Adenoid Cystic Carcinoma of the Breast May Be Exempt from Adjuvant Chemotherapy
Abstract
1. Introduction
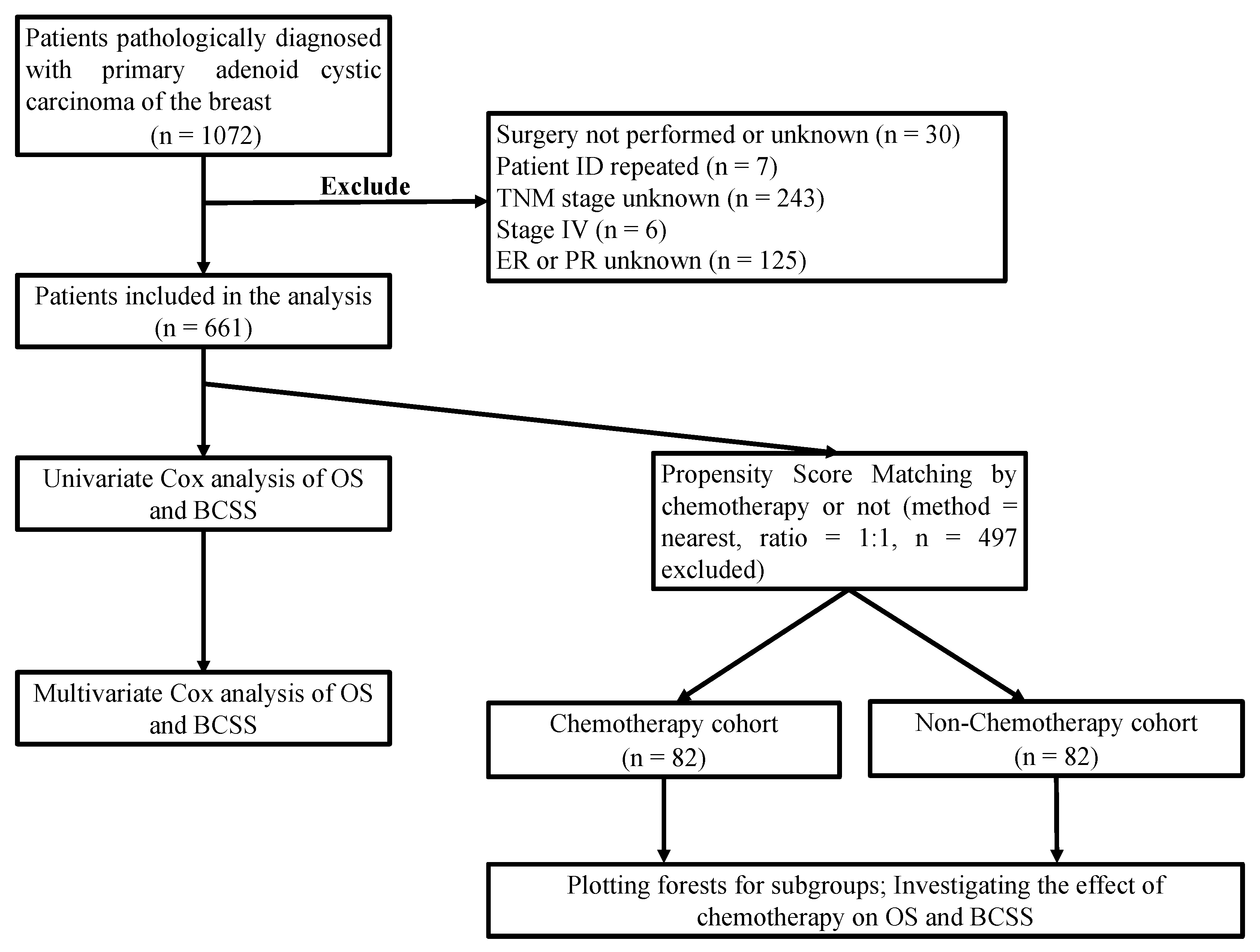
2. Materials and Methods
2.1. Data Collection
2.2. Statistical Analysis
3. Results
3.1. Clinicopathological Characteristics
3.2. Prognostic Factors
3.3. Propensity Matching Score and Subgroup Analysis
3.4. Clinical Practice in China
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cozzi, S.; Bardoscia, L.; Najafi, M.; Botti, A.; Blandino, G.; Augugliaro, M.; Manicone, M.; Iori, F.; Giaccherini, L.; Sardaro, A.; et al. Adenoid Cystic Carcinoma/Basal Cell Carcinoma of the Prostate: Overview and Update on Rare Prostate Cancer Subtypes. Curr. Oncol. 2022, 29, 1866–1876. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Fang, Y.; Zhang, Z.; Wang, J. Management of adenoid cystic carcinoma of the breast: A single-institution study. Front. Oncol. 2021, 11, 621012. [Google Scholar] [CrossRef] [PubMed]
- Varghese, A.; Suneha, S.; Watkinson, J.C. Adenoid Cystic Carcinoma of Trachea. Indian J. Surg. 2017, 79, 67–69. [Google Scholar] [CrossRef][Green Version]
- Epstein, J.I.; Sears, D.L.; Tucker, R.S.; Eagan, J.W., Jr. Carcinoma of the esophagus with adenoid cystic differentiation. Cancer 1984, 53, 1131–1136. [Google Scholar] [CrossRef]
- Liao, H.Y.; Zhang, W.W.; Sun, J.Y.; Li, F.Y.; He, Z.Y.; Wu, S.G. The clinicopathological features and survival outcomes of different histological subtypes in triple-negative breast cancer. J. Cancer 2018, 9, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Elimimian, E.B.; Samuel, T.A.; Liang, H.; Elson, L.; Bilani, N.; Nahleh, Z.A. Clinical and demographic factors, treatment patterns, and overall survival associated with rare triple-negative breast carcinomas in the US. JAMA Netw. Open 2021, 4, e214123. [Google Scholar] [CrossRef]
- Ghabach, B.; Anderson, W.F.; Curtis, R.E.; Huycke, M.M.; Lavigne, J.A.; Dores, G.M. Adenoid cystic carcinoma of the breast in the United States (1977 to 2006): A population-based cohort study. Breast Cancer Res. 2010, 12, R54. [Google Scholar] [CrossRef]
