Potential of Multiplex Polymerase Chain Reaction Performed on Protected Telescope Catheter Samples for Early Adaptation of Antimicrobial Therapy in ARDS Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. VAP Definition
2.2. Culture
2.3. mPCR
2.4. Antibiotic
2.5. Statistical Analysis
3. Results
3.1. Patients
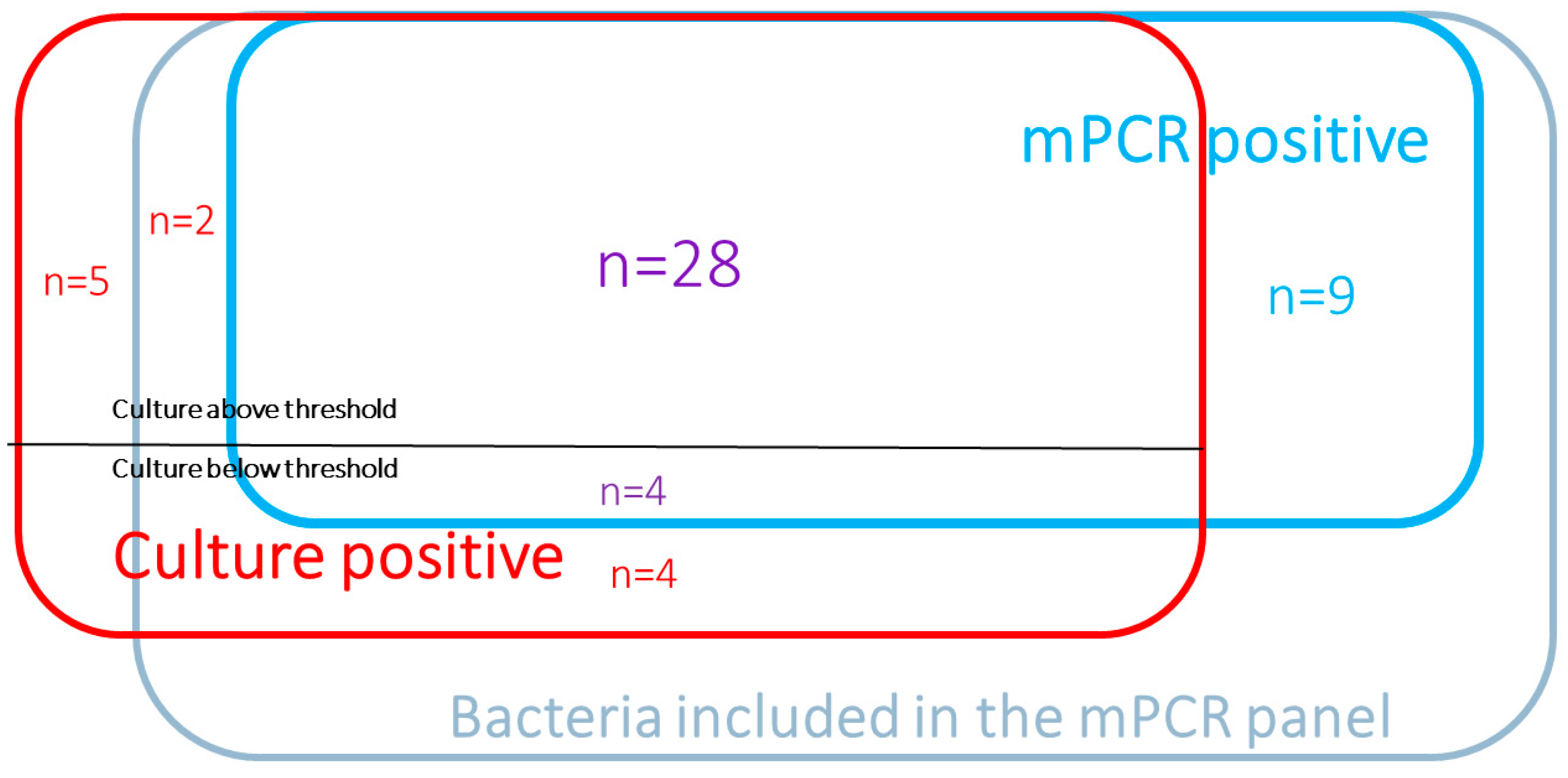
3.2. Culture and mPCR
3.3. Diagnostic Value of mPCR
3.4. Antibiotic Resistance and Therapy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Versporten, A.; Zarb, P.; Caniaux, I.; Gros, M.F.; Drapier, N.; Miller, M.; Jarlier, V.; Nathwani, D.; Goossens, H.; Koraqi, A.; et al. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: Results of an internet-based global point prevalence survey. Lancet Glob. Health 2018, 6, e619–e629. [Google Scholar] [CrossRef] [Green Version]
- Rouzé, A.; Martin-Loeches, I.; Povoa, P.; Makris, D.; Artigas, A.; Bouchereau, M.; Lambiotte, F.; Metzelard, M.; Cuchet, P.; Boulle Geronimi, C.; et al. Relationship between SARS-CoV-2 infection and the incidence of ventilator-associated lower respiratory tract infections: A European multicenter cohort study. Intensive Care Med. 2021, 47, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Luyt, C.-E.; Bouadma, L.; Morris, A.C.; Dhanani, J.A.; Kollef, M.; Lipman, J.; Martin-Loeches, I.; Nseir, S.; Ranzani, O.T.; Roquilly, A.; et al. Pulmonary infections complicating ARDS. Intensive Care Med. 2020, 46, 2168–2183. [Google Scholar] [CrossRef] [PubMed]
- Klompas, M. Does this patient have ventilator-associated pneumonia? JAMA 2007, 297, 1583–1593. [Google Scholar] [CrossRef] [PubMed]
- Barbier, F.; Bailly, S.; Schwebel, C.; Papazian, L.; Azoulay, É.; Kallel, H.; Siami, S.; Argaud, L.; Marcotte, G.; Misset, B.; et al. Infection-related ventilator-associated complications in ICU patients colonised with extended-spectrum β-lactamase-producing Enterobacteriaceae. Intensive Care Med. 2018, 44, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Vacheron, C.-H.; Lepape, A.; Savey, A.; Machut, A.; Timsit, J.F.; Vanhems, P.; Le, Q.V.; Egbeola, J.; Martin, M.; Maxime, V.; et al. Increased Incidence of Ventilator-Acquired Pneumonia in Coronavirus Disease 2019 Patients: A Multicentric Cohort Study. Crit. Care Med. Publish Ahead of Print. Available online: https://journals.lww.com/10.1097/CCM.0000000000005297(accessed on 14 March 2022).
- Koulenti, D.; Tsigou, E.; Rello, J. Nosocomial pneumonia in 27 ICUs in Europe: Perspectives from the EU-VAP/CAP study. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2017, 36, 1999–2006. [Google Scholar] [CrossRef] [PubMed]
