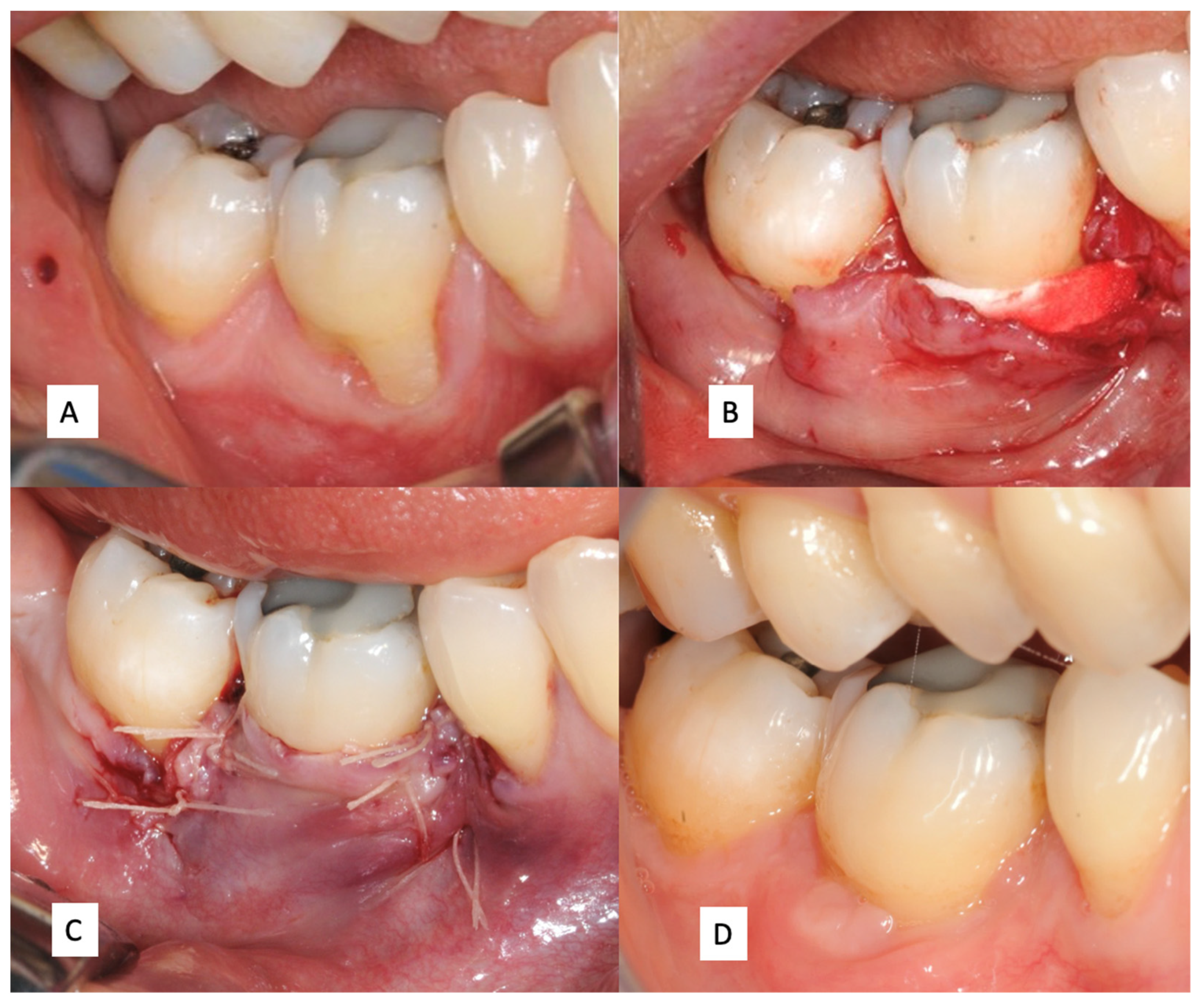
A New Matrix for Soft Tissue Management
Abstract
:1. Introduction
2. Materials and Methods
2.1. Focused Question
2.2. Information Sources
2.3. Search Strategy
2.4. Eligibility Criteria
2.4.1. Inclusion Criteria
- Randomized controlled clinical trials (RCT) and prospective studies with a minimum follow-up of 6 months, which is necessary for the complete healing and maturation of soft tissues subjected to surgery [16];
- Studies with ≥ 5 patients involved;
- Patients with single or multiple GRs classified as class I or II according to Miller 4 or class RT1 according to Cairo et al. [2];
- Studies applying these types of surgery: CAF/tunnel + XCM, CAF/tunnel + CTG, or CAF.
2.4.2. Exclusion Criteria
- In vitro studies, animal studies, retrospective studies, case reports, case series, and systematic reviews;
- Studies with a follow-up < 6 months;
- Studies with < 5 patients involved;
- Patients with single or multiple GRs classified as class III or IV according to Miller [4] or class RT2 or RT3 according to Cairo et al. [2]. We decided to exclude these types of defects as they involve a loss of attachment and bone support at the interproximal level that does not allow for complete and predictable root coverage;
- Surgical interventions other than those previously specified, with biomaterials other than the xenogeneic collagen matrix or with interventions that, although adopting XCM, aimed to compare two different surgical techniques.
