Is Evar Feasible in Challenging Aortic Neck Anatomies? A Technical Review and Ethical Discussion
Abstract
:1. Introduction
2. Material and Methods
2.1. Search Strategy
2.2. Study Selection: Study Design and Data Extraction
2.3. Outcomes
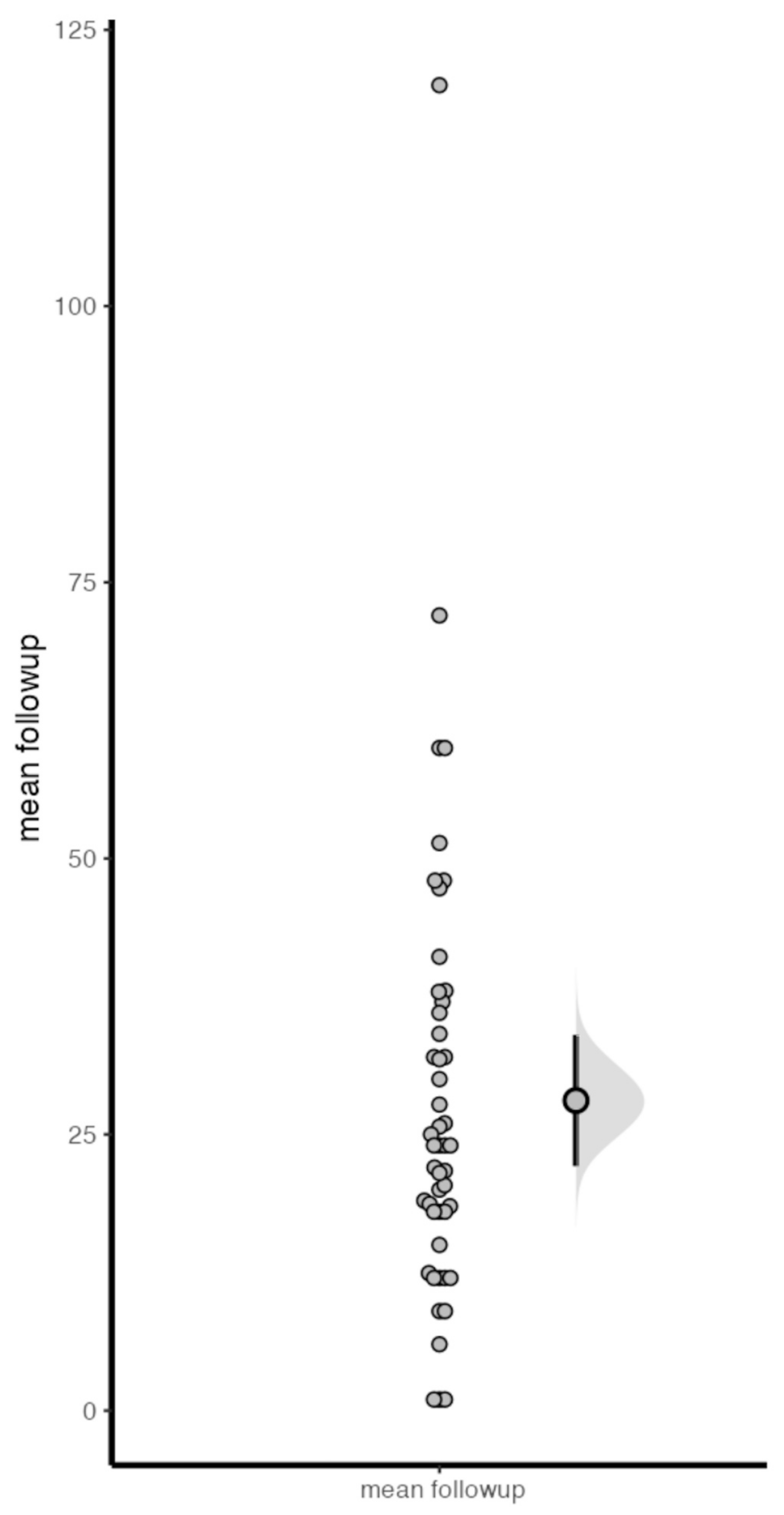
2.4. Statistical Analysis
3. Results
3.1. Study Characteristics
3.2. Technical Success and Adjunctive Procedures
3.3. Thirty-Day Results
3.4. Mid-Term Follow-Up Results
4. Discussion
4.1. Ethical Considerations
4.2. Study Limitations
4.3. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, S.; Hicks, C.W.; Malas, M.B. Neck diameter and inner curve seal zone predict endograft-related complications in highly angulated necks after endovascular aneurysm repair using the Aorfix endograft. J. Vasc. Surg. 2018, 67, 760–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kansagra, K.; Kang, J.; Taon, M.; Ganguli, S.; Gandhi, R.; Vatakencherry, G.; Lam, C. Advanced endografting techniques: Snorkels, chimneys, periscopes, fenestrations, and branched endografts. Cardiovasc. Diagn. Ther. 2018, 8 (Suppl. 1), 175–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kouvelos, G.N.; Oikonomou, K.; Antoniou, G.A.; Verhoeven, E.L.; Katsargyris, A. A systematic review of proximal neck dilatation after endovascular repair for abdominal aortic aneurysm. J. Endovasc. Ther. 2017, 24, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Igari, K.; Kudo, T.; Toyofuku, T.; Jibiki, M.; Inoue, Y. Outcomes following endovascular abdominal aortic aneurysm repair both within and outside of the instructions for use. Ann. Thorac. Cardiovasc. Surg. 2014, 20, 61–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giménez-Gaibar, A.; González-Cañas, E.; Solanich-Valldaura, T.; Herranz-Pinilla, C.; Rioja-Artal, S.; Ferraz-Huguet, E. Could Preoperative Neck Anatomy Influence Follow-up of EVAR? Ann. Vasc. Surg. 2017, 43, 127–133. [Google Scholar] [CrossRef]
- Caradu, C.; Berard, X.; Midy, D.; Ducasse, E. Influence of anatomic angulations in chimney and fenestrated endovascular aneurysm repair. Ann. Vasc. Surg. 2017, 43, 104–114. [Google Scholar] [CrossRef]
- Speziale, F.; Sirignano, P.; Setacci, F.; Menna, D.; Capoccia, L.; Mansour, W.; Galzerano, G.; Satacci, C. Immediate and two year outcomes after EVAR in ‘‘on-label’’ and ‘‘off-label’’ neck anatomies using different commercially available devices. Analysis of the experience of two Italian vascular centers. Ann. Vasc. Surg. 2014, 28, 1892–1900. [Google Scholar] [CrossRef] [PubMed]
- Muhs, B.E.; Jordan, W.; Ouriel, K.; Rajaee, S.; de Vries, J.P. Matched cohort comparison of endovascular abdominal aortic aneurysm repair with and without EndoAnchors. J. Vasc. Surg. 2018, 67, 1699–1707. [Google Scholar] [CrossRef] [PubMed]
- Troisi, N.; Torsello, G. New-generation devices and adjunctive procedures are the key elements to expanding the indications for endovascular aneurysm repair. J. Endovasc. Ther. 2015, 22, 179–181. [Google Scholar] [CrossRef]
- Aburahma, A.F.; Derderian, T.; Aburahma, Z.T.; Hass, S.M.; Yacoub, M.; Dean, L.S.; Abu-Halimah, S.; Mousa, A.Y. Comparative study of clinical outcome of endovascular aortic aneurysms repair in large diameter aortic necks (>31 mm) versus smaller necks. J. Vasc. Surg. 2018, 68, 1345–1353.e1. [Google Scholar] [CrossRef]