- Covid-19 Fiches et Documents SFM. Société Fr. Microbiol. Available online: https://www.sfm-microbiologie.org/covid-19-fiches-et-documents-sfm/ (accessed on 12 May 2022).
- Papazian, L.; Klompas, M.; Luyt, C.-E. Ventilator-associated pneumonia in adults: A narrative review. Intensive Care Med. 2020, 46, 888–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brun-Buisson, C.; Fartoukh, M.; Lechapt, E.; Honoré, S.; Zahar, J.-R.; Cerf, C.; Maitre, B. Contribution of blinded, protected quantitative specimens to the diagnostic and therapeutic management of ventilator-associated pneumonia. Chest 2005, 128, 533–544. [Google Scholar] [CrossRef] [Green Version]
- Poritz, M.A.; Blaschke, A.J.; Byington, C.L.; Meyers, L.; Nilsson, K.; Jones, D.E.; Thatcher, S.A.; Robbins, T.; Lingenfelter, B.; Amiott, E.; et al. FilmArray, an automated nested multiplex PCR system for multi-pathogen detection: Development and application to respiratory tract infection. PLoS ONE. 2011, 6, e26047. [Google Scholar] [CrossRef]
- Weiss, E.; Zahar, J.-R.; Lesprit, P.; Ruppe, E.; Leone, M.; Chastre, J.; Lucet, J.C.; Paugam-Burtz, C.; Brun-Buisson, C.; Timsit, J.F.; et al. Elaboration of a consensual definition of de-escalation allowing a ranking of β-lactams. Clin. Microbiol. Infect. 2015, 21, 649.e1–649.e10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Wreede, L.C.; Fiocco, M.; Putter, H. The mstate package for estimation and prediction in non- and semi-parametric multi-state and competing risks models. Comput. Methods Programs Biomed. 2010, 99, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Gastli, N.; Loubinoux, J.; Daragon, M.; Lavigne, J.-P.; Saint-Sardos, P.; Pailhoriès, H.; Lemarié, C.; Benmansour, H.; d’Humières, C.; Broutin, L.; et al. Multicentric evaluation of BioFire FilmArray Pneumonia Panel for rapid bacteriological documentation of pneumonia. Clin. Microbiol. Infect. 2020, 27, 1308–1314. [Google Scholar] [CrossRef]
- Enne, V.I.; Aydin, A.; Baldan, R.; Owen, D.R.; Richardson, H.; Ricciardi, F.; Russell, C.; Nomamiukor-Ikeji, B.O.; Swart, A.M.; High, J.; et al. Multicentre evaluation of two multiplex PCR platforms for the rapid microbiological investigation of nosocomial pneumonia in UK ICUs: The INHALE WP1 study. Thorax 2022. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef] [PubMed]
- Le Terrier, C.; Vinetti, M.; Bonjean, P.; Richard, R.; Jarrige, B.; Pons, B.; Madeux, B.; Piednoir, P.; Ardisson, F.; Elie, E.; et al. Impact of a restrictive antibiotic policy on the acquisition of extended-spectrum beta-lactamase-producing Enterobacteriaceae in an endemic region: A before-and-after, propensity-matched cohort study in a Caribbean intensive care unit. Crit. Care 2021, 25, 261. [Google Scholar] [CrossRef] [PubMed]