2.5. Data Items
- Complete root coverage (CRC), which is a percentage value describing the number of sites, with respect to the total number of sites treated, that obtained a complete radicular covering at a given time of follow-up. The formula to calculate it is the following: ;
- Mean root coverage (MRC), which is a percentage value that describes the rate of reduction of the recession compared to the initial recession;
- Recession reduction (RecRed), which is a millimeter value that describes the difference between the recession measure at a given follow-up and the measure of the initial recession;
- Differential clinical attachment level (ΔCAL), which reflects the gain or loss of CAL at the end of the time of a given follow-up;
- Differential keratinized tissue width (ΔKTW). KTW is the distance from the free gingival margin to muco-gingival junction;
- Differential gingival thickness (ΔGT). GT is a millimeter measurement that indicates the thickness of the attached gingiva.
2.6. Study Selection
2.7. Data Extraction
2.8. Quality Assessment
2.9. Meta-Analysis
3. Results
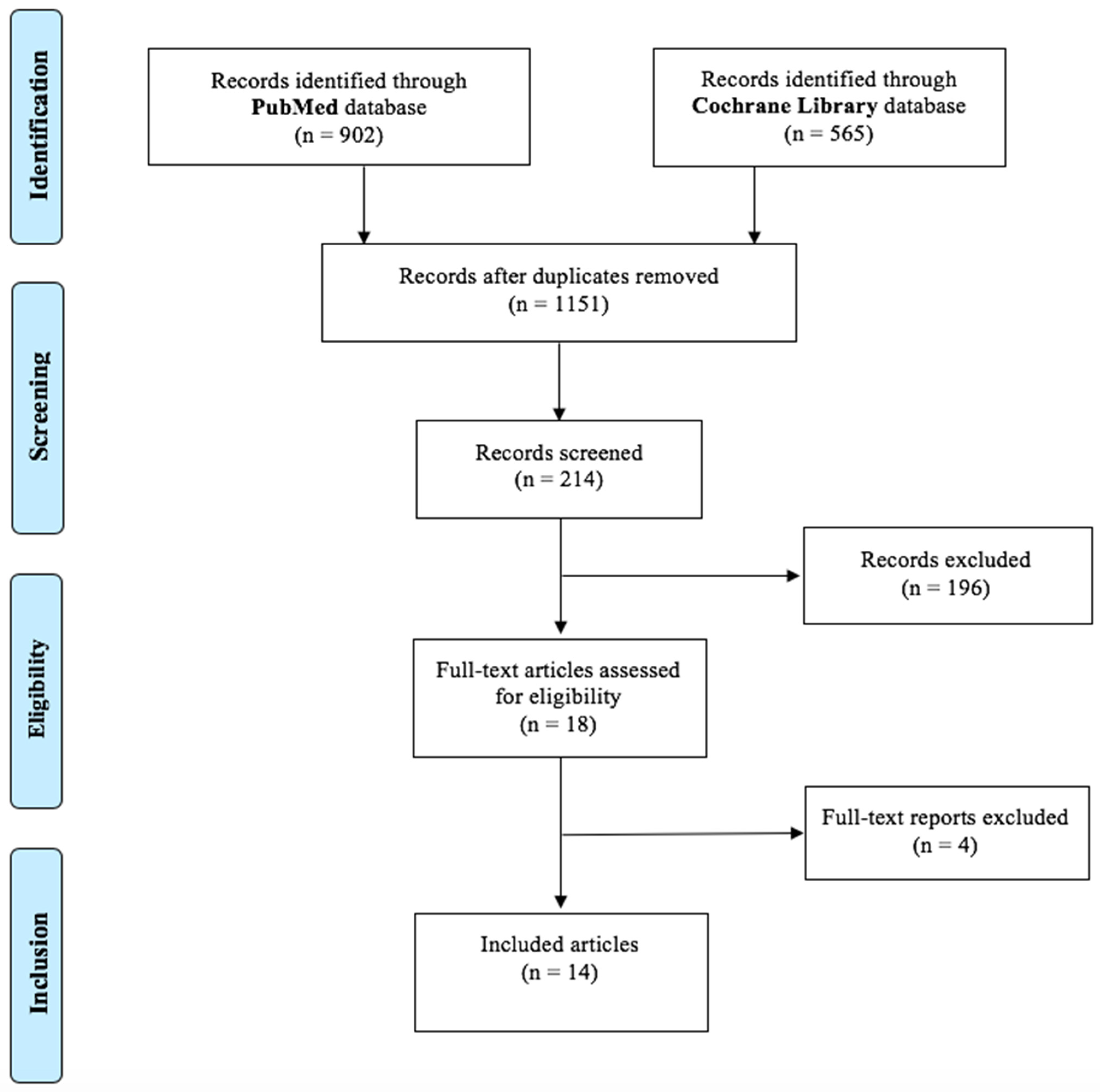
3.1. Study Selection
3.2. Characteristics of the Included Study
3.3. Risk of Bias
3.4. Results of Individual Studies
3.5. Synthesis of Results
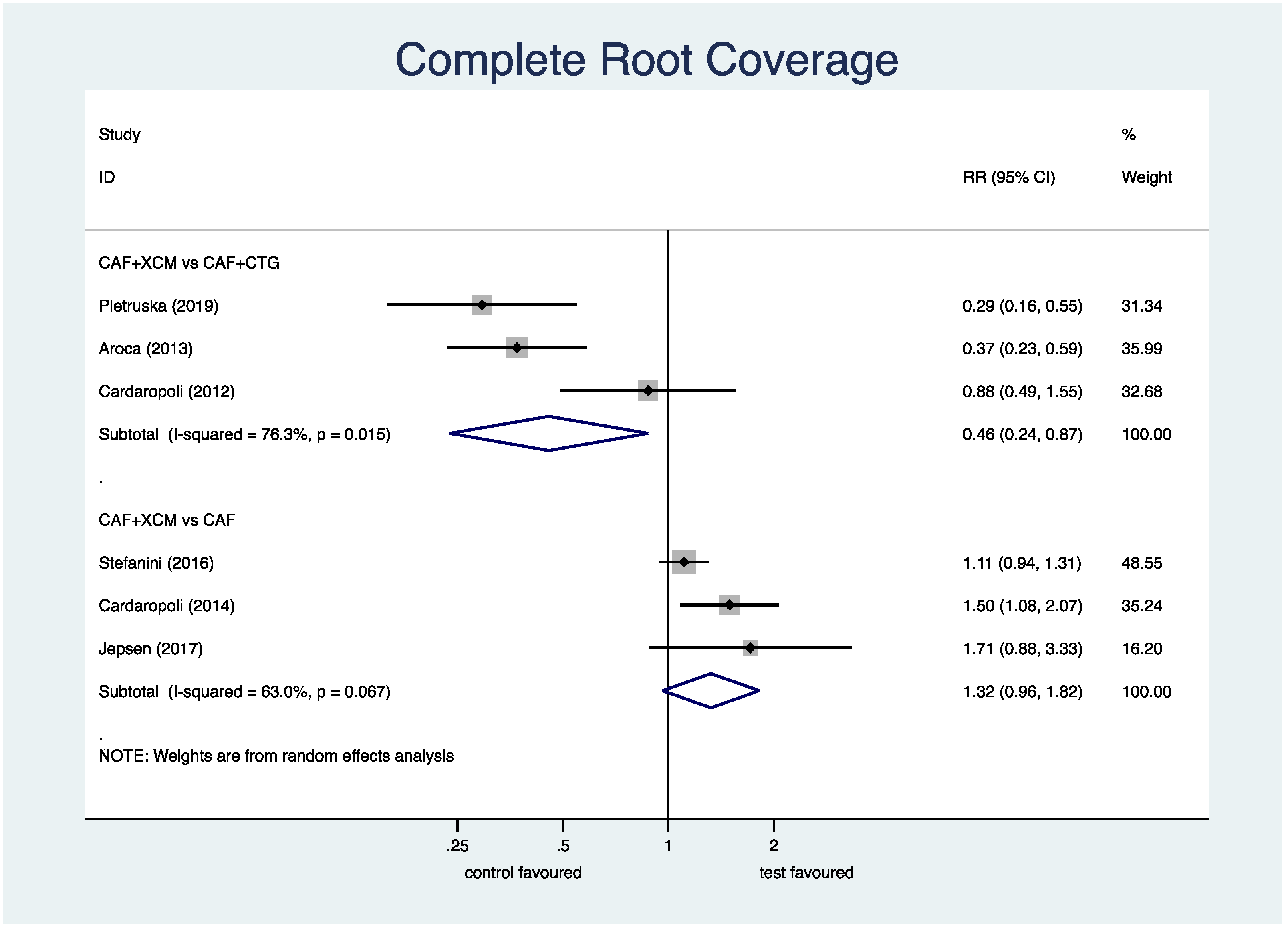
- Complete root coverage (Figure 4): the meta-analysis shows a statistically significant difference in favor of CAF + CTG compared to CAF + XCM relative to the parameter of complete root coverage at the 12-month follow-up: RR (relative risk) 0.46; 95% CI (confidence interval) from 0.24 to 0.87; p = 0.018. On the contrary, the difference between CAF + XCM and CAF alone is not statistically significant: RR 1.32; 95% CI from 0.96 to 1.82; p = 0.085.Figure 4. Forest plot relating to complete root coverage.