- Sanges, F.; Floris, M.; Cossu-Rocca, P.; Muroni, M.R.; Pira, G.; Urru, S.A.M.; Barrocu, R.; Gallus, S.; Bosetti, C.; D’Incalci, M.; et al. Histologic subtyping affecting outcome of triple negative breast cancer: A large Sardinian population-based analysis. BMC Cancer 2020, 20, 491. [Google Scholar] [CrossRef]
- Sun, J.Y.; Wu, S.G.; Chen, S.Y.; Li, F.Y.; Lin, H.X.; Chen, Y.X.; He, Z.Y. Adjuvant radiation therapy and survival for adenoid cystic carcinoma of the breast. Breast 2017, 31, 214–218. [Google Scholar] [CrossRef]
- Treitl, D.; Radkani, P.; Rizer, M.; El Hussein, S.; Paramo, J.C.; Mesko, T.W. Adenoid cystic carcinoma of the breast, 20 years of experience in a single center with review of literature. Breast Cancer 2018, 25, 28–33. [Google Scholar] [CrossRef]
- Coates, J.M.; Martinez, S.R.; Bold, R.J.; Chen, S.L. Adjuvant radiation therapy is associated with improved survival for adenoid cystic carcinoma of the breast. J. Surg. Oncol. 2010, 102, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Arpino, G.; Clark, G.M.; Mohsin, S.; Bardou, V.J.; Elledge, R.M. Adenoid cystic carcinoma of the breast: Molecular markers, treatment, and clinical outcome. Cancer 2002, 94, 2119–2127. [Google Scholar] [CrossRef]
- Jaso, J.; Malhotra, R. Adenoid cystic carcinoma. Arch. Pathol. Lab. Med. 2011, 135, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Hanna, G.J.; Bae, J.E.; Lorch, J.H.; Schoenfeld, J.D.; Margalit, D.N.; Tishler, R.B.; Haddad, R.I.; Chau, N.G. Long-term outcomes and clinicogenomic correlates in recurrent, metastatic adenoid cystic carcinoma. Oral Oncol. 2020, 106, 104690. [Google Scholar] [CrossRef] [PubMed]
- Slodkowska, E.; Xu, B.; Kos, Z.; Bane, A.; Barnard, M.; Zubovits, J.; Iyengar, P.; Faragalla, H.; Turbin, D.; Williams, P.; et al. Predictors of outcome in mammary adenoid cystic carcinoma: A multi-institutional study. Am. J. Surg. Pathol. 2020, 44, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Fusco, N.; Geyer, F.C.; De Filippo, M.R.; Martelotto, L.G.; Ng, C.K.; Piscuoglio, S.; Guerini-Rocco, E.; Schultheis, A.M.; Fuhrmann, L.; Wang, L.; et al. Genetic events in the progression of adenoid cystic carcinoma of the breast to high-grade triple-negative breast cancer. Mod. Pathol. Off. J. United States Can. Acad. Pathol. Inc. 2016, 29, 1292–1305. [Google Scholar] [CrossRef] [PubMed]
