Treatment Options in AF Patients with Cancer; Focus on Catheter Ablation
Abstract
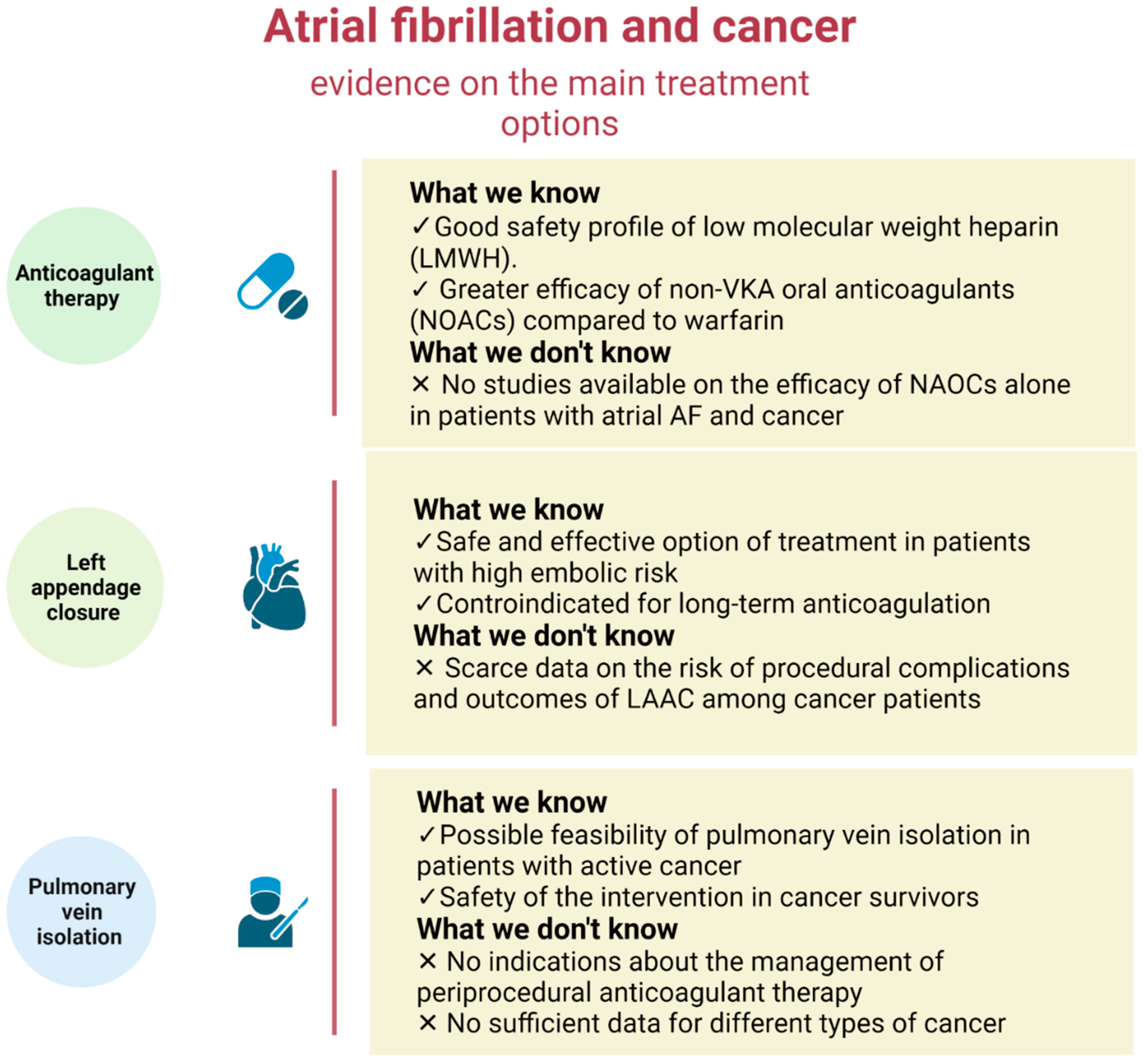
:1. Introduction
2. Epidemiology
3. Anticoagulant Therapy in Cancer Patients
4. Left Appendage Closure in Cancer Patients
5. Pulmonary Vein Isolation (PVI) Ablation
5.1. Radiation Exposure during Atrial Fibrillation Catheter Ablation
5.2. Catheter Ablation of Atrial Fibrillation in Cancer Patients
5.3. Periprocedural Anticoagulant
6. Discussion
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the Diagnosis and Management of Atrial Fibrillation Developed in Collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Chu, G.; Versteeg, H.H.; Verschoor, A.J.; Trines, S.A.; Hemels, M.E.W.; Ay, C.; Huisman, M.V.; Klok, F.A. Atrial Fibrillation and Cancer—An Unexplored Field in Cardiovascular Oncology. Blood Rev. 2019, 35, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Rahman, F.; Ko, D.; Benjamin, E.J. Association of Atrial Fibrillation and Cancer. JAMA Cardiol. 2016, 1, 384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farmakis, D.; Parissis, J.; Filippatos, G. Insights into Onco-Cardiology. J. Am. Coll. Cardiol. 2014, 63, 945–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conen, D.; Wong, J.A.; Sandhu, R.K.; Cook, N.R.; Lee, I.-M.; Buring, J.E.; Albert, C.M. Risk of Malignant Cancer Among Women With New-Onset Atrial Fibrillation. JAMA Cardiol. 2016, 1, 389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostenfeld, E.B.; Erichsen, R.; Pedersen, L.; Farkas, D.K.; Weiss, N.S.; Sørensen, H.T. Atrial Fibrillation as a Marker of Occult Cancer. PLoS ONE 2014, 9, e102861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Lip, G.Y.H.; Apostolakis, S. Inflammation in Atrial Fibrillation. J. Am. Coll. Cardiol. 2012, 60, 2263–2270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neal, W.T.; Lakoski, S.G.; Qureshi, W.; Judd, S.E.; Howard, G.; Howard, V.J.; Cushman, M.; Soliman, E.Z. Relation between Cancer and Atrial Fibrillation (from the REasons for Geographic and Racial Differences in Stroke Study). Am. J. Cardiol. 2015, 115, 1090–1094. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Liu, C.; Chang, P.M.; Tsao, H.; Lin, Y.; Chang, S.; Lo, L.; Tuan, T.; Li, C.; Chao, T.; et al. Incident Thromboembolism and Heart Failure Associated with New-Onset Atrial Fibrillation in Cancer Patients. Int. J. Cardiol. 2013, 165, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Guzzetti, S.; Costantino, G.; Vernocchi, A.; Sada, S.; Fundarò, C. First Diagnosis of Colorectal or Breast Cancer and Prevalence of Atrial Fibrillation. Intern. Emerg. Med. 2008, 3, 227–231. [Google Scholar] [CrossRef]
- Jakobsen, C.B.; Lamberts, M.; Carlson, N.; Lock-Hansen, M.; Torp-Pedersen, C.; Gislason, G.H.; Schou, M. Incidence of Atrial Fibrillation in Different Major Cancer Subtypes: A Nationwide Population-Based 12 Year Follow up Study. BMC Cancer 2019, 19, 1105. [Google Scholar] [CrossRef] [PubMed]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Muñoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. 2016 ESC Position Paper on Cancer Treatments and Cardiovascular Toxicity Developed under the Auspices of the ESC Committee for Practice Guidelines: The Task Force for Cancer Treatments and Cardiovascular Toxicity of the European Society of Cardiology (ESC). Eur. J. Heart Fail 2017, 19, 9–42. [Google Scholar] [CrossRef] [PubMed]
- Weitz, J.I. Low-Molecular-Weight Heparins. N. Engl. J. Med. 1997, 337, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.T.; Hellkamp, A.S.; Becker, R.C.; Berkowitz, S.D.; Breithardt, G.; Fox, K.A.A.; Hacke, W.; Halperin, J.L.; Hankey, G.J.; Mahaffey, K.W.; et al. Efficacy and Safety of Rivaroxaban vs. Warfarin in Patients with Non-Valvular Atrial Fibrillation and a History of Cancer: Observations from ROCKET AF. Eur. Heart J.-Qual. Care Clin. Outcomes 2019, 5, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Norby, F.L.; Datta, Y.H.; Lutsey, P.L.; MacLehose, R.F.; Chen, L.Y.; Alonso, A. Comparative Effectiveness of Direct Oral Anticoagulants and Warfarin in Patients with Cancer and Atrial Fibrillation. Blood Adv. 2018, 2, 200–209. [Google Scholar] [CrossRef] [Green Version]