- Schuurmann, R.C.L.; Van Noort, K.; Overeem, S.P.; Ouriel, K.; Jordan, J.W.D.; Muhs, B.E.; Mannetje, Y.; Reijnen, M.; Fioole, B.; Ünlü, C.; et al. Aortic curvature is a predictor of late type Ia endoleak and migration after endovascular aneurysm repair. J. Endovasc. Ther. 2017, 24, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, G.A.; Georgiadis, G.S.; Antoniou, S.A.; Kuhan, G.; Murray, D. A metaanalysis of outcomes of endovascular abdominal aortic aneurysm repair in patients with hostile and friendly neck anatomy. J. Vasc. Surg. 2013, 57, 527–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brownrigg, J.R.W.; De Bruin, J.L.; Rossi, L.; Karthikesalingam, A.; Patterson, B.; Holt, P.; Hinchliffe, R.; Morgan, R.; Loftus, I.; Thompson, M. Endovascular aneurysm sealing for infrarenal abdominal aortic aneurysms: 30-day outcomes of 105 patients in a single centre. Eur. J. Vasc. Endovasc. Surg. 2015, 50, 157–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoretsanitis, N.; Argyriou, C.; Georgiadis, G.S.; Lazaridis, M.K.; Georgakarakos, E. Hostile neck in abdominal aortic aneurysms: Does it still exist? Vasc. Endovasc. Surg. 2016, 50, 208–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, N.F.G.; Goncalves, F.B.; Ultee, K.; Pinto, J.P.; van Rijn, M.J.; Raa, S.T.; Mwipatayi, B.P.; Böckler, D.; Hoeks, S.E.; Verhagen, H.J. Patients with large neck diameter have a higher risk of type IA endoleaks and aneurysm rupture after standard endovascular aneurysm repair. J. Vasc. Surg. 2019, 69, 783–791. [Google Scholar] [CrossRef]
- Wanhainen, A.; Verzini, F.; Van Herzeele, I.; Allaire, E.; Bown, M.; Cohnert, T.; Dick, F.; van Herwaarden, J.; Karkos, C.; Koelemay, M.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 8–93, Erratum in Eur. J. Vasc. Endovasc. Surg. 2020, 59, 494. [Google Scholar] [CrossRef] [Green Version]
- Chaikof, E.L.; Dalman, R.L.; Eskandari, M.K.; Jackson, B.M.; Lee, W.A.; Mansour, M.A.; Mastracci, T.M.; Mell, M.; Murad, M.H.; Nguyen, L.L.; et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J. Vasc. Surg. 2018, 67, 2–77. [Google Scholar] [CrossRef] [Green Version]
- The National Institute for Health and Care Excellence (NICE). Abdominal Aortic Aneurysm: Diagnosis and Management [Nice Guideline 156]. Published 19 March 2020. Available online: https://www.nice.org.uk/guidance/ng156 (accessed on 25 July 2022).
- Pratesi, C.; Esposito, D.; Apostolou, D.; Attisani, L.; Bellosta, R.; Benedetto, F.; Blangetti, I.; Bonardelli, S.; Casini, A.; Fargion, A.T.; et al. Italian Guidelines for Vascular Surgery Collaborators—AAA Group. Guidelines on the management of abdominal aortic aneurysms: Updates from the Italian Society of Vascular and Endovascular Surgery (SICVE). J. Cardiovasc. Surg. 2022, 63, 328–352. [Google Scholar] [CrossRef]
- Setacci, C.; Sirignano, P.; Fineschi, V.; Frati, P.; Ricci, G.; Speziale, F. Clinical and ethical review on late results and benefits after EVAR. Ann. Med. Surg. 2017, 16, 1–6. [Google Scholar] [CrossRef]
- Albertini, J.N.; Kalliafas, S.; Travis, S.; Yusuf, S.W.; Macierewicz, J.A.; Whitaker, S.C.; Elmarasy, N.M.; Hopkinson, B.R. Anatomical risk factors for proximal perigraft endoleak and graft migration following endovascular repair of abdominal aortic aneurysms. Eur. J. Vasc. Endovasc. Surg. 2000, 19, 308–312. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.A.; Benaragama, K.S.; Pope, T.; Coughlin, P.A.; Winterbottom, A.P.; Harrison, S.C.; Boyle, J.R. Progressive Device Failure at Long Term Follow Up of the Nellix EndoVascular Aneurysm Sealing (EVAS) System. Eur. J. Vasc. Endovasc. Surg. 2021, 61, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Pereson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 6 May 2021).