- Mean root coverage (Figure 5): the statistical analysis shows a statistically significant difference in favor of CAF + CTG compared to CAF + XCM relative to the parameter mean root coverage at 12-months follow-up: SMD (standardized mean difference) −0.89; 95% CI from −1.12 to −0.66; p < 0.001. The difference between CAF + XCM and CAF alone is statistically significant in favor of the first surgical procedure: SMD 0.51; 95% CI from 0.002 to 1.01; p = 0.049. Notice that SMD is the ratio between the means and the estimated common standard deviation.Figure 5. Forest plot relating to mean root coverage.
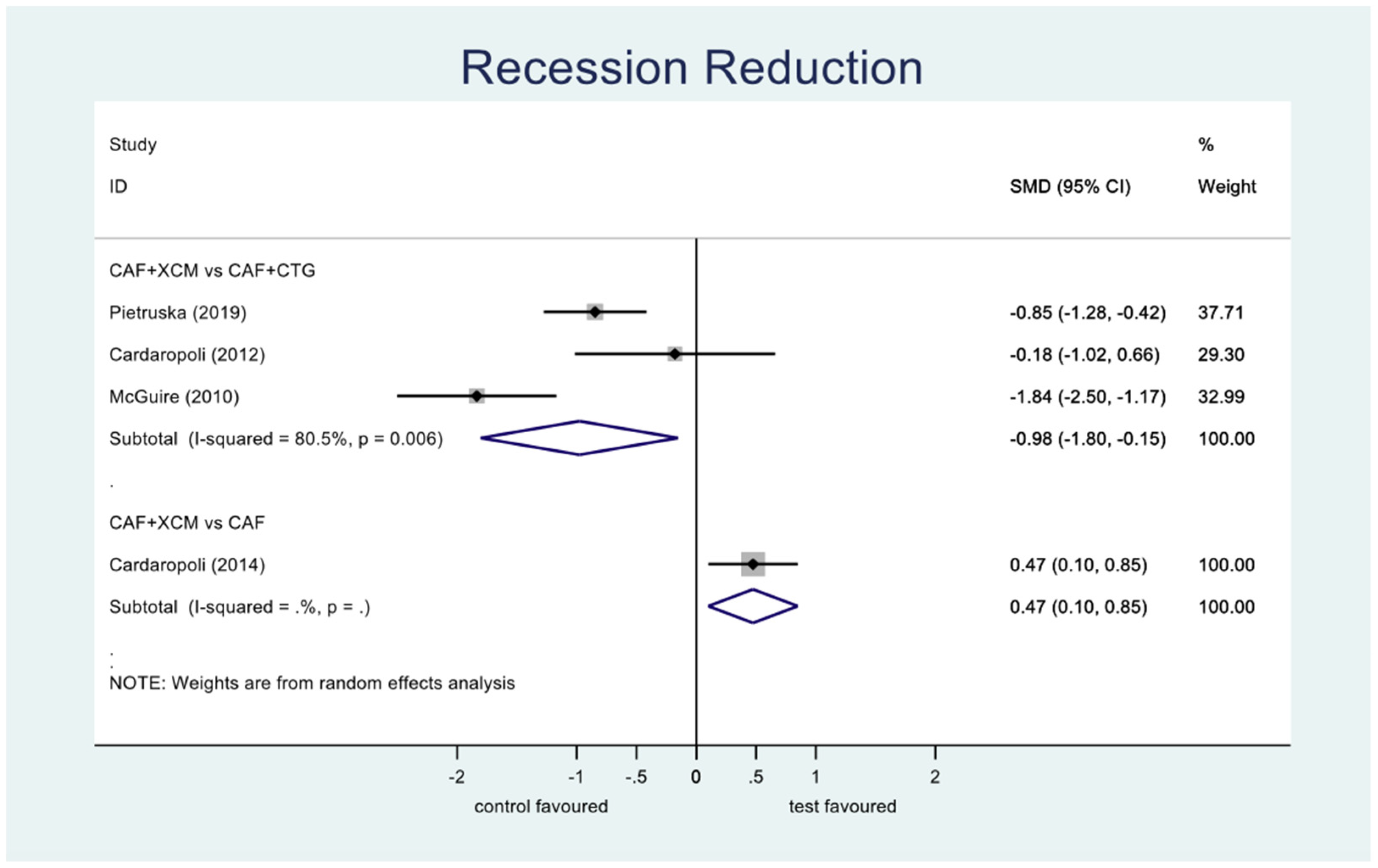
- Recession reduction (Figure 6): the difference between CAF + XCM and CAF + CTG is statistically significant in favor of the latter procedure relative to the reduction of recession depth 12 months after surgery: SMD −0.98; 95% CI from −1.80 to −0,15; p = 0.02. Furthermore, there is a statistically significant difference between CAF + XCM and CAF alone in favor of the first procedure: SMD 0.47; 95% CI from 0.10 to 0.85; p = 0.013.Figure 6. Forest plot relating to recession reduction.