- Fanola, C.L.; Ruff, C.T.; Murphy, S.A.; Jin, J.; Duggal, A.; Babilonia, N.A.; Sritara, P.; Mercuri, M.F.; Kamphuisen, P.W.; Antman, E.M.; et al. Efficacy and Safety of Edoxaban in Patients With Active Malignancy and Atrial Fibrillation: Analysis of the ENGAGE AF-TIMI 48 Trial. JAHA 2018, 7, e008987. [Google Scholar] [CrossRef]
- Kim, K.; Lee, Y.-J.; Kim, T.-H.; Uhm, J.-S.; Pak, H.-N.; Lee, M.-H.; Joung, B. Effect of Non-Vitamin K Antagonist Oral Anticoagulants in Atrial Fibrillation Patients with Newly Diagnosed Cancer. Korean Circ. J. 2018, 48, 406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melloni, C.; Dunning, A.; Granger, C.B.; Thomas, L.; Khouri, M.G.; Garcia, D.A.; Hylek, E.M.; Hanna, M.; Wallentin, L.; Gersh, B.J.; et al. Efficacy and Safety of Apixaban Versus Warfarin in Patients with Atrial Fibrillation and a History of Cancer: Insights from the ARISTOTLE Trial. Am. J. Med. 2017, 130, 1440–1448.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ording, A.G.; Horváth-Puhó, E.; Adelborg, K.; Pedersen, L.; Prandoni, P.; Sørensen, H.T. Thromboembolic and Bleeding Complications during Oral Anticoagulation Therapy in Cancer Patients with Atrial Fibrillation: A Danish Nationwide Population-Based Cohort Study. Cancer Med. 2017, 6, 1165–1172. [Google Scholar] [CrossRef]
- Flack, K.F.; Desai, J.; Kolb, J.M.; Chatterjee, P.; Wallentin, L.C.; Ezekowitz, M.; Yusuf, S.; Connolly, S.; Reilly, P.; Brueckmann, M.; et al. Major Gastrointestinal Bleeding Often Is Caused by Occult Malignancy in Patients Receiving Warfarin or Dabigatran to Prevent Stroke and Systemic Embolism From Atrial Fibrillation. Clin. Gastroenterol. Hepatol. 2017, 15, 682–690. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Tong, Y.; Deng, Y.; Zou, L.; Li, S.; Chen, H. Non–Vitamin K Antagonist Oral Anticoagulants Versus Warfarin in Patients With Cancer and Atrial Fibrillation: A Systematic Review and Meta-Analysis. JAHA 2019, 8, e012540. [Google Scholar] [CrossRef] [PubMed]
- Laube, E.S.; Yu, A.; Gupta, D.; Miao, Y.; Samedy, P.; Wills, J.; Harnicar, S.; Soff, G.A.; Mantha, S. Rivaroxaban for Stroke Prevention in Patients With Nonvalvular Atrial Fibrillation and Active Cancer. Am. J. Cardiol. 2017, 120, 213–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, V.; Rago, A.; Papa, A.; Meo, F.; Attena, E.; Golino, P.; D’Onofrio, A.; Nigro, G. Use of Non–Vitamin K Antagonist Oral Anticoagulants in Atrial Fibrillation Patients with Malignancy: Clinical Practice Experience in a Single Institution and Literature Review. Semin. Thromb. Hemost. 2018, 44, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Ianotto, J.-C.; Couturier, M.-A.; Galinat, H.; Mottier, D.; Berthou, C.; Guillerm, G.; Lippert, E.; Delluc, A. Administration of Direct Oral Anticoagulants in Patients with Myeloproliferative Neoplasms. Int. J. Hematol. 2017, 106, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.; Bottino, R.; Rago, A.; Micco, P.; D’ Onofrio, A.; Liccardo, B.; Golino, P.; Nigro, G. Atrial Fibrillation and Malignancy: The Clinical Performance of Non–Vitamin K Oral Anticoagulants—A Systematic Review. Semin. Thromb. Hemost 2019, 45, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Sorigue, M.; Miljkovic, M.D. Atrial Fibrillation and Stroke Risk in Patients With Cancer: A Primer for Oncologists. JOP 2019, 15, 641–650. [Google Scholar] [CrossRef]
- Shabtaie, S.A.; Tan, N.Y.; Ward, R.C.; Herrmann, J. Abstract 11688: Left Atrial Appendage Occlusion in Patients with Atrial Fibrillation and Cancer. Circulation 2021, 144 (Suppl. 1). [Google Scholar] [CrossRef]
- Madias, J.E. COVID-19, POCUS, and Takotsubo. Am. J. Cardiol. 2021, 141, 157. [Google Scholar] [CrossRef]
- Nishimura, M.; Sab, S.; Reeves, R.R.; Hsu, J.C. Percutaneous Left Atrial Appendage Occlusion in Atrial Fibrillation Patients with a Contraindication to Oral Anticoagulation: A Focused Review. EP Eur. 2018, 20, 1412–1419. [Google Scholar] [CrossRef]
- Haïssaguerre, M.; Jaïs, P.; Shah, D.C.; Takahashi, A.; Hocini, M.; Quiniou, G.; Garrigue, S.; Le Mouroux, A.; Le Métayer, P.; Clémenty, J. Spontaneous Initiation of Atrial Fibrillation by Ectopic Beats Originating in the Pulmonary Veins. N. Engl. J. Med. 1998, 339, 659–666. [Google Scholar] [CrossRef] [Green Version]