- Dillavou, E.D.; Muluk, S.C.; Rhee, R.Y.; Tzeng, E.; Woody, J.D.; Gupta, N.; Makaroun, M.S. Does hostile neck anatomy preclude successful endovascular aortic aneurysm repair? J. Vasc. Surg. 2003, 38, 657–663. [Google Scholar] [CrossRef] [Green Version]
- Fairman, R.M.; Velazquez, O.C.; Carpenter, J.P.; Woo, E.; Baum, R.A.; Golden, M.A.; Kritpracha, B.; Criado, F. Midterm pivotal trial results of the Talent Low Profile System for repair of abdominal aortic aneurysm: Analysis of complicated versus uncomplicated aortic necks. J. Vasc. Surg. 2004, 40, 1074–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choke, E.; Munneke, G.; Morgan, R.; Belli, A.-M.; Loftus, I.; McFarland, R.; Loosemore, T.; Thompson, M.M. Outcomes of endovascular abdominal aortic aneurysm repair in patients with hostile neck anatomy. Cardiovasc. Intervent. Radiol. 2006, 29, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.E.; Jacobs, D.L.; Motaganahalli, R.L.; Wittgen, C.M.; Peterson, G.J. Outcomes of endovascular AAA repair in patients with hostile neck anatomy using adjunctive balloon-expandable stents. Vasc. Endovasc. Surg. 2006, 40, 35–40. [Google Scholar] [CrossRef]
- McDonnell, C.O.; Halak, M.; Bartlett, A.; Baker, S.R. Abdominal aortic aneurysm neck morphology: Proposed classification system. Ir. J. Med. Sci. 2006, 175, 4–8. [Google Scholar] [CrossRef]
- Abbruzzese, T.A.; Kwolek, C.J.; Brewster, D.C.; Chung, T.K.; Kang, J.; Conrad, M.F.; LaMuraglia, G.M.; Cambria, R.P. Outcomes following endovascular abdominal aortic aneurysm repair (EVAR): An anatomic and device-specific analysis. J. Vasc. Surg. 2008, 48, 19–28. [Google Scholar] [CrossRef] [Green Version]
- Chisci, E.; Kristmundsson, T.; de Donato, G.; Resch, T.; Setacci, F.; Sonesson, B.; Setacci, C.; Malina, M. The AAA with a challenging neck: Outcome of open versus endovascular repair with standard and fenestrated stent-grafts. J. Endovasc. Ther. 2009, 16, 137–146. [Google Scholar] [CrossRef]
- Jim, J.; Rubin, B.G.; Geraghty, P.J.; Criado, F.J.; Fajardo, A.; Sanchez, L.A. A 5-year comparison of EVAR for large and small aortic necks. J. Endovasc. Ther. 2010, 17, 575–584. [Google Scholar] [CrossRef]
- Troisi, N.; Torsello, G.; Donas, K.P.; Austermann, M. Endurant stent-graft: A 2-year, single-center experience with a new commercially available device for the treatment of abdominal aortic aneurysms. J. Endovasc. Ther. 2010, 17, 439–448. [Google Scholar] [CrossRef]
- AbuRahma, A.F.; Campbell, J.E.; Mousa, A.Y.; Hass, S.M.; Stone, P.A.; Jain, A.; Nanjundappa, A.; Dean, L.S.; Keiffer, T.; Habib, J. Clinical outcomes for hostile versus favorable aortic neck anatomy in endovascular aortic aneurysm repair using modular devices. J. Vasc. Surg. 2011, 54, 13–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgiadis, G.S.; Trellopoulos, G.; Antoniou, G.A.; Gallis, K.; Nikolopoulos, E.S.; Kapoulas, K.C.; Pitta, X.; Lazarides, M.K. Early results of the Endurant endograft system in patients with friendly and hostile infrarenal abdominal aortic aneurysm anatomy. J. Vasc. Surg. 2011, 54, 616–627. [Google Scholar] [CrossRef] [Green Version]
- Hoshina, K.; Kato, M.; Hosaka, A.; Miyahara, T.; Mikuriya, A.; Ohkubo, N.; Miyata, T. Middle-term results of endovascular aneurysm repair in Japan: Does intraoperative endovascular management against the hostile aneurysmal neck prevent the proximal type I endoleak? Int. Angiol. 2011, 30, 467–473. [Google Scholar]
- Hyhlik-Durr, A.; Weber, T.F.; Kotelis, D.; Rengier, F.; Gahlen, J.; Bock, S.; Köhler, J.; Ratusinski, C.-M.; Böckler, D. The Endurant stent graft system: 15-month follow-up report in patients with challenging abdominal aortic anatomies. Langenbecks Arch. Surg. 2011, 396, 801–810. [Google Scholar] [CrossRef]
- Rouwet, E.V.; Torsello, G.; de Vries, J.P.P.M.; Cuypers, P.; van Herwaarden, J.; Eckstein, H.-H.; Beuk, R.; Florek, H.-J.; Jentjens, R.; Verhagen, H. Final results of the prospective European trial of the Endurant Stent Graft for endovascular abdominal aortic aneurysm repair. Eur. J. Vasc. Endovasc. Surg. 2011, 42, 489–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torsello, G.; Troisi, N.; Donas, K.; Austermann, M. Evaluation of the Endurant stent graft under instructions for use vs off-label conditions for endovascular aortic aneurysm repair. J. Vasc. Surg. 2011, 54, 300–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Keulen, J.W.; de Vries, J.P.; Dekker, H.; Gonçalves, F.B.; Moll, F.L.; Verhagen, H.J.; van Herwaarden, J.A. One-year multicenter results of 100 abdominal aortic aneurysm patients treated with the Endurant stent graft. J. Vasc. Surg. 2011, 54, 609–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.; Lee, D.Y.; Kim, M.D.; Lee, K.-H.; Lee, M.S.; Park, S.I.; Won, J.Y.; Choi, D.; Ko, Y.-G. Coupling bifurcated stent-grafts to overcome anatomic limitations of endovascular repair of abdominal aortic aneurysms. J. Vasc. Interv. Radiol. 2012, 23, 1065–1069. [Google Scholar] [CrossRef] [PubMed]
- Hager, E.S.; Cho, J.S.; Makaroun, M.S.; Park, S.C.; Chaer, R.; Marone, L.; Rhee, R.Y. Endografts with suprarenal fixation do not perform better than those with infrarenal fixation in the treatment of patients with short straight proximal aortic necks. J. Vasc. Surg. 2012, 55, 1242–1246. [Google Scholar] [CrossRef] [Green Version]
- Kvinlaug, K.E.; Lawlor, D.K.; Forbes, T.L.; Willoughby, R.; MacKenzie, K.S.; DeRose, G.; Corriveau, M.M.; Steinmetz, O.K. Early results from a Canadian multicenter prospective registry of the Endurant stent graft for endovascular treatment of abdominal aortic aneurysms. J. Endovasc. Ther. 2012, 19, 58–66. [Google Scholar] [CrossRef]