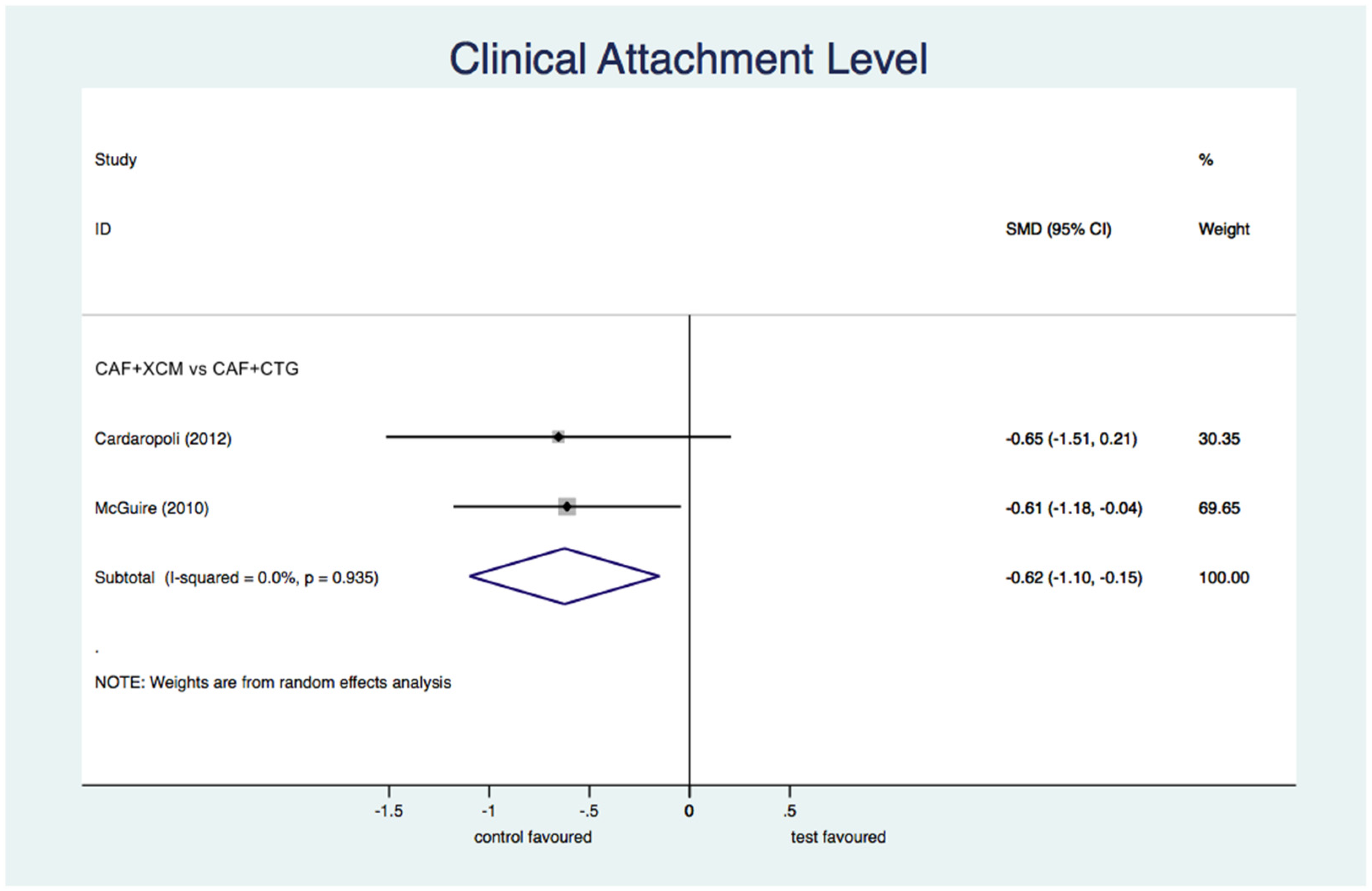
- Differential clinical attachment level (Figure 7): the meta-analysis shows a statistically significant difference in favor of CAF + CTG compared to CAF + XCM relative to the parameter of clinical attachment level at the 12-month follow-up: SMD −0.63; 95% CI from −1.10 to −0.15; p = 0.01.Figure 7. Forest plot relating to the clinical attachment level.
- Differential keratinized tissue width (Figure 8): the difference between CAF + XCM and CAF + CTG is not statistically significant relative to the parameter ΔKTW 12 months after periodontal surgery: SMD −0.68; 95% CI from −2.06 to 0.71; p = 0.34. The difference between CAF + XCM and CAF alone is not statistically significant too: SMD 0.27; 95% CI from −0.15 to 0.68; p = 0.209.Figure 8. Forest plot relating to keratinized tissue width.
- Differential gingival thickness (Figure 9): the statistical analysis finds a statistically significant difference in favor of CAF + CTG compared to CAF + XCM relative to gingival thickness gain at 12-months follow-up: SMD −1.68; 95% CI from −2.78 to −0.58; p = 0.003. The difference between CAF + XCM and CAF alone is statistically significant in favor of the first procedure: SMD 0.56; 95% CI from 0.14 to 0.98; p = 0.009.Figure 9. Forest plot relating to gingival thickness.
