Utilizing Computed Tomography to Analyze the Morphomic Change between Patients with Localized and Metastatic Renal Cell Carcinoma: Body Composition Varies According to Cancer Stage
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| CT | Computed tomography |
| SAT | Subcutaneous adipose tissue |
| VAT | Visceral adipose tissue |
| SMT | Skeletal muscle tissue |
| RCC | Renal cell carcinoma |
| ccRCC | Clear cell renal cell carcinoma |
| pRCC | Papillary renal cell carcinoma |
| chRCC | Chromophobe renal cell carcinoma |
References
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baracos, V.E.; Martin, L.; Korc, M.; Guttridge, D.C.; Fearon, K.C. Cancer-associated cachexia. Nat. Rev. Dis. Primers 2018, 4, 17105. [Google Scholar] [CrossRef] [PubMed]
- Moch, H.; Cubilla, A.L.; Humphrey, P.A.; Reuter, V.E.; Ulbright, T.M. The 2016 WHO Classification of tumours of the urinary System and male genital organs-Part A: Renal, penile, and testicu- lar tumours. Eur. Urol. 2016, 70, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, J.J.; Purdue, M.P.; Signoretti, S.; Swanton, C.; Albiges, L.; Schmidinger, M.; Heng, D.Y.; Larkin, J.; Ficarra, V. Renal cell carcinoma. Nat. Rev. Dis. Primers 2017, 3, 17009. [Google Scholar] [CrossRef] [PubMed]
- Petejova, N.; Martinek, A. Renal cell carcinoma: Review of etiology, pathophysiology and risk factors. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech Repub. 2016, 160, 183–194. [Google Scholar] [CrossRef] [Green Version]
- Otunctemur, A.; Dursun, M.; Ozer, K.; Horsanali, O.; Ozbek, E. Renal cell carcinoma and visceral adipose index: A new risk parameter. Int. Braz. J. Urol. 2016, 42, 955–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.W.; Jeong, B.C.; Seo, S.I.; Jeon, S.S.; Lee, H.M.; Choi, H.Y.; Jeon, H.G. Prognostic significance of visceral obesity in patients with advanced renal cell carcinoma undergoing nephrectomy. Int. J. Urol. 2015, 22, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizuno, R.; Miyajima, A.; Hibi, T.; Masuda, A.; Shinojima, T.; Kikuchi, E.; Jinzaki, M.; Oya, M. Impact of baseline visceral fat accumulation on prognosis in patients with metastatic renal cell carcinoma treated with systemic therapy. Med. Oncol. 2017, 34, 47. [Google Scholar] [CrossRef]