- Setacci, F.; Sirignano, P.; de Donato, G.; Chisci, E.; Iacoponi, F.; Galzerano, G.; Palasciano, G.; Cappelli, A. AAA with a challenging neck: Early outcomes using the Endurant stent-graft system. Eur. J. Vasc. Endovasc. Surg. 2012, 44, 274–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stather, P.W.; Sayers, R.D.; Cheah, A.; Wild, J.; Bown, M.; Choke, E. Outcomes of endovascular aneurysm repair in patients with hostile neck anatomy. Eur. J. Vasc. Endovasc. Surg. 2012, 44, 556–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antoniou, G.A.; Georgiadis, G.S.; Glancz, L.; Delbridge, M.; Murray, D.; Smyth, J.V.; Lazarides, M.K.; Serracino-Inglott, F. Outcomes of endovascular aneurysm repair with 2 different endograft systems with suprarenal fixation in patients with hostile infrarenal aortic anatomy. Vasc. Endovasc. Surg. 2013, 47, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Mwipatayi, B.P.; Picardo, A.; Wong, J.; Thomas, S.D.; Vijayan, V. Endovascular repair of abdominal aortic aneurysms with reverse taper neck anatomy using the Endurant stent-graft: Analysis of stent-graft oversizing. J. Endovasc. Ther. 2013, 20, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Shintani, T.; Mitsuoka, H.; Atsuta, K.; Saitou, T.; Higashi, S. Thromboembolic complications after endovascular repair of abdominal aortic an-eurysm with neck thrombus. Vasc. Endovasc. Surg. 2013, 47, 172–178. [Google Scholar] [CrossRef]
- Ierardi, A.M.; Tsetis, D.; Ioannou, C.; Laganà, D.; Floridi, C.; Petrillo, M.; Pinto, A.; Re, G.L.; Carrafiello, G. Ultra-low profile polymer-filled stent graft for abdominal aortic aneurysm treatment: A two-year follow-up. Radiol. Med. 2015, 120, 542–548. [Google Scholar] [CrossRef]
- Iwakoshi, S.; Ichihashi, S.; Higashiura, W.; Itoh, H.; Sakaguchi, S.; Tabayashi, N.; Uchida, H.; Kichikawa, K. A decade of outcomes and predictors of sac enlargement after endovascular abdominal aortic aneurysm repair using zenith endografts in a Japanese population. J. Vasc. Interv. Radiol. 2014, 25, 694–701. [Google Scholar] [CrossRef]
- Setacci, F.; Sirignano, P.; de Donato, G.; Galzerano, G.; Messina, G.; Guerrini, S.; A Mazzei, M.; Setacci, C. Two-year-results of Endurant stent-graft in challenging aortic neck morphologies versus standard anatomies. J. Cardiovasc. Surg. 2014, 55, 85–92. [Google Scholar]
- Kaladji, A.; Steintmetz, E.; Gouëffic, Y.; Bartoli, M.; Cardon, A. Academic Association for Surgical Research (AURC). Long-Term Results of Large Stent Grafts to Treat Abdominal Aortic Aneurysms. Ann. Vasc. Surg. 2015, 29, 1416–1425. [Google Scholar] [CrossRef]
- Saha, P.; Hughes, J.; Patel, A.S.; Donati, T.; Sallam, M.; Patel, S.D.; Bell, R.E.; Katsanos, K.; Modarai, B.; Zayed, H.A. Medium-term outcomes following endovascular repair of infrarenal abdominal aortic an-eurysms with an infavourable proximal neck. Cardiovasc. Interv. Radiol. 2015, 38, 840–845. [Google Scholar] [CrossRef]
- Cerini, P.; Guzzardi, G.; Divenuto, I.; Parziale, G.; Brustia, P.; Carriero, A.; Fossaceca, R. Are abdominal aortic aneurysms with hostile neck really unsuitable for EVAR? Our experience. Radiol. Med. 2016, 121, 528–535. [Google Scholar] [CrossRef] [PubMed]
- De Donato, G.; Setacci, F.; Bresadola, L.; Castelli, P.; Chiesa, R.; Mangialardi, N.; Nano, G.; Setacci, C.; Ricci, C.; Gasparini, D.; et al. TriVascular Ovation Italian Study. Aortic neck evolution after endovascular repair with TriVascular Ovation stent graft. J. Vasc. Surg. 2016, 63, 8–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallitto, E.; Gargiulo, M.; Freyrie, A.; Massoni, C.B.; Pini, R.; Mascoli, C.; Faggioli, G.; Stella, A. Results of standard suprarenal fixation endografts for abdominal aortic aneurysms with neck length ≤10 mm in high-risk patients unfit for open repair and fenestrated endograft. J. Vasc. Surg. 2016, 64, 563–570.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirignano, P.; Capoccia, L.; Menna, D.; Mansour, W.; Speziale, F. Pushing forward the limits of EVAR: New therapeutic solutions for extremely chal-lenging AAAs using the Ovation® stent-graft. J. Cardiovasc. Surg. 2016, 57, 839–845. [Google Scholar]
- De Donato, G.; Setacci, F.; Bresadola, L.; Castelli, P.; Chiesa, R.; Mangialardi, N.; Nano, G.; Setacci, C. Midterm Results of Proximal Aneurysm Sealing with the Ovation Stent-Graft Ac-cording to On- vs Off-Label Use. J. Endovasc. Ther. 2017, 24, 191–197. [Google Scholar] [CrossRef] [Green Version]
- Gargiulo, M.; Gallitto, E.; Wattez, H.; Wattez, H.; Verzini, F.; Massoni, C.B.; Loschi, D.; Freyrie, A.; Haulon, S. Outcomes of endovascular aneurysm repair performed in abdominal aortic aneurysms with large infrarenal necks. J. Vasc. Surg. 2017, 66, 1065–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kontopodis, N.; Papadopoulos, G.; Galanakis, N.; Tsetis, D.; Ioannou, C.V. Improvement of patient eligibility with the use of new generation endo-grafts for the treatment of abdominal aortic aneurysms. A comparison study among currently used endografts and literature review. Expert Rev. Med. Devices 2017, 14, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, K.H. Endovascular Aneurysm Repair in Patients with Conical Neck Anatomy. Vasc. Spec. Int. 2017, 33, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Pitoulias, G.A.; Valdivia, A.R.; Hahtapornsawan, S.; Torsello, G.; Pitoulias, A.G.; Austermann, M.; Gandarias, C.; Donas, K.P. Conical neck is strongly associated with proximal failure in standard endovascular aneurysm repair. J. Vasc. Surg. 2017, 66, 1686–1695. [Google Scholar] [CrossRef] [Green Version]
- Sirignano, P.; Mansour, W.; Pranteda, C.; Siani, A.; Accrocca, F.; D’Adamo, A.; Capoccia, L.; Speziale, F. Real-Life Experience with Ovation Stent Graft:Lesson Learned from the First One Hundred Fifty Treated Patients. Ann. Vasc. Surg. 2017, 45, 253–261. [Google Scholar] [CrossRef]