4. Discussion
4.1. Quantitative Analysis of Evidence
4.2. Qualitative Analysis of Evidence
4.3. Quality and Limitations of Included Studies
5. Conclusions
- CAF/tunnel + CTG;
- CAF/tunnel + XCM;
- CAF.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cortellini, P.; Bissada, N.F. Mucogingival conditions in the natural dentition: Narrative review, case definitions, and diagnostic considerations. J. Periodontol. 2018, 89, S204–S213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cairo, F.; Nieri, M.; Cincinelli, S.; Mervelt, J.; Pagliaro, U. The interproximal clinical attachment level to classify gingival recessions and predict root coverage outcomes: An explorative and reliability study. J. Clin. Periodontol. 2011, 38, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Bernimoulin, J.-P.; Luscher, B.; Muhlemann, H.R. Coronally repositioned periodontal flap. Clinical evaluation after one year. J. Clin. Periodontol. 1975, 2, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.D., Jr. A classification of marginal tissue recession. Int. J. Periodontics Restor. Dent. 1985, 5, 8–13. [Google Scholar]
- Langer, B.; Langer, L. Subepithelial Connective Tissue Graft Technique for Root Coverage. J. Periodontol. 1985, 56, 715–720. [Google Scholar] [CrossRef]
- Zucchelli, G. Chirurgia Estetica Mucogengivale; Quintessenza Edizioni: Verona, Italy, 2014; ISBN 978-88-7492-196-6. [Google Scholar]
- Cairo, F.; Nieri, M.; Pagliaro, U. Efficacy of periodontal plastic surgery procedures in the treatment of localized facial gingival recessions. A systematic review. J. Clin. Periodontol. 2014, 41 (Suppl. 15), S44–S62. [Google Scholar] [CrossRef]
- Griffin, T.J.; Cheung, W.S.; Zavras, A.I.; Damoulis, P.D. Postoperative complications following gingival augmentation procedures. J. Periodontol. 2006, 77, 2070–2079. [Google Scholar] [CrossRef]
- Cairo, F. Periodontal plastic surgery of gingival recessions at single and multiple teeth. Periodontology 2000 2017, 75, 296–316. [Google Scholar] [CrossRef]
- Wei, P.-C.; Laurell, L.; Geivelis, M.; Lingen, M.W.; Maddalozzo, D. Acellular Dermal Matrix Allografts to Achieve Increased Attached Gingiva. Part 1. A Clinical Study. J. Periodontol. 2000, 71, 1297–1305. [Google Scholar] [CrossRef]
- Wei, P.-C.; Laurell, L.; Lingen, M.W.; Geivelis, M. Acellular Dermal Matrix Allografts to Achieve Increased Attached Gingiva. Part 2. A Histological Comparative Study. J. Periodontol. 2002, 73, 257–265. [Google Scholar] [CrossRef]
- Sanz, M.; Lorenzo, R.; Aranda, J.J.; Martin, C.; Orsini, M. Clinical evaluation of a new collagen matrix (Mucografts prototype) to enhance the width of keratinized tissue in patients with fixed prosthetic restorations: A randomized prospective clinical trial. J. Clin. Periodontol. 2009, 36, 868–876. [Google Scholar] [CrossRef]
- Costa, D.R.; Nicolau, R.A.; Raniero, L.J.; Oliveira, M.A. FTIR and SEM analysis applied in tissue engineering for root recovering surgery. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 105, 1326–1329. [Google Scholar] [CrossRef]
- Ghanaati, S.; Schlee, M.; Webber, M.J.; Willershausen, I.; Barbeck, M.; Balic, E.; Görlach, C.; I Stupp, S.; A Sader, R.; Kirkpatrick, C.J. Evaluation of the tissue reaction to a new bilayered collagen matrix in vivo and its translation to the clinic. Biomed. Mater. 2011, 6, 015010. [Google Scholar] [CrossRef]
- Thoma, D.S.; Nänni, N.; Benic, G.I.; Weber, F.E.; Hämmerle, C.H.F.; Jung, R.E. Effect of platelet-derived growth factor-BB on tissue integration of cross-linked and non-cross-linked collagen matrices in a rat ectopic model. Clin. Oral. Implants Res. 2015, 26, 263–270. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef] [Green Version]
- Wilderman, M.N.; Wentz, F.M. Repair of a Dentogingival Defect with a Pedicle Flap. J. Periodontol. 1965, 36, 218–231. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; Version 5.1.0 [updated March 2011]; The Cochrane Collaboration: London, UK, 2011. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Matoh, U.; Petelin, M.; Gašperšič, R. Split-Mouth Comparison of Coronally Advanced Flap with Connective Tissue Graft or Collagen Matrix for Treatment of Isolated Gingival Recessions. Int. J. Periodontics Restor. Dent. 2019, 39, 439–446. [Google Scholar] [CrossRef]