- Nelke, C.; Dziewas, R.; Minnerup, J.; Meuth, S.G.; Ruck, T. Skeletal muscle as potential central link between sarcopenia and immune senescence. EBioMedicine 2019, 49, 381–388. [Google Scholar] [CrossRef] [Green Version]
- Buchler, T.; Kopecka, M.; Zemankova, A.; Wiesnerová, M.; Streckova, E.; Rozsypalova, A.; Melichar, B.; Poprach, A.; Richter, I. Sarcopenia in metastatic renal cell carcinoma patients treated with cabozantinib. Target. Oncol. 2020, 15, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Darbas, T.; Forestier, G.; Leobon, S.; Pestre, J.; Jesus, P.; Lachatre, D.; Tubiana-Mathieu, N.; Descazeaud, A.; Deluche, E. Impact of body composition in overweight and obese patients with localised renal cell carcinoma. In Vivo 2020, 34, 2873–2881. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Ishihara, H.; Takagi, T.; Kondo, T.; Ishiyama, R.; Fukuda, H.; Yoshida, K.; Iizuka, J.; Kobayashi, H.; Ishida, H.; et al. Impact of sarcopenia on post-operative outcomes following nephrectomy and tumor thrombectomy for renal cell carci- noma with inferior vena cava thrombus. Jpn. J. Clin. Oncol. 2021, 51, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.-C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef] [Green Version]
- Yoshizumi, T.; Nakamura, T.; Yamane, M.; Islam, A.H.M.W.; Menju, M.; Yamasaki, K.; Arai, T.; Kotani, K.; Funahashi, T.; Yamashita, S.; et al. Abdominal fat: Standardized technique for measurement at CT. Radiology 1999, 211, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Batista, M.L., Jr.; Henriques, F.S.; Neves, R.X.; Olivan, M.R.; Matos-Neto, E.M.; Alcântara, P.S.; Maximiano, L.F.; Otoch, J.P.; Alves, M.J.; Seelaender, M. Cachexia-associated adipose tissue morphological rearrangement in gastrointestinal cancer patients. J. Cachexia Sar- Copenia Muscle 2016, 7, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Leibovitz, E.; Ben-David, N.; Shibanov, L.; Elias, S.; Shimonov, M. Visceral adiposity but not subcutaneous fat associated with improved outcome of patients with acute cholecystitis. J. Surg. Res. 2018, 225, 15–20. [Google Scholar] [CrossRef]
- Sato, F.; Maeda, N.; Yamada, T.; Namazui, H.; Fukuda, S.; Natsukawa, T.; Nagao, H.; Murai, J.; Masuda, S.; Tanaka, Y.; et al. Association of epicardial, visceral, and subcutaneous fat with cardiometabolic diseases. Circ. J. 2018, 82, 502–508. [Google Scholar] [CrossRef] [Green Version]