- Haïssaguerre, M.; Jaïs, P.; Shah, D.C.; Garrigue, S.; Takahashi, A.; Lavergne, T.; Hocini, M.; Peng, J.T.; Roudaut, R.; Clémenty, J. Electrophysiological End Point for Catheter Ablation of Atrial Fibrillation Initiated from Multiple Pulmonary Venous Foci. Circulation 2000, 101, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Pappone, C.; Oreto, G.; Lamberti, F.; Vicedomini, G.; Loricchio, M.L.; Shpun, S.; Rillo, M.; Calabrò, M.P.; Conversano, A.; Ben-Haim, S.A.; et al. Catheter Ablation of Paroxysmal Atrial Fibrillation Using a 3D Mapping System. Circulation 1999, 100, 1203–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arentz, T.; Weber, R.; Bürkle, G.; Herrera, C.; Blum, T.; Stockinger, J.; Minners, J.; Neumann, F.J.; Kalusche, D. Small or Large Isolation Areas Around the Pulmonary Veins for the Treatment of Atrial Fibrillation?: Results From a Prospective Randomized Study. Circulation 2007, 115, 3057–3063. [Google Scholar] [CrossRef] [Green Version]
- Calkins, H.; Hindricks, G.; Cappato, R.; Kim, Y.-H.; Saad, E.B.; Aguinaga, L.; Akar, J.G.; Badhwar, V.; Brugada, J.; Camm, J.; et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation. Heart Rhythm. 2017, 14, e275–e444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fürnkranz, A.; Brugada, J.; Albenque, J.-P.; Tondo, C.; Bestehorn, K.; Wegscheider, K.; Ouyang, F.; Kuck, K.-H. Rationale and Design of FIRE AND ICE: A Multicenter Randomized Trial Comparing Efficacy and Safety of Pulmonary Vein Isolation Using a Cryoballoon versus Radiofrequency Ablation with 3D-Reconstruction: Rationale and Design of Fire and Ice. J. Cardiovasc. Electrophysiol. 2014, 25, 1314–1320. [Google Scholar] [CrossRef]
- Aryana, A.; Singh, S.M.; Kowalski, M.; Pujara, D.K.; Cohen, A.I.; Singh, S.K.; Aleong, R.G.; Banker, R.S.; Fuenzalida, C.E.; Prager, N.A.; et al. Acute and Long-Term Outcomes of Catheter Ablation of Atrial Fibrillation Using the Second-Generation Cryoballoon versus Open-Irrigated Radiofrequency: A Multicenter Experience: Second-Generation Cryoballoon versus RF. J. Cardiovasc. Electrophysiol. 2015, 26, 832–839. [Google Scholar] [CrossRef]
- Andrade, J.G.; Champagne, J.; Dubuc, M.; Deyell, M.W.; Verma, A.; Macle, L.; Leong-Sit, P.; Novak, P.; Badra-Verdu, M.; Sapp, J.; et al. Cryoballoon or Radiofrequency Ablation for Atrial Fibrillation Assessed by Continuous Monitoring: A Randomized Clinical Trial. Circulation 2019, 140, 1779–1788. [Google Scholar] [CrossRef]
- Luik, A.; Radzewitz, A.; Kieser, M.; Walter, M.; Bramlage, P.; Hörmann, P.; Schmidt, K.; Horn, N.; Brinkmeier-Theofanopoulou, M.; Kunzmann, K.; et al. Cryoballoon Versus Open Irrigated Radiofrequency Ablation in Patients With Paroxysmal Atrial Fibrillation: The Prospective, Randomized, Controlled, Noninferiority FreezeAF Study. Circulation 2015, 132, 1311–1319. [Google Scholar] [CrossRef]
- Kuck, K.-H.; Brugada, J.; Fürnkranz, A.; Metzner, A.; Ouyang, F.; Chun, K.R.J.; Elvan, A.; Arentz, T.; Bestehorn, K.; Pocock, S.J.; et al. Cryoballoon or Radiofrequency Ablation for Paroxysmal Atrial Fibrillation. N. Engl. J. Med. 2016, 374, 2235–2245. [Google Scholar] [CrossRef]
- Kleemann, T.; Brachmann, J.; Lewalter, T.; Andresen, D.; Willems, S.; Spitzer, S.G.; Hoffmann, E.; Eckardt, L.; Hochadel, M.; Senges, J.; et al. Development of Radiation Exposure in Patients Undergoing Pulmonary Vein Isolation in Germany between 2007 and 2014: Great Potential to Minimize Radiation Dosage. Clin. Res. Cardiol. 2016, 105, 858–864. [Google Scholar] [CrossRef]
- Park, T.H.; Eichling, J.O.; Schechtman, K.B.; Bromberg, B.I.; Smith, J.M.; Lindsay, B.D. Risk of Radiation Induced Skin Injuries from Arrhythmia Ablation Procedures. Pacing. Clin. Electron. 1996, 19, 1363–1369. [Google Scholar] [CrossRef] [PubMed]
- Perisinakis, K.; Damilakis, J.; Theocharopoulos, N.; Manios, E.; Vardas, P.; Gourtsoyiannis, N. Accurate Assessment of Patient Effective Radiation Dose and Associated Detriment Risk From Radiofrequency Catheter Ablation Procedures. Circulation 2001, 104, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Rehani, M.M.; Ortiz-Lopez, P. Radiation Effects in Fluoroscopically Guided Cardiac Interventions—Keeping Them under Control. Int. J. Cardiol. 2006, 109, 147–151. [Google Scholar] [CrossRef] [PubMed]