- Atallah, S.; Casiraghi, O.; Fakhry, N.; Wassef, M.; Uro-Coste, E.; Espitalier, F.; Sudaka, A.; Kaminsky, M.C.; Dakpe, S.; Digue, L.; et al. A prospective multicentre refcor study of 470 cases of head and neck Adenoid cystic carcinoma: Epidemiology and prognostic factors. Eur. J. Cancer 2020, 130, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Lorini, L.; Ardighieri, L.; Bozzola, A.; Romani, C.; Bignotti, E.; Buglione, M.; Guerini, A.; Lombardi, D.; Deganello, A.; Tomasoni, M.; et al. Prognosis and management of recurrent and/or metastatic head and neck adenoid cystic carcinoma. Oral Oncol. 2021, 115, 105213. [Google Scholar] [CrossRef]
- Dillon, P.M.; Chakraborty, S.; Moskaluk, C.A.; Joshi, P.J.; Thomas, C.Y. Adenoid cystic carcinoma: A review of recent advances, molecular targets, and clinical trials. Head Neck 2016, 38, 620–627. [Google Scholar] [CrossRef]
- Papaspyrou, G.; Hoch, S.; Rinaldo, A.; Rodrigo, J.P.; Takes, R.P.; van Herpen, C.; Werner, J.A.; Ferlito, A. Chemotherapy and targeted therapy in adenoid cystic carcinoma of the head and neck: A review. Head Neck 2011, 33, 905–911. [Google Scholar] [CrossRef]
- Vander Poorten, V.; Hunt, J.; Bradley, P.J.; Haigentz, M., Jr.; Rinaldo, A.; Mendenhall, W.M.; Suarez, C.; Silver, C.; Takes, R.P.; Ferlito, A. Recent trends in the management of minor salivary gland carcinoma. Head Neck 2014, 36, 444–455. [Google Scholar] [CrossRef] [PubMed]
- Dodd, R.L.; Slevin, N.J. Salivary gland adenoid cystic carcinoma: A review of chemotherapy and molecular therapies. Oral Oncol. 2006, 42, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, N.; Moran, M.S.; Li, Y.; Liang, Y.; Su, P.; Haffty, B.G.; Yang, Q. Special subtypes with favorable prognosis in breast cancer: A registry-based cohort study and network meta-analysis. Cancer Treat. Rev. 2020, 91, 102108. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | Total (n = 661) | Chemotherapy | χ2 | p | |
|---|---|---|---|---|---|
| Yes (n = 82) | No (n = 579) | ||||
| Age | 6.824 | 0.009 | |||
| <60 | 306 (21.2) | 49 (30.5) | 257 (19.9) | ||
| ≥60 | 355 (78.8) | 33 (69.5) | 322 (80.1) | ||
| Sex | - | 1 | |||
| Female | 655 (99.1) | 82 (100) | 573 (99) | ||