- Merlotti, C.; Ceriani, V.; Morabito, A.; Pontiroli, A.E. Subcutaneous fat loss is greater than visceral fat loss with diet and exercise, weight-loss promoting drugs and bariatric surgery: A critical review and meta-analysis. Int. J. Obes. 2017, 41, 672–682. [Google Scholar] [CrossRef]
- Baracos, V.E.; Mazurak, V.C.; Bhullar, A.S. Cancer cachexia is defined by an ongoing loss of skeletal muscle mass. Ann Palliat. Med. 2019, 8, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Friesen, D.E.; Baracos, V.E.; Tuszynski, J.A. Modeling the energetic cost of cancer as a result of altered energy metabolism: Implications for cachexia. Theor. Biol. Med. Model. 2015, 12, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bal, N.C.; Maurya, S.K.; Sopariwala, D.H.; Sahoo, S.K.; Gupta, S.C.; Shaikh, S.A.; Pant, M.; Rowland, L.A.; Bombardier, E.; Goonasekera, S.A.; et al. Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nat. Med. 2012, 18, 1575–1579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reig Garcia-Galbis, M.; Rizo Baeza, M.; Cortes Castell, E. [Predictors of weight loss and fat in the dietary management: Sex, age, BMI and consulting assistance]. Nutr. Hosp. 2015, 32, 1028–1035. [Google Scholar] [PubMed] [Green Version]
- Chang, E.; Varghese, M.; Singer, K. Gender and sex differences in adipose tssue. Curr. Diab. Rep. 2018, 18, 69. [Google Scholar] [CrossRef] [PubMed]
- Pillon, N.J.; Krook, A. Innate immune receptors in skeletal muscle metabolism. Exp. Cell Res. 2017, 360, 47–54. [Google Scholar] [CrossRef]
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2010, 11, 11–18. [Google Scholar] [CrossRef]
- Schaffler, A.; Scholmerich, J.; Buechler, C. Mechanisms of disease: Adipokines and breast cancer—endocrine and paracrine mechanisms that connect adiposity and breast cancer. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 345–354. [Google Scholar] [CrossRef]
- Park, J.; Euhus, D.M.; Scherer, P.E. Paracrine and endocrine effects of adipose tissue on cancer development and progression. Endocr. Rev. 2011, 32, 550–570. [Google Scholar] [CrossRef] [Green Version]
- Greco, F.; Mallio, C.A.; Grippo, R.; Messina, L.; Vallese, S.; Rabitti, C.; Quarta, L.G.; Grasso, R.F.; Beomonte Zobel, B. Increased visceral adipose tissue in male patients with non-clear cell renal cell carcinoma. Radiol. Med. 2020, 125, 538–543. [Google Scholar] [CrossRef]

| Variables | Mean | SD | Range/ Percentage | |||
|---|---|---|---|---|---|---|