- McFadden, S.L.; Mooney, R.B.; Shepherd, P.H. X-Ray Dose and Associated Risks from Radiofrequency Catheter Ablation Procedures. BJR 2002, 75, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Clay, M.A.; Campbell, R.M.; Strieper, M.; Frias, P.A.; Stevens, M.; Mahle, W.T. Long-Term Risk of Fatal Malignancy Following Pediatric Radiofrequency Ablation. Am. J. Cardiol. 2008, 102, 913–915. [Google Scholar] [CrossRef]
- Balter, S. Stray Radiation in the Cardiac Catheterisation Laboratory. Radiat. Prot. Dosim. 2001, 94, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Vano, E. Radiation Exposure to Cardiologists: How It Could Be Reduced. Heart 2003, 89, 1123–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cousins, C.; Miller, D.L.; Bernardi, G.; Rehani, M.M.; Schofield, P.; Vañó, E.; Einstein, A.J.; Geiger, B.; Heintz, P.; Padovani, R.; et al. ICRP Publication 120: Radiological Protection in Cardiology. Ann. ICRP 2013, 42, 1–125. [Google Scholar] [CrossRef]
- Macle, L.; Weerasooriya, R.; Jais, P.; Scavee, C.; Raybaud, F.; Choi, K.-J.; Hocini, M.; Clementy, J.; Haissaguerre, M. Radiation Exposure During Radiofrequency Catheter Ablation for Atrial Fibrillation. Pacing Clin. Electrophysiol. 2003, 26, 288–291. [Google Scholar] [CrossRef]
- Lickfett, L.; Mahesh, M.; Vasamreddy, C.; Bradley, D.; Jayam, V.; Eldadah, Z.; Dickfeld, T.; Kearney, D.; Dalal, D.; Lüderitz, B.; et al. Radiation Exposure During Catheter Ablation of Atrial Fibrillation. Circulation 2004, 110, 3003–3010. [Google Scholar] [CrossRef] [PubMed]
- Estner, H.L.; Deisenhofer, I.; Luik, A.; Ndrepepa, G.; von Bary, C.; Zrenner, B.; Schmitt, C. Electrical Isolation of Pulmonary Veins in Patients with Atrial Fibrillation: Reduction of Fluoroscopy Exposure and Procedure Duration by the Use of a Non-Fluoroscopic Navigation System (NavX®). EP Eur. 2006, 8, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Kistler, P.M.; Rajappan, K.; Harris, S.; Earley, M.J.; Richmond, L.; Sporton, S.C.; Schilling, R.J. The Impact of Image Integration on Catheter Ablation of Atrial Fibrillation Using Electroanatomic Mapping: A Prospective Randomized Study. Eur. Heart J. 2008, 29, 3029–3036. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, B.; Balmforth, D.C.; Hunter, R.J.; Schilling, R.J. Fluoroscopy-Free AF Ablation Using Transesophageal Echocardiography and Electroanatomical Mapping Technology. J. Interv. Card. Electrophysiol. 2017, 50, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.Y.; Morales, G.; Ahmed, H.; Neuzil, P.; Dukkipati, S.; Kim, S.; Clemens, J.; D’Avila, A. Catheter Ablation of Atrial Fibrillation without the Use of Fluoroscopy. Heart Rhythm. 2010, 7, 1644–1653. [Google Scholar] [CrossRef] [PubMed]
- Bulava, A.; Hanis, J.; Eisenberger, M. Catheter Ablation of Atrial Fibrillation Using Zero-Fluoroscopy Technique: A Randomized Trial: ATRIAL FIBRILLATION ABLATION WITHOUT FLUOROSCOPY. Pacing Clin. Electrophysiol. 2015, 38, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Balmforth, D.C.; Smith, A.; Schilling, R.; O’Brien, B. Fluoroscopy-Free Cryoablation of Atrial Fibrillation Guided Solely by Transoesophageal Echocardiography: A Case Report. Eur. Heart J.-Case Rep. 2018, 2, yty137. [Google Scholar] [CrossRef] [PubMed]
- Caponi, D.; Corleto, A.; Scaglione, M.; Blandino, A.; Biasco, L.; Cristoforetti, Y.; Cerrato, N.; Toso, E.; Morello, M.; Gaita, F. Ablation of Atrial Fibrillation: Does the Addition of Three-Dimensional Magnetic Resonance Imaging of the Left Atrium to Electroanatomic Mapping Improve the Clinical Outcome?: A Randomized Comparison of Carto-Merge vs. Carto-XP Three-Dimensional Mapping Ablation in Patients with Paroxysmal and Persistent Atrial Fibrillation. Europace 2010, 12, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Della Bella, P.; Fassini, G.; Cireddu, M.; Riva, S.; Carbucicchio, C.; Giraldi, F.; Maccabelli, G.; Trevisi, N.; Moltrasio, M.; Pepi, M.; et al. Image Integration-Guided Catheter Ablation of Atrial Fibrillation: A Prospective Randomized Study. J. Cardiovasc. Electrophysiol. 2009, 20, 258–265. [Google Scholar] [CrossRef]