- Maataoui, N.; Chemali, L.; Patrier, J.; Tran Dinh, A.; Le Fèvre, L.; Lortat-Jacob, B.; Marzouk, M.; d’Humières, C.; Rondinaud, E.; Ruppé, E.; et al. Impact of rapid multiplex PCR on management of antibiotic therapy in COVID-19-positive patients hospitalized in intensive care unit. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2021, 40, 2227–2234. [Google Scholar] [CrossRef] [PubMed]
- Rouze, A.; Martin-Loeches, I.; Povoa, P.; Metzelard, M.; Du Cheyron, D.; Lambiotte, F.; Tamion, F.; Labruyere, M.; Boulle Geronimi, C.; Nieszkowska, A.; et al. Early Bacterial Identification Among Intubated Patients with COVID-19 or Influenza Pneumonia: A European Multicenter Comparative Cohort Study. Am. J. Respir. Crit. Care Med. 2021, 204, 546–556. [Google Scholar] [CrossRef] [PubMed]
- COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: A prospective cohort study. Intensive Care Med. 2021, 47, 60–73. [Google Scholar] [CrossRef] [PubMed]
| Clinical Characteristics and Comorbidities | Patients n = 95 |
|---|---|
| Age, years, median [IQR] | 60 [52–71] |
| Male gender, n (%) | 79 (80%) |
| SAPS II at ICU admission, median [IQR] | 38 [30–50] |
| Charlson Comorbidity index, median [IQR] | 3 [2–5] |
| Diabetes mellitus, n (%) | 40 (40%) |
| Congestive heart failure (NYHA 3–4), n (%) | 6 (6%) |
| COPD, n (%) | 9 (9%) |
| Immunosuppression condition, n (%) | 21 (22%) |
| Organ failures and outcome | |
| ARDS | 95 (100%) |
| Extracorporeal membrane oxygenation | 28 (29%) |
| Dialysis | 42 (44%) |
| White blood cell count (×109/L) | 11.4 [8.7–15.9] |
| C-Reactive Protein, mg/L | 143 [91–216] |
| Procalcitonin, µg/L | 1.0 [0.3–4.8] |
| Death in ICU | 42 (44%) |
| Bacterial Target | No. of Specimens | mPCR Performance | Cohen’s Kappa Coefficient | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Culture+/FA-PP+ | Culture+/FA-PP− | Culture−/FA-PP+ | Culture−/FA-PP− | Se (95% CI), % | Sp (95% CI), % | PPV (95% CI), % | NPV (95% CI), % | ||
| Acineterobacter calcoaceticus-baumannii complex | 0 | 0 | 0 | 125 | NA | 100 | NA | 100 | |
| Enterobacter cloacae complex | 2 | 0 | 1 | 122 | 100 | 99 | 67 | 100 | |
| Escherichiacoli | 2 | 0 | 2 | 121 | 100 | 98 | 50 | 100 | |
| Haemophilus influenzae | 0 | 0 | 1 | 124 | NA | 99 | 0 | 100 | |
| Klebsiella aerogenes | 2 | 1 | 1 | 121 | 67 | 99 | 99 | 98 | |
| Klebsiellaoxytoca | 0 | 0 | 0 | 125 | NA | 100 | NA | 100 | |
| Klebsiellapneumoniae group | 0 | 0 | 0 | 125 | NA | 100 | NA | 100 | |
| Moraxellacatarrhalis | 1 | 0 | 0 | 124 | 100 | 100 | 100 | 100 | |