- Reyes Valdivia, A.; Pitoulias, G.; Criado, F.J.; Torsello, G.; Gandarias, C.; Austermann, M.; Pitoulias, A.G.; Donas, K. Multicenter European Registry for Patients with AAA Undergoing EVAR Evaluating the Performance of the 36-mm-Diameter Endurant Stent-Graft. Cardiovasc. Intervent. Radiol. 2017, 40, 1514–1521. [Google Scholar] [CrossRef] [PubMed]
- Bryce, Y.; Kim, W.; Katzen, B.; Benenati, J.; Samuels, S. Outcomes over Time in Patients with Hostile Neck Anatomy Undergoing Endovascular Repair of Abdominal Aortic Aneurysm. J. Vasc. Interv. Radiol. 2018, 29, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Greaves, N.S.; Moore, A.; Seriki, D.; Ghosh, J. Outcomes of Endovascular Aneurysm Repair using the Ovation Stent Graft System in Adverse Anatomy. Eur. J. Vasc. Endovasc. Surg. 2018, 55, 512–517. [Google Scholar] [CrossRef] [Green Version]
- Howard, D.P.J.; Marron, C.D.; Sideso, E.; Puckridge, P.J.; Verhoeven EL, G.; Spark, J.I. Editor’s choice—Influence of proximal aortic neck diameter on durability of an-eurysm sealing and overall survival in patients undergoing endovascular aneurysm repair. Real world data from the Gore Global Registry for Endovascular Aortic Treatment (GREAT). Eur. J. Vasc. Endovasc. Surg. 2018, 56, 189–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, M.; Wang, Y.; Ding, Y.; Cai, L.; Lin, C.; Shi, Z.; Fu, W. Prognostic Nomogram for Patients with Hostile Neck Anatomy after Endovascular Ab-dominal Aortic Aneurysm Repair. Ann. Vasc. Surg. 2019, 56, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Kouvelos, G.N.; Spanos, K.; Nana, P.; Koutsias, S.; Rousas, N.; Giannoukas, A.; Matsagkas, M. Large Diameter (≥29 mm) Proximal Aortic Necks Are Associated with Increased Complication Rates after Endovascular Repair for Abdominal Aortic Aneurysm. Ann. Vasc. Surg. 2019, 60, 70–75. [Google Scholar] [CrossRef] [PubMed]
- McFarland, G.; Tran, K.; Virgin-Downey, W.; Sgroi, M.D.; Chandra, V.; Mell, M.W.; Harris, E.J.; Dalman, R.L.; Lee, J.T. Infrarenal endovascular aneurysm repair with large device (34- to 36-mm) diameters is associated with higher risk of proximal fixation failure. J. Vasc. Surg. 2019, 69, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Sirignano, P.; Mansour, W.; Capoccia, L.; Cuozzo, S.; Camparini, S.; de Donato, G.; Mangialardi, N.; Ronchey, S.; Talarico, F.; Setacci, C.; et al. Endovascular aortic repair in patients with challenging anatomies: The EXTREME study. EuroIntervention 2021, 16, e1544–e1550. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.R.; Ormesher, D.C.; Griffin, R.; Jackson, R.J.; Lip, G.Y.; Vallabhaneni, S.R. UK-COMPASS Trial. Editor’s Choice—Comparison of Open, Standard, and Complex Endovascular Aortic Repair Treatments for Juxtarenal/Short Neck Aneurysms: A Systematic Review and Network Meta-Analysis. Eur. J. Vasc. Endovasc. Surg. 2022, 63, 696–706. [Google Scholar] [CrossRef]
- Berman, L.; Curry, L.; Gusberg, R.; Dardik, A.; Fraenkel, L. Informed consent for abdominal aortic aneurysm repair: The patient’s perspective. J. Vasc. Surg. 2008, 48, 296–302.e1. [Google Scholar] [CrossRef] [Green Version]
- Bulder, R.M.A.; Hamming, J.F.; van Schaik, J.; Lindeman, J.H. Towards Patient Centred Outcomes for Elective Abdominal Aortic Aneurysm Repair: A Scoping Review of Quality of Life Scales. Eur. J. Vasc. Endovasc. Surg. 2021, 62, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Spatz, E.S.; Krumholz, H.M.; Moulton, B.W. The New Era of Informed Consent: Getting to a Reasonable-Patient Standard Through Shared Decision Making. JAMA 2016, 315, 2063–2064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powell, J.T.; Ambler, G.K.; Svensjö, S.; Wanhainen, A.; Bown, M.J. Beyond the AAA Guidelines: Core Outcome Sets to Make Life Better for Patients. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 6–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braithwaite, B.; Greenhalgh, R.M.; Grieve, R.; Hassan, T.B.; Hinchliffe, R.; Howell, S.; Moore, F.; Nicholson, A.A.; Soong, C.V.; Thompson, M.M.; et al. Endovascular strategy or open repair for ruptured abdominal aortic aneurysm: One-year outcomes from the IMPROVE randomized trial. Eur. Heart J. 2015, 36, 2061–2069. [Google Scholar]
- Beauchamp, T.; Childress, J. Principles of Biomedical Ethics, 5th ed.; Oxford University Press: New York, NY, USA, 2001. [Google Scholar]
- Marone, E.M.; Freyrie, A.; Ruotolo, C.; Michelagnoli, S.; Antonello, M.; Speziale, F.; Veroux, P.; Gargiulo, M.; Gaggiano, A. Expert Opinion on Hostile Neck Definition in Endovascular Treatment of Abdominal Aortic Aneurysms (a Delphi Consensus). Ann. Vasc. Surg. 2020, 62, 173–182. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.E.; Siegler, M.; Angelos, P. Ethical issues in surgical innovation. World J. Surg. 2014, 38, 1638–1643. [Google Scholar] [CrossRef]
- Convie, L.J.; Carson, E.; McCusker, D.; McCain, R.S.; McKinley, N.; Campbell, W.J.; Kirk, S.J.; Clarke, M. The patient and clinician experience of informed consent for surgery: A systematic review of the qualitative evidence. BMC Med. Ethics 2020, 21, 58. [Google Scholar] [CrossRef]
- Whitney, S.N.; McGuire, A.L.; McCullough, L.B. A typology of shared decision making, informed consent, and simple consent. Ann. Intern. Med. 2004, 140, 54–59. [Google Scholar] [CrossRef]
- Tunzi, M.; Satin, D.J.; Day, P.G. The Consent Continuum: A New Model of Consent, Assent, and Nondissent for Primary Care. Hastings Cent. Rep. 2021, 51, 33–40. [Google Scholar] [CrossRef]
- Berman, L.; Curry, L.; Goldberg, C.; Gusberg, R.; Fraenkel, L. Pilot testing of a decision support tool for patients with abdominal aortic aneurysms. J. Vasc. Surg. 2011, 53, 285–292.e1. [Google Scholar] [CrossRef] [Green Version]