- Tahin, T.; Riba, A.; Nemeth, B.; Arvai, F.; Lupkovics, G.; Szeplaki, G.; Geller, L. Implementation of a Zero Fluoroscopic Workflow Using a Simplified Intracardiac Echocardiography Guided Method for Catheter Ablation of Atrial Fibrillation, Including Repeat Procedures. BMC Cardiovasc. Disord. 2021, 21, 407. [Google Scholar] [CrossRef]
- Casella, M.; Dello Russo, A.; Russo, E.; Catto, V.; Pizzamiglio, F.; Zucchetti, M.; Majocchi, B.; Riva, S.; Vettor, G.; Dessanai, M.A.; et al. X-Ray Exposure in Cardiac Electrophysiology: A Retrospective Analysis in 8150 Patients Over 7 Years of Activity in a Modern, Large-Volume Laboratory. JAHA 2018, 7, e008233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steffel, J.; Collins, R.; Antz, M.; Cornu, P.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; Rowell, N.; et al. 2021 European Heart Rhythm Association Practical Guide on the Use of Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation. EP Eur. 2021, 23, 1612–1676. [Google Scholar] [CrossRef] [PubMed]
- López-Fernández, T.; Martín-García, A.; Roldán Rabadán, I.; Mitroi, C.; Mazón Ramos, P.; Díez-Villanueva, P.; Escobar Cervantes, C.; Alonso Martín, C.; Alonso Salinas, G.L.; Arenas, M.; et al. Atrial Fibrillation in Active Cancer Patients: Expert Position Paper and Recommendations. Rev. Española De Cardiol. íA (Engl. Ed.) 2019, 72, 749–759. [Google Scholar] [CrossRef]
- Kanmanthareddy, A.; Vallakati, A.; Reddy Yeruva, M.; Dixit, S.; Di Biase, L.; Mansour, M.; Boolani, H.; Gunda, S.; Bunch, T.J.; Day, J.D.; et al. Pulmonary Vein Isolation for Atrial Fibrillation in the Postpneumonectomy Population: A Feasibility, Safety, and Outcomes Study: AF Ablation in Pulmonary Vein Stumps. J. Cardiovasc. Electrophysiol. 2015, 26, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Shabtaie, S.A.; Luis, S.A.; Ward, R.C.; Karki, R.; Connolly, H.M.; Pellikka, P.A.; Kapa, S.; Asirvatham, S.J.; Packer, D.L.; DeSimone, C.V. Catheter Ablation in Patients With Neuroendocrine (Carcinoid) Tumors and Carcinoid Heart Disease. JACC Clin. Electrophysiol. 2021, 7, 151–160. [Google Scholar] [CrossRef]
- Giustozzi, M.; Ali, H.; Reboldi, G.; Balla, C.; Foresti, S.; de Ambroggi, G.; Lupo, P.P.; Agnelli, G.; Cappato, R. Safety of Catheter Ablation of Atrial Fibrillation in Cancer Survivors. J. Interv. Card Electrophysiol. 2021, 60, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Eitel, C.; Sciacca, V.; Bartels, N.; Saraei, R.; Fink, T.; Keelani, A.; Gaßmann, A.; Kuck, K.-H.; Vogler, J.; Heeger, C.-H.; et al. Safety and Efficacy of Cryoballoon Based Pulmonary Vein Isolation in Patients with Atrial Fibrillation and a History of Cancer. JCM 2021, 10, 3669. [Google Scholar] [CrossRef]
- Ganatra, S.; Abraham, S.; Parikh, R.; Kamenetsky, D.; Patel, R.; Dani, S.; Chaudhry, G.; Resnic, F.; Shah, S.; Venesy, D.; et al. Efficacy and Safety of Catheter Ablation for Atrial Fibrillation in Patients with Cancer. Eur. Heart J. 2020, 41 (Suppl. 2), ehaa946.3278. [Google Scholar] [CrossRef]
- Briceno, D.F.; Madan, N.; Villablanca, P.A.; Lupercio, F.; Cyrille, N.; Ramakrishna, H.; Di Biase, L. Periprocedural Anticoagulation for Catheter Ablation of Atrial Fibrillation: Practical Implications for Perioperative Management. J. Cardiothorac. Vasc. Anesth. 2017, 31, 1519–1526. [Google Scholar] [CrossRef]
- Santangeli, P.; Di Biase, L.; Horton, R.; Burkhardt, J.D.; Sanchez, J.; Al-Ahmad, A.; Hongo, R.; Beheiry, S.; Bai, R.; Mohanty, P.; et al. Ablation of Atrial Fibrillation Under Therapeutic Warfarin Reduces Periprocedural Complications: Evidence From a Meta-Analysis. Circ Arrhythmia Electrophysiol. 2012, 5, 302–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Biase, L.; Burkhardt, J.D.; Santangeli, P.; Mohanty, P.; Sanchez, J.E.; Horton, R.; Gallinghouse, G.J.; Themistoclakis, S.; Rossillo, A.; Lakkireddy, D.; et al. Periprocedural Stroke and Bleeding Complications in Patients Undergoing Catheter Ablation of Atrial Fibrillation With Different Anticoagulation Management: Results From the Role of Coumadin in Preventing Thromboembolism in Atrial Fibrillation (AF) Patients Undergoing Catheter Ablation (COMPARE) Randomized Trial. Circulation 2014, 129, 2638–2644. [Google Scholar] [CrossRef] [Green Version]