- Pietruska, M.; Skurska, A.; Podlewski, L.; Milewski, R.; Pietruski, J. Clinical evaluation of Miller class I and II recessions treatment with the use of modified coronally advanced tunnel technique with either collagen matrix or subepithelial connective tissue graft: A randomized clinical study. J. Clin. Periodontol. 2019, 46, 86–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonetti, M.; Cortellini, P.; Pellegrini, G.; Nieri, M.; Bonaccini, D.; Allegri, M.; Bouchard, P.; Cairo, F.; Conforti, G.; Fourmousis, I.; et al. Xenogenic collagen matrix or autologous connective tissue graft as adjunct to coronally advanced flaps for coverage of multiple adjacent gingival recession: Randomized trial assessing non-inferiority in root coverage and superiority in oral health-related quality of life. J. Clin. Periodontol. 2018, 45, 78–88. [Google Scholar] [PubMed]
- Jepsen, K.; Stefanini, M.; Sanz, M.; Zucchelli, G.; Jepsen, S. Long-Term Stability of Root Coverage by Coronally Advanced Flap Procedures. J. Periodontol. 2017, 88, 626–633. [Google Scholar] [CrossRef]
- Cieślik-Wegemund, M.; Wierucka-Młynarczyk, B.; Tanasiewicz, M.; Gilowski, L. Tunnel Technique with Collagen Matrix Compared with Connective Tissue Graft for Treatment of Periodontal Recession: A Randomized Clinical Trial. J. Periodontol. 2016, 87, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- McGuire, M.K.; Scheyer, E.T. Long-Term Results Comparing Xenogeneic Collagen Matrix and Autogenous Connective Tissue Grafts with Coronally Advanced Flaps for Treatment of Dehiscence-Type Recession Defects. J. Periodontol. 2016, 87, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Stefanini, M.; Jepsen, K.; de Sanctis, M.; Baldini, N.; Greven, B.; Heinz, B.; Wennström, J.; Cassel, B.; Vignoletti, F.; Sanz, M.; et al. Patient-reported outcomes and aesthetic evaluation of root coverage procedures: A 12-month follow-up of a randomized controlled clinical trial. J. Clin. Periodontol. 2016, 43, 1132–1141. [Google Scholar] [CrossRef]
- Cardaropoli, D.; Tamagnone, L.; Roffredo, A.; Gaveglio, L. Coronally Advanced Flap with and without a Xenogenic Collagen Matrix in the Treatment of Multiple Recessions: A Randomized Controlled Clinical Study. Restor. Dent. 2014, 34, S97–S102. [Google Scholar]
- Aroca, S.; Molnár, B.; Windisch, P.; Gera, I.; Salvi, G.E.; Nikolidakis, D.; Sculean, A. Treatment of multiple adjacent Miller class I and II gingival recessions with a Modified Coronally Advanced Tunnel (MCAT) technique and a collagen matrix or palatal connective tissue graft: A randomized, controlled clinical trial. J. Clin. Periodontol. 2013, 40, 713–720. [Google Scholar] [CrossRef]
- Cardaropoli, D.; Tamagnone, L.; Roffredo, A.; Gaveglio, L. Treatment of Gingival Recession Defects Using Coronally Advanced Flap with a Porcine Collagen Matrix Compared to Coronally Advanced Flap with Connective Tissue Graft: A Randomized Controlled Clinical Trial. J. Periodontol. 2012, 83, 321–328. [Google Scholar] [CrossRef]
- McGuire, M.K.; Scheyer, E.T. Xenogeneic Collagen Matrix with Coronally Advanced Flap Compared to Connective Tissue with Coronally Advanced Flap for the Treatment of Dehiscence-Type Recession Defects. J. Periodontol. 2010, 81, 1108–1117. [Google Scholar] [CrossRef]
- Moreira, A.R.O.; Santamaria, M.P.; Silvério, K.G.; Casati, M.Z.; Junior, F.H.N.; Sculean, A.; Sallum, E.A. Coronally advanced flap with or without porcine collagen matrix for root coverage: A randomized clinical trial. Clin. Oral Investig. 2016, 20, 2539–2549. [Google Scholar] [CrossRef] [Green Version]
- Tatarakis, N.; Gkranias, N.; Darbar, U.; Donos, N. Blood flow changes using a 3D xenogeneic collagen matrix or a subepithelial connective tissue graft for root coverage procedures: A pilot study. Clin. Oral Investig. 2017, 22, 1697–1705. [Google Scholar] [CrossRef]
- Jepsen, K.; Jepsen, S.; Zucchelli, G.; Stefanini, M.; de Sanctis, M.; Baldini, N.; Greven, B.; Heinz, B.; Wennström, J.; Cassel, B.; et al. Treatment of gingival recession defects with a coronally advanced flap and a xenogeneic collagen matrix: A multicenter randomized clinical trial. J. Clin. Periodontol. 2013, 40, 82–89. [Google Scholar] [CrossRef]