| Age | 57.1 | 13.11 | 11–82 | Years old | ||
| Body weight | 67.71 | 12.68 | 29–117 | kg | ||
| Height | 164.1 | 9.02 | 139.0–186.8 | cm | ||
| BMI | 25.17 | 4.01 | 15.01–39.55 | |||
| Tumor-Related Parameters | ||||||
| Clinical T stage | 1a | 109 | 58.0% | |||
| 1b | 33 | 17.6% | ||||
| 2a | 7 | 3.7% | ||||
| 2b | 1 | 0.5% | ||||
| 3a | 35 | 18.6% | ||||
| 3b | 2 | 1.1% | ||||
| 3c | 1 | 0.5% | ||||
| Clinical N stage | 0 | 121 | 64.4% | |||
| 1 | 29 | 15.4% | ||||
| 2 | 1 | 0.5% | ||||
| x | 35 | 18.6% | ||||
| Clinical M stage | 0 | 141 | 75% | |||
| 1 | 47 | 25% | ||||
| Tumor pathology | ccRCC | Stage I | 107 | 72.3% | ||
| Stage II | 2 | 1.4% | ||||
| Stage III | 11 | 7.4% | ||||
| Stage IV | 28 | 18.9% | ||||
| pRCC | Stage I | 13 | 76.5% | |||
| Stage II | 0 | 0% | ||||
| Stage III | 0 | 0% | ||||
| Stage IV | 4 | 23.5% | ||||
| chRCC | Stage I | 12 | 66.7% | |||
| Stage II | 0 | 0% | ||||
| Stage III | 0 | 0% | ||||
| Stage IV | 6 | 33.3% | ||||
| mcRCC | Stage I | 1 | 100% | |||
| Xp11.2 RCC | Stage I | 2 | 100% | |||
| Other | Stage I | 2 | 100% | |||
| Body Composition Parameters | ||||||
| SMT | 1895.19 | 520.83 | 705.69–4148.16 | cm3 | ||
| SAT | 1799.69 | 1070.70 | 5.61–7617.65 | cm3 | ||
| VAT | 1870.86 | 1130.64 | 23.92–5725.61 | cm3 | ||
| SMT at L3 | 140.31 | 31.96 | 57.76–216.15 | cm2 | ||
| SAT at L3 | 138.02 | 70.65 | 38.1–254.4 | cm2 | ||
| VAT at L3 | 139.49 | 79.63 | 1.16–119.6 | cm2 | ||
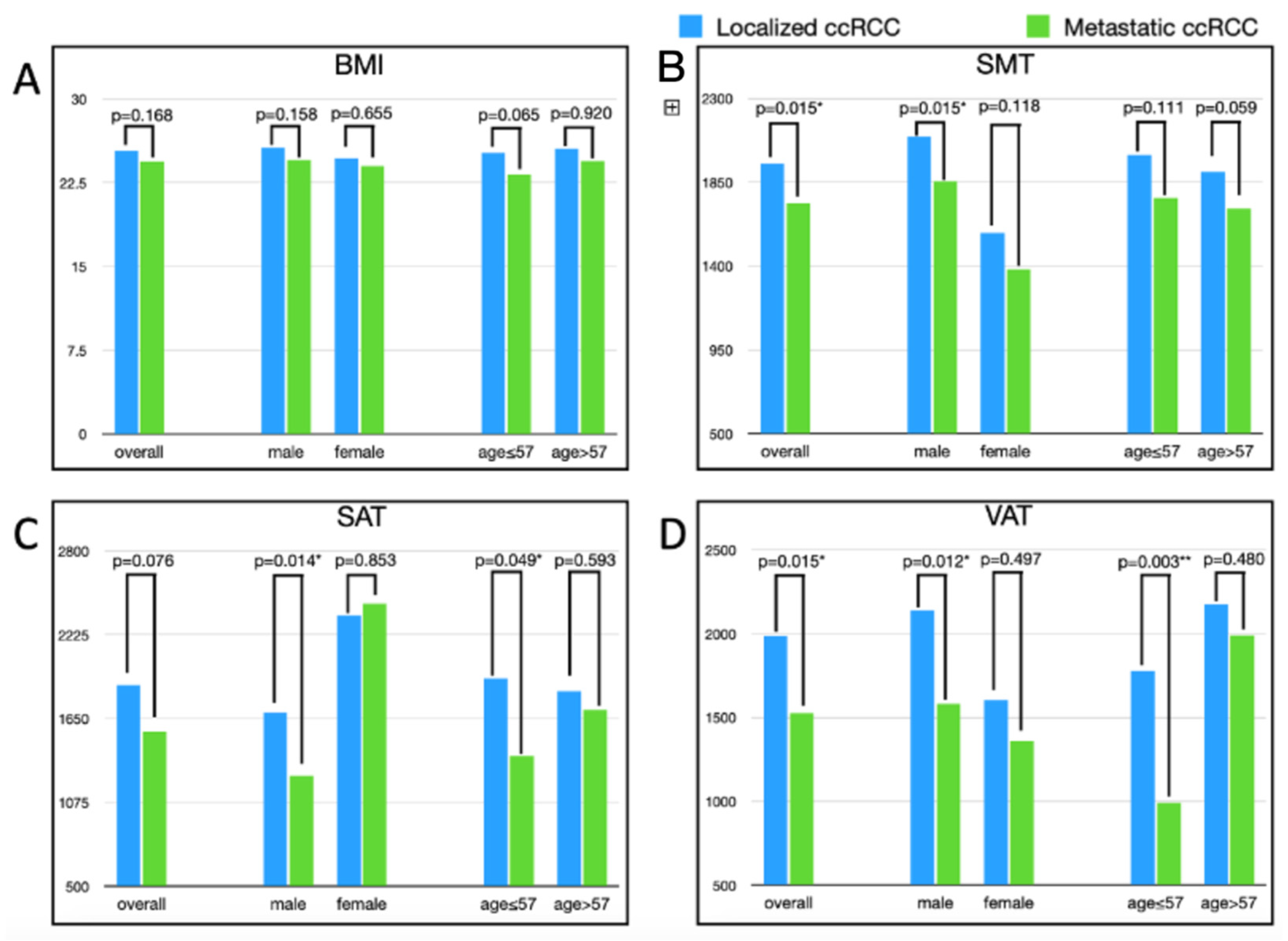
| Localized RCC (N = 141) | Metastatic RCC (N = 47) | Univariate Analysis | Multi-Variate Analysis | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean Difference | p-Value | p-Value | ||
| Body weight | kg | 68.7 | 13.14 | 64.96 | 13.43 | −3.74 | 0.096 | 0.799 |
| BMI | kg/m2 | 25.36 | 4.11 | 24.39 | 4.27 | −0.97 | 0.168 | 0.357 |
| SMT | cm3 | 1948.49 | 511.89 | 1735.28 | 520 | −213.21 | 0.015 * | 0.981 |
| SAT | cm3 | 1879.64 | 1038.50 | 1559.81 | 1140 | −319.83 | 0.076 | 0.123 |
| VAT | cm3 | 1986.74 | 1083.09 | 1523.2 | 1209.07 | −463.54 | 0.015 * | 0.020 * |
| SMT-L3 | cm2 | 142.65 | 31.83 | 133.27 | 31.66 | −9.38 | 0.082 | 0.471 |
| SAT-L3 | cm2 | 142.3 | 66.71 | 125.18 | 80.78 | −17.12 | 0.151 | 0.573 |
| VAT-L3 | cm2 | 146.48 | 75.39 | 118.52 | 88.77 | −27.96 | 0.037 * | 0.736 |
| Localized RCC | Metastatic RCC | ||||||
| (Male:Female = 101:40) | (Male:Female = 35:12) | ||||||