| Proteusspp. | 2 | 0 | 0 | 123 | 100 | 100 | 100 | 100 | |
| Pseudomonasaeruginosa | 11 | 0 | 1 | 113 | 100 | 99 | 92 | 99 | |
| Serratia marcescens | 1 | 0 | 0 | 124 | 100 | 100 | 100 | 100 | |
| Streptococcuspneumoniae | 1 | 0 | 1 | 123 | 100 | 99 | 50 | 100 | |
| Staphylococcus aureus | 5 | 1 | 3 | 116 | 83 | 97 | 63 | 99 | |
| Streptococcuspyogenes | 0 | 0 | 0 | 125 | NA | 100 | NA | 100 | |
| Streptococcusagalactiae | 0 | 0 | 2 | 123 | NA | 98 | 0 | 100 | |
| Legionella pneumophila | 1 | 0 | 1 | 123 | 100 | 99 | 50 | 100 | |
| TOTAL | 28 | 2 | 13 | 1957 | 93 [84–100] | 99 [99–100] | 68 [54–83] | 100 [100] | 0.8 [0.68–0.89] |
| Suspected CAP/HAP Cases (n= 48) | Suspected VAP Cases (n= 77) | ||||
|---|---|---|---|---|---|
| mPCR − (n = 45) | mPCR + (n = 3) | mPCR − (n = 49) | mPCR + (n = 28) | ||
| Antibiotic modification after mPCR | 1 | 3 | 2 | 12 | |
| 1 | 3 | 2 | 1 | |
| Narrower spectrum antibiotic | 0 | 3 | 1 | 1 | |
| Stop antibiotic | 1 | 0 | 1 | 0 | |
| 0 | 11 | |||
| Escalation/Adaptation | 0 | 4 | |||
| Escalation usefulness | 0 | 2 | |||
| Initiation | 0 | 5 | |||
| No change after mPCR results | 44 | 0 | 47 | 16 | |
| 15 | 0 | 20 | 14 | |
| Continuation of antibiotic initiated before suspecting pneumonia * | 27 | 0 | 19 | 2 |
| No antibiotic initiation | 2 | 0 | 8 | 0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razazi, K.; Delamaire, F.; Fihman, V.; Boujelben, M.A.; Mongardon, N.; Gendreau, S.; de Roux, Q.; de Prost, N.; Carteaux, G.; Woerther, P.-L.; et al. Potential of Multiplex Polymerase Chain Reaction Performed on Protected Telescope Catheter Samples for Early Adaptation of Antimicrobial Therapy in ARDS Patients. J. Clin. Med. 2022, 11, 4366. https://doi.org/10.3390/jcm11154366
Razazi K, Delamaire F, Fihman V, Boujelben MA, Mongardon N, Gendreau S, de Roux Q, de Prost N, Carteaux G, Woerther P-L, et al. Potential of Multiplex Polymerase Chain Reaction Performed on Protected Telescope Catheter Samples for Early Adaptation of Antimicrobial Therapy in ARDS Patients. Journal of Clinical Medicine. 2022; 11(15):4366. https://doi.org/10.3390/jcm11154366
Chicago/Turabian StyleRazazi, Keyvan, Flora Delamaire, Vincent Fihman, Mohamed Ahmed Boujelben, Nicolas Mongardon, Ségolène Gendreau, Quentin de Roux, Nicolas de Prost, Guillaume Carteaux, Paul-Louis Woerther, and et al. 2022. "Potential of Multiplex Polymerase Chain Reaction Performed on Protected Telescope Catheter Samples for Early Adaptation of Antimicrobial Therapy in ARDS Patients" Journal of Clinical Medicine 11, no. 15: 4366. https://doi.org/10.3390/jcm11154366