- Providencia, R.; Marijon, E.; Albenque, J.-P.; Combes, S.; Combes, N.; Jourda, F.; Hireche, H.; Morais, J.; Boveda, S. Rivaroxaban and Dabigatran in Patients Undergoing Catheter Ablation of Atrial Fibrillation. Europace 2014, 16, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Cappato, R.; Marchlinski, F.E.; Hohnloser, S.H.; Naccarelli, G.V.; Xiang, J.; Wilber, D.J.; Ma, C.-S.; Hess, S.; Wells, D.S.; Juang, G.; et al. Uninterrupted Rivaroxaban vs. Uninterrupted Vitamin K Antagonists for Catheter Ablation in Non-Valvular Atrial Fibrillation. Eur. Heart J. 2015, 36, 1805–1811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calkins, H.; Willems, S.; Gerstenfeld, E.P.; Verma, A.; Schilling, R.; Hohnloser, S.H.; Okumura, K.; Serota, H.; Nordaby, M.; Guiver, K.; et al. Uninterrupted Dabigatran versus Warfarin for Ablation in Atrial Fibrillation. N. Engl. J. Med. 2017, 376, 1627–1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Biase, L.; Callans, D.; Hæusler, K.G.; Hindricks, G.; Al-Khalidi, H.; Mont, L.; Cosedis Nielsen, J.; Piccini, J.P.; Schotten, U.; Kirchhof, P. Rationale and Design of AXAFA-AFNET 5: An Investigator-Initiated, Randomized, Open, Blinded Outcome Assessment, Multi-Centre Trial to Comparing Continuous Apixaban to Vitamin K Antagonists in Patients Undergoing Atrial Fibrillation Catheter Ablation. EP Eur. 2017, 19, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Hohnloser, S.H.; Camm, J.; Cappato, R.; Diener, H.-C.; Heidbüchel, H.; Mont, L.; Morillo, C.A.; Abozguia, K.; Grimaldi, M.; Rauer, H.; et al. Uninterrupted Edoxaban vs. Vitamin K Antagonists for Ablation of Atrial Fibrillation: The ELIMINATE-AF Trial. Eur. Heart J. 2019, 40, 3013–3021. [Google Scholar] [CrossRef]
- De Heide, J.; Vroegh, C.J.; Bhagwandien, R.E.; Wijchers, S.A.; Szili-Torok, T.; Zijlstra, F.; Lenzen, M.J.; Yap, S.C. Minimally Interrupted Novel Oral Anticoagulant versus Uninterrupted Vitamin K Antagonist during Atrial Fibrillation Ablation. J. Interv. Card Electrophysiol. 2018, 53, 341–346. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, K.; Naito, S.; Sasaki, T.; Take, Y.; Minami, K.; Kitagawa, Y.; Motoda, H.; Inoue, M.; Otsuka, Y.; Niijima, K.; et al. Uninterrupted vs. Interrupted Periprocedural Direct Oral Anticoagulants for Catheter Ablation of Atrial Fibrillation: A Prospective Randomized Single-Centre Study on Post-Ablation Thrombo-Embolic and Haemorrhagic Events. EP Eur. 2019, 21, 259–267. [Google Scholar] [CrossRef]
- Yanagisawa, S.; Inden, Y.; Fujii, A.; Ando, M.; Funabiki, J.; Murase, Y.; Takenaka, M.; Otake, N.; Ikai, Y.; Sakamoto, Y.; et al. Uninterrupted Direct Oral Anticoagulant and Warfarin Administration in Elderly Patients Undergoing Catheter Ablation for Atrial Fibrillation. JACC Clin. Electrophysiol. 2018, 4, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Romero, J.; Cerrud-Rodriguez, R.C.; Diaz, J.C.; Michaud, G.F.; Taveras, J.; Alviz, I.; Grupposo, V.; Cerna, L.; Avendano, R.; Kumar, S.; et al. Uninterrupted Direct Oral Anticoagulants vs. Uninterrupted Vitamin K Antagonists during Catheter Ablation of Non-Valvular Atrial Fibrillation: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. EP Eur. 2018, 20, 1612–1620. [Google Scholar] [CrossRef]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation. J. Am. Coll. Cardiol. 2019, 74, 104–132. [Google Scholar] [CrossRef]
- Cheng, W.-L.; Kao, Y.-H.; Chen, S.-A.; Chen, Y.-J. Pathophysiology of Cancer Therapy-Provoked Atrial Fibrillation. Int. J. Cardiol. 2016, 219, 186–194. [Google Scholar] [CrossRef]
- Reissmann, B.; Metzner, A.; Kuck, K.-H. Cryoballoon Ablation versus Radiofrequency Ablation for Atrial Fibrillation. Trends Cardiovasc. Med. 2017, 27, 271–277. [Google Scholar] [CrossRef]
- Calkins, H.; Kuck, K.H.; Cappato, R.; Brugada, J.; Camm, A.J.; Chen, S.-A.; Crijns, H.J.G.; Damiano, R.J.; Davies, D.W.; DiMarco, J.; et al. 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: Recommendations for Patient Selection, Procedural Techniques, Patient Management and Follow-up, Definitions, Endpoints, and Research Trial Design: A Report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in Partnership with the European Heart Rhythm Association (EHRA), a Registered Branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in Collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the Governing Bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Europace 2012, 14, 528–606. [Google Scholar] [CrossRef]