- Knops, A.M.; Goossens, A.; Ubbink, D.T.; Balm, R.; Koelemay, M.; Vahl, A.; de Nie, A.; Akker, P.V.D.; Willems, M.; Koedam, N.; et al. A decision aid regarding treatment options for patients with an asymptomatic abdominal aortic aneurysm: A randomised clinical trial. Eur. J. Vasc. Endovasc. Surg. 2014, 48, 276–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowers, N.; Eisenberg, E.; Montbriand, J.; Jaskolka, J.; Roche-Nagle, G. Using a multimedia presentation to improve patient understanding and sat-isfaction with informed consent for minimally invasive vascular procedures. Surgeon 2017, 15, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Kinnersley, P.; Phillips, K.; Savage, K.; Kelly, M.J.; Farrell, E.; Morgan, B.; Whistance, R.; Lewis, V.; Mann, M.K.; Stephens, B. Interventions to promote informed consent for patients undergoing surgical and other invasive healthcare procedures. Cochrane Database Syst. Rev. 2013, 2013, CD009445. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. WMA Declaration of Lisbon on the Rights of the Patient; World Medical Association: Ferney-Voltaire, France, 2015. [Google Scholar]

| First Author | Year of Publication | Country | Study Period | Number of Patients | Mean Follow-Up (Month) | Hostile Neck Anatomy Definition | Implanted Endografts | Mean Age (Years) | Technical Success (%) | Need for Adjunctive Procedures (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Dillavou ED [24] | 2003 | United States of America | 1999–2002 | 91 | 18 | short neck <10 mm; bulge: focal enlargement of the aneurysm neck; reverse taper: dilation >2 mm within the first 10 mm; angulated neck >60 degrees; significant neck thrombus covering > 50% of the circumference | Ancure | 75.7 | 95.61 | 4.39 |
| Fairman RM [25] | 2004 | United States of America | NA | 153 | 21 | short neck: <15 mm; very short neck: <10 mm, dilated neck >28 mm, angulated neck > 45 degrees; calcified, and thrombus-lined, with or without ulceration | Talent | NA | NA | NA |
| Choke E [26] | 2006 | United Kingdom | 1997–2005 | 60 | 21.7 | short neck:< 10 mm; wide neck: >28 mm; angulated neck: >60 degrees; significant neck thrombus covering > 50% of the circumference | AneuRX, Excluder, Zenith, Talent, Fortron, Endofit, Vanguard, Lifepath | 74.4 | 98 | 18 |
| Cox DE [27] | 2006 | United States of America | 2000–2004 | 19 | 12 | short neck: <15 mm; wide neck: >26 mm; angulated neck: >60 degrees; circumferential neck thrombus; neck bulge; reverse taper neck: dilated >2 mm within 10 mm | Zenith, Aneurx | 72 | 100 | 15.78 |
| Mc Donnell CO [28] | 2006 | Australia | 2001–2004 | 46 | 20.2 | Flared neck; Barrel neck; cone neck; irregular neck; hourglass neck | Talent, Zenith | NA | NA | NA |
| Abbruzzese TA [29] | 2008 | United States of America | 1999–2005 | 222 | 29.6 | any deviation from single-device IFU | Zenith, Excluder, AneuRX | NA | NA | NA |
| Chisci E [30] | 2009 | Italy, Sweden | 2005–2007 | 74 | 19 | neck diameter >28 mm; neck length <15 mm, neck angulation >60 degrees; reverse, tapered or bulging neck, circumferential neck thrombus >50% | Talent, Zenith | 77.5 | 95.9 | 41.9 |
| Jim J [31] | 2010 | United States of America | 2002–2003 | 53 | 60 | wide neck: diameter >28 mm; angulated neck: >60 degrees; short neck: length <15 mm; significant thrombus: >50% of neck circumference; reverse tapered neck: neck dilated >2 mm within 10 mm; neck bulge: focal neck enlargement >3 mm within 15 mm | Talent | 76.5 | 96.2 | 0 |
| Troisi N [32] | 2010 | Germany | 2007–2009 | 106 | 9 | short neck: <10 mm; neck bulge: focal dilatation >3 mm within 15 mm; tapered neck: enlargement >2 mm within 10 mm; angulated neck: >60 degrees; neck thrombus: >50% of neck circumference | Endurant | 73.6 | 100 | 9 |
| Aburahma AF [33] | 2011 | USA | 2004–2010 | 149 | 22 | short neck: <10 mm; angulated neck: >60 degrees; wide diameter: >28 mm; calcified neck: >50% of neck circumference; neck thrombus: >2 mm thick; reverse tapered neck: neck dilatation >2 mm within the first 10 mm | AneuRX, Excluder, Zenith, Talent | 74.3 | 99 | 22 |
| Georgiadis GS [34] | 2011 | Greece | 2009–2010 | 34 | 12.44 | neck length between 5 and 12 mm; neck angulation between 60 and 90 degrees | Endurant | 72.8 | 100 | 8.8 |
| Hoshina K [35] | 2011 | Japan | 2006–2008 | 49 | 26 | short neck: <15 mm; angulated neck: >60 degrees | Zenith, Excluder | NA | NA | 51 |
| Hyhlik-Dürr A [36] | 2011 | Germany | 2008–2009 | 50 | 15 | short neck: <15 mm | Endurant | 75 | 96 | 4 |
| Rouwet EV [37] | 2011 | International | 2007–2008 | 80 | 12 | infrarenal angulation >60 degrees | Endurant | 76 | 100 | 0 |
| Torsello G [38] | 2011 | Germany | 2007–2010 | 56 | 12 | short neck: <10 mm; angulated neck: >60 degrees | Endurant | 75.3 | 100 | 1.8 |
| Van Keulen JW [39] | 2011 | The Netherlands | 2007–2009 | 19 | 12 | any deviation from single-device IFU | Endurant | 73 | 100 | 0 |
| Lee M [40] | 2012 | Republic of Korea | 2007–2010 | 19 | 18.7 | angulated neck: >60 degrees; conical neck: diameter at 15 mm below the lowest renal artery >10% larger than the diameter at the lowest renal artery | Zenith, Talent | 73.3 | 100 | NA |
| Hager ES [41] | 2012 | United States of America | 2002–2009 | 84 | 18.5 | short neck: <15 mm | Excluder, Zenith | 75.5 | 100 | 16.6 |
| Kvinlaug KE [42] | 2012 | Canada | 2008–2010 | 37 | 6 | short neck: <15 mm; wide neck: >28 mm; angulated neck: >60 degrees | Endurant | 75.3 | 100 | NA |
| Setacci F [43] | 2012 | Italy | 2010 | 72 | 1 | hourglass neck; angulated neck: >60 degrees; short neck: <15 mm; thrombosed neck: >50% of the neck circumference; reverse conical neck: dilatation > 2 mm within 10 mm; barrel neck: focal enlargement >3 mm within 15 mm | Endurant | 77 | 100 | 11.11 |
| Stather PW [44] | 2012 | United Kingdom | 1999–2010 | 199 | 48 | angulated neck: >60 degrees; short neck: <15 mm; wide neck: >28 mm; thrombosed neck; flared neck | Zenith, Talent, Excluder, Endurant, Jotec Tube | 73.9 | 98 | NA |