| Male | 6 (0.9) | 0 (0) | 6 (1) | ||
| Stage | 40.453 | <0.001 | |||
| I | 383 (57.9) | 29 (35.4) | 354 (61.1) | ||
| IIA | 236 (35.7) | 36 (43.9) | 200 (34.5) | ||
| IIB and III | 42 (5.9) | 17 (17.1) | 25 (4.3) | ||
| Tumor size | 15.444 | <0.001 | |||
| ≤2 cm | 390 (59.0) | 32 (39) | 358 (61.8) | ||
| >2 cm | 271 (41.0) | 50 (61) | 221 (38.2) | ||
| Lymph node | - | <0.001 | |||
| N0 | 634 (95.9) | 65 (79.3) | 569 (98.3) | ||
| N1 | 27 (4.1) | 17 (20.7) | 10 (1.7) | ||
| ER status | 0.086 | 0.77 | |||
| Positive | 137 (20.7) | 18 (22) | 119 (20.6) | ||
| Negative | 524 (79.3) | 64 (78) | 460 (79.4) | ||
| PR status | 5.177 | 0.023 | |||
| Positive | 85 (12.9) | 17 (20.7) | 68 (11.7) | ||
| Negative | 576 (87.1) | 65 (79.3) | 511 (88.3) | ||
| Grade | 12.285 | 0.002 | |||
| 1 | 213 (32.2) | 17 (20.7) | 196 (33.9) | ||
| ≥2 | 263 (28) | 47 (32.9) | 216 (27.3) | ||
| Unknown | 185 (28) | 18 (22) | 167 (28.8) | ||
| Radiotherapy | 2.041 | 0.153 | |||
| Yes | 314 (47.5) | 45 (54.9) | 269 (46.5) | ||
| No | 347 (52.5) | 37 (45.1) | 310 (53.5) | ||
| Variable | OS | BCSS | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Age | ||||||||
| <60 (ref) | ||||||||
| ≥60 | 3.418 (2.354–4.961) | <0.001 | 1.08 (0.571–2.042) | 0.814 | ||||
| Stage | ||||||||
| I (ref) | ||||||||
| IIA | 1.196 (0.849–1.686) | 0.306 | 3.334 (1.538–7.226) | 0.002 | ||||
| IIB and III | 2.071 (1.212–3.538) | 0.008 | 10.24 (4.253–24.659) | <0.001 | ||||
| Tumor size | ||||||||
| ≤2cm (ref) | ||||||||
| >2 cm | 1.173 (0.851–1.618) | 0.33 | 3.527 (1.779–6.993) | <0.001 | 2.678 (1.332–5.386) | 0.006 | ||
| Lymph node | ||||||||
| N0 (ref) | ||||||||
| N1 | 4.689 (2.818-7.802) | <0.001 | 0.207 (0.125–0.345) | <0.001 | 9.756 (4.589–20.743) | <0.001 | 6.889 (3.126–15.18) | <0.001 |
| ER status | ||||||||
| Positive (ref) | ||||||||
| Negative | 1.089 (0.89–1.331) | 0.408 | 1.506 (0.897–2.529) | 0.121 | ||||
| PR status | ||||||||
| Positive (ref) | ||||||||
| Negative | 0.978 (0.626–1.528) | 0.923 | 1.365 (0.757–2.462) | 0.301 | ||||
| Grade | ||||||||
| 1 (ref) | ||||||||
| ≥2 | 1.614 (1.077–2.418) | 0.02 | 3.563 (1.471–8.633) | 0.005 | 3.081 (1.256–7.56) | 0.014 | ||