- Providencia, R.; Defaye, P.; Lambiase, P.D.; Pavin, D.; Cebron, J.-P.; Halimi, F.; Anselme, F.; Srinivasan, N.; Albenque, J.-P.; Boveda, S. Results from a Multicentre Comparison of Cryoballoon vs. Radiofrequency Ablation for Paroxysmal Atrial Fibrillation: Is Cryoablation More Reproducible? Europace 2016, euw080. [Google Scholar] [CrossRef] [PubMed]
| Reference | Study Design | Mean Age (year) | Male Sex (%) | AF Patients with History of Cancer and/or Active Cancer (%) | Type of Cancer | Efficacy Outcomes | Safety Outcomes | NOAC | Follow Up (Year) |
|---|---|---|---|---|---|---|---|---|---|
| Chen et al. (2019) [14] | Post-hoc analysis from ROCKET AF trial | 77 | 423 (66%) | 640 (%4.5) | Prostate (28.6%), breast (14.7%), colorectal (16.1%), gastrointestinal (3%), lung (3.1%), melanoma (5.9%), leukemia or lymphoma (5.2%), gynecological (6.6%), genitourinary (12.2%), head and neck (3.9%), thyroid (2.5%), brain (0.3%), others (3%), unspecified cancer type (3.9%) | Stroke or systemic embolism, ischemic stroke, hemorrhagic stroke, myocardial infarction, venous thromboembolism, all-cause death, cardiovascular death | Major bleeding *, intracranial bleeding, non-major clinically relevant (NMCR) bleeding, any bleeding | Rivaroxaban | 1.9 |
| Shah et al. (2018) [15] | Retrospective population-based cohort study | 74 | 2430 (40%) | 6075 (100%) | Breast (19.2%), gastrointestinal (12.7%), lung (12.3%), genitourinary (29.2%), gyneco-oncological (2.4%), hematological (9.8%), others (14.4%) | Ischemic stroke, venous thromboembolism | Severe bleeding (intracranial or gastrointestinal), other bleeding | Rivaroxaban, dabigatran, apixaban | 1.0 |
| Fanola et al. (2018) [16] | Post-hoc analysis from ENGAGE AF-TIMI 48 trial | 75 | 794 (68.9%) | 1153 (5.5%) | Prostate (13.7%), breast (6.5%), bladder (7.5%), gastrointestinal (20.5%), lung or pleura (11%), skin (5.9%), pancreatic (3.8%), liver, gallbladder, or bile ducts (3.8%), esophageal (2.5%), oropharyngeal (2.6%), renal (2.5%), uterine (2.1%), brain (2.1%), genital (1.3%), thyroid (1.1%), leukemia (2.8%), lymphoma (2.2%), others (1.3%), unspecified cancer type (1.5%) | Stroke or systemic embolism, ischemic stroke, myocardial infarction, all-cause death, cardiovascular death | Major bleeding *, gastrointestinal bleeding, NMCR bleeding, any bleeding | Edoxaban | 2.8 |
| Kim et al. (2018) [17] | Retrospective population-based cohort study | 72.4 | 267 (68.8%) | 388 (100%) | Stomach (20.6%), colorectal (14.9%), thyroid (10.8%), prostate (9.3%), lung (12.2%), melanoma (5.9%), biliary tract (5.4%), urinary tract (6.1%), genitourinary (12.2%), head and neck (4.1%), hepatocellular carcinoma (3.0%), breast (2.4%), ovary and endometrial (2.6%), renal cell carcinoma (3.1%), hematologic malignancy (2.2%), others (3.2%) | Stroke or systemic embolism, ischemic stroke, all-cause death | Major bleeding *, gastrointestinal bleeding, intracranial bleeding, other bleeding | Rivaroxaban, dabigatran, apixaban | 1.8 |
| Melloni et al. (2017) [18] | Post-hoc analysis from ARISTOTLE trial | 75 | 831 (67.2%) | 1236 (6.8%) | Bladder (7%), breast (16%), colon (11%), gastric (2%), lung (3%), melanoma (6%), others (10%), ovarian/uterus (6%), prostate (29%), rectal (3%), renal cell carcinoma (4%), Hodgkin’s lymphoma (1%), leukemia (<1%), lymphoma (1%), non-Hodgkin’s lymphoma (1%) | Stroke or systemic embolism, myocardial infarction, all-cause death | Major bleeding *, NMCR bleeding, any bleeding | Apixaban | 1.8 |
| Ording et al. 2017 [19] | Retrospective population-based cohort study | <65 (168) 65–74 (580)75–79 (336)≥80 (725) | 886 (49%) | 1809 (15.2%) | Urological (15%), breast cancer (12%), GI (12%), lung (4%), hematological (3%), intracranial (0.1%), other sites (54%) | Recurrence of ischemic stroke, VTE, other arterial embolism, or myocardial infarction | Diagnosis of hemorrhagic stroke or GI, lung, or urinary hemorrhage | Not referred | 1 |