| Antoniou GA [45] | 2013 | International | NA | 60 | 18 | short neck: <15 mm; angulated neck: >60 degrees | Endurant, Zenith | 74 | 100 | 8 |
| Mwipatayi BP [46] | 2013 | Australia | 2008–2011 | 31 | 20 | short neck: <10 mm; angulated neck: >60 degrees; reverse tapered neck: diameter >2 mm for every 5 mm distal from the most caudal renal artery | Endurant | 75 | 100 | 12.9 |
| Shintan T [47] | 2013 | Japan | 2007–2011 | 20 | 25.7 | short neck: <10 mm; angulated neck: >60 degrees; reverse tapered neck: dilation >2 mm within the first 10 mm; thrombosed neck: thrombus in the first 10 mm of the neck, with thickness >2 mm and covering >25% of the circumference | Excluder, Zenith | 75.6 | 100 | 10 |
| Ierardi AM [48] | 2014 | Italy | 2009–2011 | 36 | 27.7 | short neck: between 7 and 10 mm | Ovation | 73.6 | 100 | 0 |
| Igari K [4] | 2014 | Japan | 2008–2010 | 12 | 25 | short neck: <15 mm; angulated neck: >60 degrees | Excluder, Zenith, Powerlink | 77.5 | NA | 0 |
| Iwakoshi S [49] | 2014 | Japan | 2009–2011 | 44 | 120 | short neck: <15 mm; angulated neck: >60 degrees; reverse tapered neck: dilation >2 mm within the first 10 mm | Zenith | 77 | 92.1 | 16 |
| Setacci F [50] | 2014 | Italy | 2010 | 72 | 24 | hourglass neck; angulated neck: >60 degrees; short neck: <15 mm; thrombosed neck: >50% of the neck circumference; reverse conical neck: dilatation > 2 mm within 10 mm; barrel neck: focal enlargement >3 mm within 15 mm | Endurant | 77 | NA | NA |
| Speziale F [7] | 2014 | Italy | 2010–2011 | 133 | 24 | noncylindrical neck: hourglass, reverse conical neck (dilation > 2 mm within 10 mm), or barrel neck (focal enlargement >3 mm within 15 mm); angulated neck: >65 degrees; short neck <15 mm, wide neck: >28 mm | Endurant, Excluder, Zenith | NA | 100 | 12 |
| Kaladji A [51] | 2015 | France | 1998–2012 | 170 | 38 | wide neck: need for a stent graft >32 mm in diameter | Talent, Zenith, Excluder, Anaconda, Endurant, Vanguard, AFX, AneuRx, Zenith, Lifepath | 75 | 100 | 0 |
| Saha P [52] | 2015 | United Kingdom | 2006–2008 | 27 | 72 | wide neck: need for a stent graft >36 mm in diameter | Zenith | 76 | 93 | 0 |
| Cerini P [53] | 2016 | Italy | 2005–2013 | 90 | 37 | any deviation from single-device IFU | Zenith, Endurant, Evita | 75.8 | 95.3 * | 1.1 |
| de Donato G [54] | 2016 | Italy | 2010–2012 | 161 | 32 | short neck: >7 mm; thrombosed neck: >50% of the neck circumference; calcified neck: >50% of the neck circumference | Ovation | 75.2 | 99.3 | 0.6 |
| Gallitto E [55] | 2016 | Italy | 2005–2010 | 60 | 51.4 | short neck: <10 mm | Zenith, Endurant | 74.9 | 95 * | 7 |
| Gimenez-Gaibar A [5] | 2016 | Spain | 2006–2013 | 52 | 24 | short neck: 15 mm; angulated neck: >60 degrees thrombosed neck: >50% of the neck circumference; calcified neck: >50% of the neck circumference | Excluder, Talent, Anaconda, Zenith, Endurant | 75.9 | 100 | 13.4 |
| Sirignano P [56] | 2016 | Italy | 2012–2014 | 21 | 9 | noncylindrical neck: hourglass, reverse conical (dilated >2 mm within 10 mm, barrel (focal enlargement >3 mm within 15 mm); angulated neck: >65 degrees; short neck: <10 mm; enlarged neck: diameter >30 mm; thrombosed neck: mural thrombosis >3 mm | Ovation | 75.6 | 100 | 0 |
| de Donato G [57] | 2017 | Italy | 2010–2012 | 89 | 32 | short neck: <7 mm | Ovation | 76.4 | 97.7 | 2.2 |
| Gargiulo M [58] | 2017 | International | 2009–2012 | 118 | 37.9 | wide neck: >28 mm | Zenith, Endurant, Excluder, Ovation, Anaconda | 73.9 | 98 | 5 |
| Kontopodis N [59] | 2017 | Greece | NA | 106 | 18 | short neck: >7 mm | Ovation | NA | 97.2 | 0 |
| Lee JH [60] | 2017 | Republic of Korea | 2010–2013 | 38 | 1 | conical neck: neck coefficient calculated using the following formula (diameter, D): Arctangent ([D3-D1]/[neck length]) × 180/π, if the absolute value of the neck coefficient was >10, it was defined as conical or inverted conical | Zenith, Endurant | 73.8 | 100 | 23.7 |
| Pitoulias GA [61] | 2017 | International | 2007–2015 | 156 | 41.1 | short neck: <15 mm; angulated neck: >60 degrees; wide neck: <32 mm; circumferential thrombus with >2-mm thickness; circumferential calcification >50%; reverse tapered neck: neck dilation >2 mm within 10 mm; neck bulge | Endurant | 73.4 | 100 | 2.5 |
| Sirignano P [62] | 2017 | Italy | 2012–2015 | 156 | 20.4 | short neck: <10 mm; noncylindrical aortic neck | Ovation | 74.83 | 100 | 10.25 |
| Reyes Valdiva A [63] | 2017 | International | 2007–2015 | 73 | 30 | need for a stent graft >36 mm in diameter; any deviation from single-device IFU | Endurant | 74.4 | 98.6 | 6.8 |
| Aburahma AF [10] | 2018 | United States of America | 2003–2015 | 33 | 31.8 | wide neck: >31 mm | Excluder, Zenith, AneuRX | 74.7 | 100 | NA |
| Bryce Y [64] | 2018 | United States of America | 2004–2013 | 125 | 47.3 | short neck: <10 mm; angulated neck: >60 degrees; reverse conical neck (neck dilated > 2 mm within 10 mm, barrel neck (focal enlargement > 3 mm within 15 mm); thrombosed neck: >50% of the neck circumference; calcified neck: >50% of the neck circumference | Endurant, Excluder, Zenith, Ovation, AFX | 75.4 | 100 | 20 |
| Greaves NS [65] | 2018 | United Kingdom | 2012–2017 | 52 | 21.5 | short neck: between 7 and 10 mm | Ovation | 75.7 | 100 | 1.9 |
| Howard DPJ [66] | 2018 | International | 2011–2017 | 1189 | 60 | wide neck: >25 mm | Excluder | 73.9 | 99.9 | 10.4 |
| Oliveira NFG [15] | 2018 | International | 2009–2011 | 97 | 48 | wide neck: >30 mm | Endurant | 73.3 | 100 | NA |
| Zhou M [67] | 2018 | China | 2010–2015 | 323 | 36 | short neck: <15 mm; very short neck: <10 mm; wide neck: >28 mm; conical neck: neck dilated over 2 mm within 10 mm below; angulated neck: >60 degrees thrombosed neck: the widest part of thrombus (≥2 mm thick) covering at least 50% of the circumference; calcified neck: calcification accounting for more than or equal to 50% of proximal neck | Endurant, Excluder, Zenith | 73 | 89.2 | 10.2 |