| Mean | SD | Mean | SD | Mean Difference | p-Value | ||
| Body weight | Male | 72.29 | 12.18 | 66.65 | 13.16 | −5.64 | 0.023 * |
| Female | 59.82 | 11.17 | 60.05 | 13.56 | 0.23 | 0.953 | |
| BMI | Male | 25.63 | 3.9 | 24.53 | 4.07 | −1.1 | 0.158 |
| Female | 24.69 | 4.58 | 24 | 4.99 | −0.69 | 0.655 | |
| SMT | Male | 2094.8 | 487.26 | 1856.75 | 513 | −238.09 | 0.015 * |
| Female | 1579 | 369.23 | 1381 | 365.33 | −197.98 | 0.118 | |
| SAT | Male | 1690.2 | 924.69 | 1259.32 | 751.6 | −430.87 | 0.014 * |
| Female | 2358 | 1162.48 | 2436.23 | 1601.06 | 78.23 | 0.853 | |
| VAT | Male | 2137.8 | 1070.63 | 1579.8 | 1254.89 | −557.95 | 0.012 * |
| Female | 1605.5 | 1031.41 | 1358.13 | 1098.13 | −247.34 | 0.497 | |
| SMT-L3 | Male | 156.09 | 25.62 | 143.51 | 28.71 | −12.58 | 0.017 * |
| Female | 108.71 | 17.48 | 103.42 | 18.45 | −5.29 | 0.39 | |
| SAT-L3 | Male | 133.75 | 62.58 | 106.88 | 54.8 | −26.87 | 0.026 * |
| Female | 72.55 | 72.56 | 117.6 | 117.61 | 45.05 | 0.6 | |
| VAT-L3 | Male | 158.28 | 74.29 | 123.09 | 92.5 | −35.19 | 0.025 * |
| Female | 70.58 | 70.58 | 79.01 | 79.01 | 8.43 | 0.656 | |
| Age subgroup | |||||||
| Localized RCC | Metastatic RCC | ||||||
| (Young:Old = 67:74) | (Young:Old = 22:25) | ||||||
| Mean | SD | Mean | SD | Mean Difference | p-Value | ||
| Bodyweight | Young | 70.3 | 13.73 | 64.3 | 16.33 | −6 | 0.094 |
| Old | 67.25 | 12.5 | 65.55 | 10.57 | −1.7 | 0.543 | |
| BMI | Young | 25.2 | 4.12 | 23.23 | 4.76 | −1.97 | 0.065 |
| Old | 25.51 | 4.13 | 25.41 | 3.57 | −0.1 | 0.92 | |
| SMT | Young | 1996.5 | 565.92 | 1766.46 | 628.48 | −230.08 | 0.111 |
| Old | 1905 | 457.1 | 1707.85 | 413.5 | −197.15 | 0.059 | |
| SAT | Young | 1925.9 | 1080.34 | 1390.64 | 1142.58 | −535.27 | 0.05 |
| Old | 1837.8 | 1004.68 | 1708.68 | 1139.86 | −129.07 | 0.593 | |
| VAT | Young | 1778.5 | 1091.88 | 991.53 | 800.84 | −786.99 | 0.003 ** |
| Old | 2175.3 | 1046.91 | 1991.07 | 1324.23 | −184.21 | 0.48 | |
| SMT-L3 | Young | 148.34 | 33.05 | 135.88 | 39.37 | −12.46 | 0.147 |
| Old | 137.5 | 29.99 | 130.98 | 23.54 | −6.52 | 0.326 | |
| SAT-L3 | Young | 149.13 | 69.96 | 117.54 | 95.22 | −31.59 | 0.098 |
| Old | 136.1 | 63.46 | 131.9 | 66.88 | −4.2 | 0.778 | |
| VAT-L3 | Young | 131.68 | 77.41 | 75.67 | 60.02 | −56.01 | 0.003 ** |
| Old | 159.88 | 71.41 | 156.22 | 93.74 | −3.66 | 0.839 | |
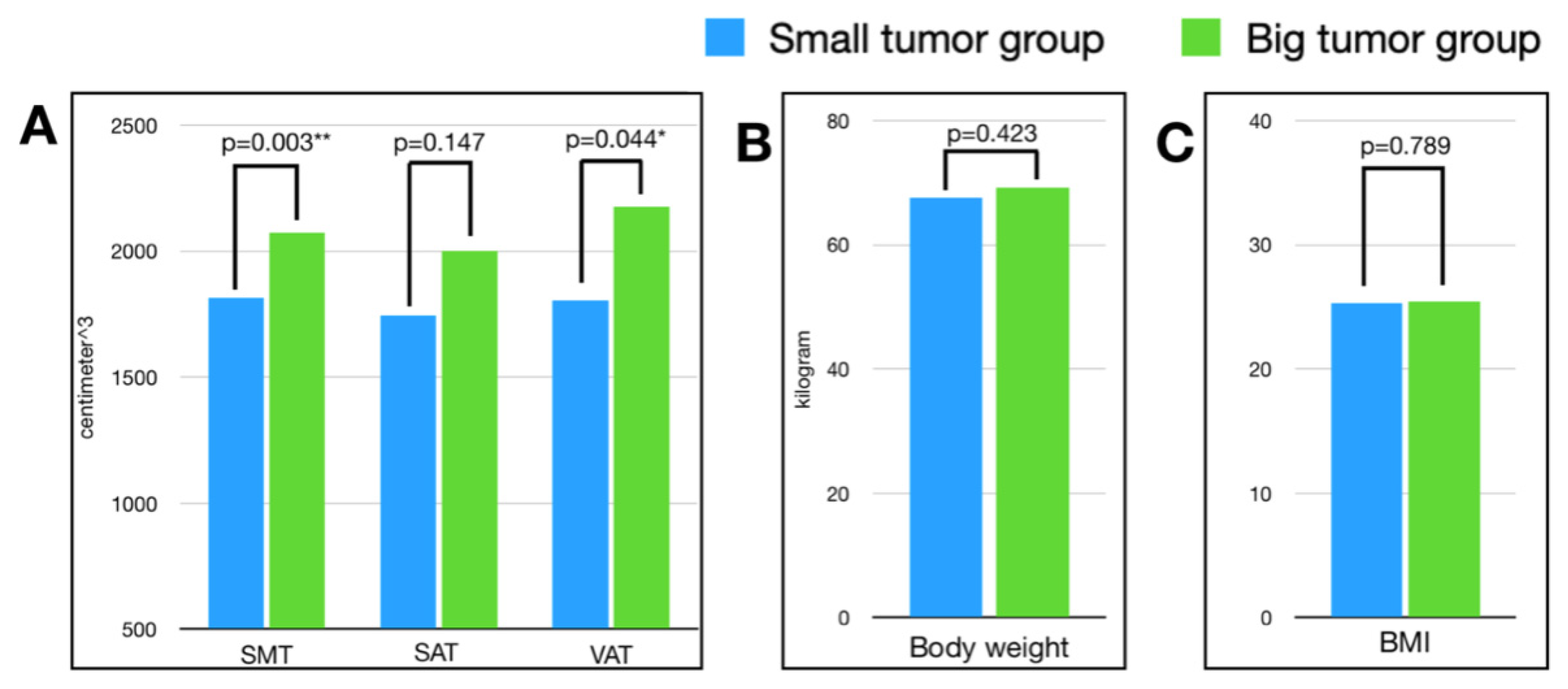