| Flack et al. 2017 [20] | Post-hoc analysis from RE-LY trial | 76.4 | 22 (64.7%) | 34 (77.2%) | Not specified | Major bleeding * due to a GI cancer | Dabigatran | 2.2 | |
| Laube et al. 2017 [22] | Retrospective cohort study, single center | 72 | 92 (56%) | 163 (100%) | Lung (19%), hematologic (15%), GI (12%), genitourinary (11%), breast (10%), other (33%) | Stroke, systemic embolism | Death, CRNMB leading to discontinuation of the drug for at least 7 d Major bleeding * | Rivaroxaban | 2 |
| Russo et al. 2018 [23] | Retrospective cohort study, single center | 73.2 | 48 (63%) | 76 (100%) | Prostatic (22%), breast, (18%), colorectal (15%), gastric (3%), lung (8%), bladder (8%), kidney (4%), esophageal (3%), skin (4%), laryngeal (3%) | Ischemic stroke TIAd, Systemic embolism | Major bleeding *. All other bleedings were classified as minor | Dabigatran (37) Apixaban (21) Rivaroxaban (18) | 4 |
| Ianotto et al. 2017 [24] | Case–control study | 68.6 | 6 (46%) | 13 (1.7%) | Myeloproliferative neoplasm; | Any documented thrombosis | Major bleeding *. All other bleedings were classified as minor | Rivaroxaban (6) Apixaban (6) Switch from apixaban to rivaroxaban (1) | 2.1 |
| Study (year) | Mean Age (year) | Male n (%) | History of Cancer/Active Cancer n | Type of Tumor (%) | Type of Arrhythmia n (%) | Procedure Length (min) | Adverse Event (n) | Restoration of Sinus Rhythm after Ablation n | Follow Up (Year) |
|---|---|---|---|---|---|---|---|---|---|
| Kanmanthareddy et al. (2015) [63] | 63 ± 7 | 10 (100) | 10 | Not specified | Atrial fibrillation (AF) 15 (100) | 200 ± 33 | Groin hematoma (2) | 12 | 1 |
| Shabtaie et al. (2021) [64] | 62.4 ± 9.3 | 9 (53) | 17 | Neuroendocrine tumors (100) | Atrial flutter 2 (11.7) AF 4 (23.5) Atrioventricular nodal reentrant tachycardia 7 (41.2) Premature ventricular contractions 3 (17.6) Ventricular Tachycardia 1 (5.9) | 196.4 ± 108.5 | Deep venous thrombosis (1) Cardiac tamponade (1) access site bleeding A1) | 2 | 1.6 ± 2.2 |
| Giustozzi et al. (2021) [65] | 64.3 ± 7.5 | 14 (67) | 21 | Solid tumors (95.2) Haematologic tumor (4.8) | AF 21 (100) | Not specified | Clinically relevant bleedings (4) Peri-procedural thromboembolic event (1) | 13 | 0.08 ± 0.013 |
| Eitel et al. (2021) [66] | 71.3 ± 8.3 | 39 (55.7) | 70 | Genitourinary cancer (30), breast cancer (28.6), haemato-oncologic cancer (12.9), gastrointestinal cancer (11.4), head or neck cancer (5.7), lung cancer (2.9) | AF 7 (100), | 128.7 ± 36.1 | Phrenic nerve palsy (4) Pseudoaneurysm (2) | 47 * | 1.68 ± 0.97 |
| Ganatra et al. (2020) [67] | 65.5 | 81 (50) | 162 | Breast cancer (30.8) Other types of cancer (69.2) | AF 162 (100) | Not specified | Access site bleeding (5) Non-access site bleeding (4) strokes (2) Cardiac tamponade (2) pulmonary vein stenosis (1) | 133 * | Not specified |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garibaldi, S.; Chianca, M.; Fabiani, I.; Emdin, M.; Piacenti, M.; Passino, C.; Aimo, A.; Fedele, A.; Cipolla, C.M.; Cardinale, D.M. Treatment Options in AF Patients with Cancer; Focus on Catheter Ablation. J. Clin. Med. 2022, 11, 4452. https://doi.org/10.3390/jcm11154452
Garibaldi S, Chianca M, Fabiani I, Emdin M, Piacenti M, Passino C, Aimo A, Fedele A, Cipolla CM, Cardinale DM. Treatment Options in AF Patients with Cancer; Focus on Catheter Ablation. Journal of Clinical Medicine. 2022; 11(15):4452. https://doi.org/10.3390/jcm11154452
Chicago/Turabian StyleGaribaldi, Silvia, Michela Chianca, Iacopo Fabiani, Michele Emdin, Marcello Piacenti, Claudio Passino, Alberto Aimo, Antonella Fedele, Carlo Maria Cipolla, and Daniela Maria Cardinale. 2022. "Treatment Options in AF Patients with Cancer; Focus on Catheter Ablation" Journal of Clinical Medicine 11, no. 15: 4452. https://doi.org/10.3390/jcm11154452
APA StyleGaribaldi, S., Chianca, M., Fabiani, I., Emdin, M., Piacenti, M., Passino, C., Aimo, A., Fedele, A., Cipolla, C. M., & Cardinale, D. M. (2022). Treatment Options in AF Patients with Cancer; Focus on Catheter Ablation. Journal of Clinical Medicine, 11(15), 4452. https://doi.org/10.3390/jcm11154452