| Kouvelos GN [68] | 2019 | Greece | 2009–2016 | 64 | 24 | wide neck: 29–32 mm | Endurant | 72.7 | 100 | 1.5 |
| McFarland G [69] | 2019 | United States of America | 2000–2016 | 108 | 34.1 | wide neck: >28 mm | Excluder, Zenith, Talent, Endurant, Ovation, AFX | 76.5 | NA | NA |
| Sirignano P [70] | 2021 | International | 2017–2018 | 122 | 1 | Anatomy outside IFU for any commercially available endografts, while inside the IFU for the Ovation stent graft | Ovation | 78.65 | 100 | 0 |
| First Author | 30 Days | Mean Follow-Up | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Conversion to Open Repair (%) | Reintervention (%) | Migration (%) | Type Ia Endoleak (%) | AAA-Related Mortality (%) | Conversion to Open Repair (%) | Reintervention (%) | Migration (%) | Type Ia Endoleak (%) | AAA-Related Mortality (%) | |
| Dillavou ED [24] | 0 | 1.09 | NA | 2.18 | 1.09 | 0 | 8.8 | 0 | 2.18 | 1.09 |
| Fairman RM [25] | NA | NA | NA | NA | NA | 3.1 | NA | 13 | 10.5 | NA |
| Choke E [26] | 0 | 3 | 0 | 3 | 0 | 0 | 1.5 | 0 | 3 | 0 |
| Cox DE [27] | NA | 0 | NA | 0 | NA | NA | 10.5 | 5.26 | 5.26 | NA |
| Mc Donnell CO [28] | 0 | 2.17 | 0 | 2.17 | 0 | NA | 0 | 2.17 | 0 | 0 |
| Abbruzzese TA [29] | NA | NA | NA | NA | NA | 1.4 | 24 | 1.4 | 0.9 | 11 |
| Chisci E [30] | 0 | 0 | 0 | 4.1 | 0 | 2.7 | 20.3 | 2.7 | 5.4 | 4.1 |
| Jim J [31] | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2.7 | 2.7 | 2.7 |
| Troisi N [32] | NA | 1.3 | NA | 1.3 | 1.3 | 0 | 0 | 0 | 0.65 | 0.65 |
| Aburahma AF [33] | 0 | 1 | 0 | 1 | 0 | 0 | 7 | 1.3 | 11 | 1 |
| Georgiadis GS [34] | NA | NA | NA | NA | NA | 0 | 0 | 0 | 0 | 0 |
| Hoshina K [35] | NA | NA | NA | NA | NA | NA | NA | NA | NA | 0 |
| Hyhlik-Dürr A [36] | 0 | 2 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rouwet EV [37] | 0 | 0 | 0 | 0 | 1.25 | 0 | 0 | 0 | 0 | 1.25 |
| Torsello G [38] | 0 | 1.8 | 0 | 3.6 | 0 | 0 | 1.8 | 0 | 3.6 | 1.8 |
| Van Keulen JW [39] | 0 | 0 | 0 | 0 | 0 | NA | 0 | NA | 0 | 0 |
| Lee M [40] | NA | NA | NA | NA | NA | 0 | 10.5 | 0 | 10.5 | 0 |
| Hager ES [41] | 0 | 1.2 | 0 | 7.14 | 0 | 0 | 0 | 0 | 2.4 | 0 |
| Kvinlaug KE [42] | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Setacci F [43] | 0 | 0 | 0 | 0 | 0 | NA | NA | NA | NA | NA |
| Stather PW [44] | NA | 5 | NA | 2.5 | 0.5 | NA | 2.5 | 3 | 9.5 | 2 |
| Antoniou GA [45] | NA | NA | NA | NA | NA | 0 | 0 | 0 | 1.7 | 0 |
| Mwipatayi BP [46] | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Shintan T [47] | NA | NA | NA | NA | NA | 0 | 0 | 25 | 0 | 0 |
| Ierardi AM [48] | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Igari K [4] | 0 | 0 | 0 | 8 | 0 | 0 | 0 | 0 | 0 | 0 |
| Iwakoshi S [49] | 0 | 0 | 0 | 0 | 0 | 0 | 3.14 | 0 | 3.14 | 2.36 |
| Setacci F [50] | NA | NA | NA | NA | NA | 0 | 5.5 | 0 | 5.5 | 0 |
| Speziale F [7] | 0 | 0 | 0 | 0 | 0 | 0 | 7.7 | 4.6 | 3 | 0 |
| Kaladji A [51] | 0 | 8.3 | 0 | 4.1 | 0 | 0 | 24.1 | 0 | 13 | 3.5 |
| Saha P [52] | 0 | 0 | 0 | 3.7 | 0 | 0 | 7.4 | 0 | 7.4 | 7.4 |
| Cerini P [53] | 2.2 | 11.1 | NA | 8.8 | 0 | 0 | 0 | 0 | 0 | 0 |
| de Donato G [54] | 0 | 0 | 0 | 0.6 | 0 | 0 | 1.8 | 0 | 1.8 | 0 |
| Gallitto E [55] | NA | 1.5 | NA | 3 | NA | NA | 3 | NA | 1.5 | 3 |
| Gimenez-Gaibar A [5] | 0 | 1.9 | 0 | 1.9 | 0 | 0 | 4.5 | 0 | 2.2 | 0 |
| Sirignano P [56] | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| de Donato G [57] | 0 | 0 | 0 | 0 | 0 | 0 | 2.2 | 0 | 2.2 | 0 |
| Gargiulo M [58] | NA | NA | NA | NA | NA | 6 | 7 | 3 | 12 | 3.4 |
| Kontopodis N [59] | 1.9 | NA | NA | NA | NA | 0 | 0 | 0 | 0 | NA |
| Lee JH [60] | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Pitoulias GA [61] | 0 | 1.2 | 0 | 1.9 | 0 | 0 | 1.2 | 0 | 1.2 | 0 |
| Sirignano P [62] | 0 | 0.7 | 0 | 1.3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Reyes Valdiva A [63] | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.3 |
| Aburahma AF [10] | 0 | 15.2 | 0 | 27.3 | 0 | 0 | 17.2 | 0 | 13.8 | 0 |
| Bryce Y [64] | 0 | 1.6 | 0 | 1.6 | 0 | 0.8 | 1.6 | 0 | 1.6 | 0 |
| Greaves NS [65] | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Howard DPJ [66] | NA | NA | NA | NA | NA | 0.2 | 3 | 0.1 | 0.3 | 0 |
| Oliveira NFG [15] | NA | NA | NA | NA | NA | NA | 3.1 | NA | 7.6 | 1 |
| Zhou M [67] | NA | NA | NA | NA | NA | NA | 5.6 | NA | 7.1 | NA |
| Kouvelos GN [68] | 0 | 0 | 0 | 1.5 | 0 | 7.2 | 10.14 | 2.9 | 4.3 | 1.5 |
| McFarland G [69] | NA | NA | NA | NA | NA | 1.85 | 11.1 | 14.8 | 14.8 | 0 |
| Sirignano P [70] | 0 | 1.6 | 0 | 1.6 | 0 | NA | NA | NA | NA | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirignano, P.; Ceruti, S.; Aloisi, F.; Sirignano, A.; Picozzi, M.; Taurino, M. Is Evar Feasible in Challenging Aortic Neck Anatomies? A Technical Review and Ethical Discussion. J. Clin. Med. 2022, 11, 4460. https://doi.org/10.3390/jcm11154460
Sirignano P, Ceruti S, Aloisi F, Sirignano A, Picozzi M, Taurino M. Is Evar Feasible in Challenging Aortic Neck Anatomies? A Technical Review and Ethical Discussion. Journal of Clinical Medicine. 2022; 11(15):4460. https://doi.org/10.3390/jcm11154460
Chicago/Turabian StyleSirignano, Pasqualino, Silvia Ceruti, Francesco Aloisi, Ascanio Sirignano, Mario Picozzi, and Maurizio Taurino. 2022. "Is Evar Feasible in Challenging Aortic Neck Anatomies? A Technical Review and Ethical Discussion" Journal of Clinical Medicine 11, no. 15: 4460. https://doi.org/10.3390/jcm11154460
APA StyleSirignano, P., Ceruti, S., Aloisi, F., Sirignano, A., Picozzi, M., & Taurino, M. (2022). Is Evar Feasible in Challenging Aortic Neck Anatomies? A Technical Review and Ethical Discussion. Journal of Clinical Medicine, 11(15), 4460. https://doi.org/10.3390/jcm11154460