| Unknown | 1.152 (0.741–1.791) | 0.529 | 0.827 (0.252–2.711) | 0.754 | 0.792 (0.241–2.603) | 0.7 | ||
| Radiotherapy | ||||||||
| Yes | 0.666 (0.48–0.923) | 0.015 | 0.649 (0.467-0.901) | 0.010 | 0.568 (0.290–1.11) | 0.098 | 0.452 (0.228–0.897) | 0.023 |
| No (ref) | ||||||||
| Chemotherapy | ||||||||
| Yes | 1.105 (0.703–1.736) | 0.665 | 3.079 (1.553–6.105) | 0.001 | ||||
| No (ref) | ||||||||
| Characteristics | Chemotherapy | χ2 | p | |
|---|---|---|---|---|
| Yes (n = 82) (%) | No (n = 82) (%) | |||
| Age | 0.025 | 0.874 | ||
| <60 | 49 (59.8) | 48 (58.5) | ||
| ≥60 | 33 (40.2) | 34 (41.5) | ||
| Stage | 1.627 | 0.443 | ||
| I | 29 (35.4) | 30 (36.6) | ||
| IIA | 36 (43.9) | 41 (50) | ||
| IIB and III | 17 (20.7) | 11 (13.4) | ||
| Tumor size | 0.101 | 0.75 | ||
| ≤2 cm | 32 (39) | 34 (41.5) | ||
| >2 cm | 50 (61) | 48 (58.5) | ||
| Lymph node | 2.172 | 0.14 | ||
| N0 | 65 (79.3) | 72 (87.8) | ||
| N1 | 17 (20.7) | 10 (12.2) | ||
| ER status | 0.137 | 0.711 | ||
| Positive | 18 (22) | 20 (24.4) | ||
| Negative | 64 (78) | 62 (75.6) | ||
| PR status | 1.593 | 0.207 | ||
| Positive | 24 (29.3) | 17 (20.7) | ||
| Negative | 58 (70.7) | 65 (79.3) | ||
| Grade | 0.922 | 0.631 | ||
| 1 | 17 (20.7) | 24 (29.3) | ||
| ≥2 | 47 (57.3) | 27 (32.9) | ||
| Unknown | 18 (22) | 15 (18.3) | ||
| Radiotherapy | 0.098 | 0.754 | ||
| Yes | 45 (54.9) | 43 (52.4) | ||
| No | 37 (45.1) | 39 (47.6) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Zhang, D.; Ma, F. Adenoid Cystic Carcinoma of the Breast May Be Exempt from Adjuvant Chemotherapy. J. Clin. Med. 2022, 11, 4477. https://doi.org/10.3390/jcm11154477
Li L, Zhang D, Ma F. Adenoid Cystic Carcinoma of the Breast May Be Exempt from Adjuvant Chemotherapy. Journal of Clinical Medicine. 2022; 11(15):4477. https://doi.org/10.3390/jcm11154477
Chicago/Turabian StyleLi, Lixi, Di Zhang, and Fei Ma. 2022. "Adenoid Cystic Carcinoma of the Breast May Be Exempt from Adjuvant Chemotherapy" Journal of Clinical Medicine 11, no. 15: 4477. https://doi.org/10.3390/jcm11154477
APA StyleLi, L., Zhang, D., & Ma, F. (2022). Adenoid Cystic Carcinoma of the Breast May Be Exempt from Adjuvant Chemotherapy. Journal of Clinical Medicine, 11(15), 4477. https://doi.org/10.3390/jcm11154477





