 | 57.0 | 63.4 | 65.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yashima, K.; Shabana, M.; Kurumi, H.; Kawaguchi, K.; Isomoto, H. Gastric Cancer Screening in Japan: A Narrative Review. J. Clin. Med. 2022, 11, 4337. https://doi.org/10.3390/jcm11154337
Yashima K, Shabana M, Kurumi H, Kawaguchi K, Isomoto H. Gastric Cancer Screening in Japan: A Narrative Review. Journal of Clinical Medicine. 2022; 11(15):4337. https://doi.org/10.3390/jcm11154337
Chicago/Turabian StyleYashima, Kazuo, Michiko Shabana, Hiroki Kurumi, Koichiro Kawaguchi, and Hajime Isomoto. 2022. "Gastric Cancer Screening in Japan: A Narrative Review" Journal of Clinical Medicine 11, no. 15: 4337. https://doi.org/10.3390/jcm11154337
APA StyleYashima, K., Shabana, M., Kurumi, H., Kawaguchi, K., & Isomoto, H. (2022). Gastric Cancer Screening in Japan: A Narrative Review. Journal of Clinical Medicine, 11(15), 4337. https://doi.org/10.3390/jcm11154337