| Small Tumor Group | Large Tumor Group | Univariate Analysis | Multi-Variate Analysis | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean Difference | p-Value | p-Value | ||
| Body weight | kilogram | 67.59 | 12.35 | 69.18 | 13.05 | −1.59 | 0.423 | 0.802 |
| BMI | kg/cm2 | 25.28 | 4.12 | 25.44 | 4.02 | −0.16 | 0.789 | 0.955 |
| SMT | cm3 | 1815.22 | 478.37 | 2072.38 | 516.07 | −257.16 | 0.003 ** | 0.319 |
| SAT | cm3 | 1744.79 | 826.72 | 2001.81 | 1213.50 | −257.02 | 0.147 | 0.866 |
| VAT | cm3 | 1804.57 | 964.8 | 2176.96 | 1178.50 | −372.39 | 0.044 * | 0.003 ** |
| SMT-L3 | cm2 | 136.79 | 32.14 | 147.44 | 30.6 | −10.65 | 0.047 * | 0.109 |
| SAT-L3 | cm2 | 135.57 | 58.78 | 147.46 | 73.76 | −11.89 | 0.295 | 0.914 |
| VAT-L3 | cm2 | 141.23 | 75.3 | 151.94 | 76.6 | −10.71 | 0.407 | 0.002 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, C.-C.; Sheng, T.-W.; Chang, Y.-H.; Wang, L.-J.; Chuang, C.-K.; Wu, C.-T.; Pang, S.-T.; Shao, I.-H. Utilizing Computed Tomography to Analyze the Morphomic Change between Patients with Localized and Metastatic Renal Cell Carcinoma: Body Composition Varies According to Cancer Stage. J. Clin. Med. 2022, 11, 4444. https://doi.org/10.3390/jcm11154444
Tan C-C, Sheng T-W, Chang Y-H, Wang L-J, Chuang C-K, Wu C-T, Pang S-T, Shao I-H. Utilizing Computed Tomography to Analyze the Morphomic Change between Patients with Localized and Metastatic Renal Cell Carcinoma: Body Composition Varies According to Cancer Stage. Journal of Clinical Medicine. 2022; 11(15):4444. https://doi.org/10.3390/jcm11154444
Chicago/Turabian StyleTan, Chin-Chieh, Ting-Wen Sheng, Ying-Hsu Chang, Li-Jen Wang, Cheng-Keng Chuang, Chun-Te Wu, See-Tong Pang, and I-Hung Shao. 2022. "Utilizing Computed Tomography to Analyze the Morphomic Change between Patients with Localized and Metastatic Renal Cell Carcinoma: Body Composition Varies According to Cancer Stage" Journal of Clinical Medicine 11, no. 15: 4444. https://doi.org/10.3390/jcm11154444
APA StyleTan, C.-C., Sheng, T.-W., Chang, Y.-H., Wang, L.-J., Chuang, C.-K., Wu, C.-T., Pang, S.-T., & Shao, I.-H. (2022). Utilizing Computed Tomography to Analyze the Morphomic Change between Patients with Localized and Metastatic Renal Cell Carcinoma: Body Composition Varies According to Cancer Stage. Journal of Clinical Medicine, 11(15), 4444. https://doi.org/10.3390/jcm11154444





.jpg)