- Atieh, M.A.; Alsabeeha, N.; Tawse-Smith, A.; Payne, A.G.T. Xenogeneic collagen matrix for periodontal plastic surgery procedures: A systematic review and meta-analysis. J. Periodontal Res. 2016, 51, 438–452. [Google Scholar] [CrossRef]
- Huang, J.P.; Liu, J.M.; Wu, Y.M.; Chen, L.L.; Ding, P.H. Efficacy of xenogeneic collagen matrix in the treatment of gingival recessions: A systematic review and meta-analysis. Oral. Dis. 2019, 25, 996–1008. [Google Scholar] [CrossRef]
- Barootchi, S.; Tavelli, L.; Zucchelli, G.; Giannobile, W.V.; Wang, H.L. Gingival phenotype modification therapies on natural teeth: A network meta-analysis. J. Periodontol. 2020, 91, 1386–1399. [Google Scholar] [CrossRef]
- Chambrone, L.; Salinas Ortega, M.A.; Sukekava, F.; Rotundo, R.; Kalemaj, Z.; Buti, J.; Pini Prato, G.P. Cochrane Database. Syst. Rev. 2018, 10, CD007161. [Google Scholar]
- Tavelli, L.; Ravidà, A.; Lin, G.-H.; Del Amo, F.S.-L.; Tattan, M.; Wang, H.-L. Comparison between Subepithelial Connective Tissue Graft and De-epithelialized Gingival Graft: A systematic review and a meta-analysis. J. Int. Acad. Periodontol. 2019, 21, 82–96. [Google Scholar]
- Tavelli, L.; Barootchi, S.; Nguyen, T.V.N.; Tattan, M.; Ravidà, A.; Wang, H.-L. Efficacy of tunnel technique in the treatment of localized and multiple gingival recessions: A systematic review and meta-analysis. J. Periodontol. 2018, 89, 1075–1090. [Google Scholar] [CrossRef]
- Xu, C.; Wang, Q.; Chen, J.; Wu, Y.; Zhao LXu, C. Collagen Matrix for Periodontal Plastic Surgery Procedures: A Meta-analysis Update. Int. J. Periodontics Restor. Dent. 2019, 39, e129–e155. [Google Scholar] [CrossRef]
- Konflanz, W.; Orth, C.C.; Celeste, R.K.; Muniz, F.W.M.G.; Haas, A.N. Influence of Donor Site and Harvesting Technique of Connective Tissue Graft on Root Coverage Outcomes of Single Gingival Recessions: Systematic Review and Meta-analyses. J. Int. Acad. Periodontol. 2021, 23, 79–98. [Google Scholar] [PubMed]
- Alsarhan, M.A.; Al Jasser, R.; Tarish, M.A.; AlHuzaimi, A.I.; Alzoman, H. Xenogeneic collagen matrix versus connective tissue graft for the treatment of multiple gingival recessions: A systematic review and meta-analysis. Clin. Exp. Dent. Res. 2019, 5, 566–579. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.; Wei, T.; Wang, Y.; Cai, Y.-J. Autologous Platelet Concentrates as Clinical Substitutes for Connective Tissue Graft in the Treatment of Miller Class I and II Gingival Recessions: An Updated Meta-Analysis. Int. J. Periodontics Restor. Dent. 2020, 40, e53–e63. [Google Scholar] [CrossRef] [PubMed]
- Moraschini, V.; de Almeida, D.C.F.; Sartoretto, S.; Bailly Guimarães, H.; Chaves Cavalcante, I.; Diuana Calasans-Maia, M. Clinical efficacy of xenogeneic collagen matrix in the treatment of gingival recession: A systematic review and meta-analysis. Acta Odontol. Scand. 2019, 77, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, A.; Yadav, V.S.; Tewari, N.; Kumar, A.; Sharma, R.K. Efficacy of modified coronally advanced flap in the treatment of multiple adjacent gingival recessions: A systematic review and meta-analysis. Acta Odontol. Scand. 2021, 79, 562–572. [Google Scholar] [CrossRef]
- Mancini, L.; Tarallo, F.; Quinzi, V.; Fratini, A.; Mummolo, S.; Marchetti, E. Platelet-Rich Fibrin in Single and Multiple Coronally Advanced Flap for Type 1 Recession: An Updated Systematic Review and Meta-Analysis. Medicina 2021, 57, 144. [Google Scholar] [CrossRef]
- Elangovan, S. Tunneling Technique in Conjunction with Autogenous Graft or Graft Substitutes Is a Predictable Surgical Approach to Achieve Root Coverage in Isolated or Multiple Gingival Recession Defects. J. Evid. Based Dent. Pract. 2019, 19, 189–191. [Google Scholar] [CrossRef]
- Li, R.; Liu, Y.; Xu, T.; Zhao, H.; Hou, J.; Wu, Y.; Zhang, D. The Additional Effect of Autologous Platelet Concentrates to Coronally Advanced Flap in the Treatment of Gingival Recessions: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2019, 2019, 2587245. [Google Scholar] [CrossRef] [Green Version]
- Panda, S.; Khijmatgar, S.; Arbildo-Vega, H.; Das, A.C.; Kumar, M.; Das, M.; Mancini, L.; Del Fabbro, M. Stability of biomaterials used in adjunct to coronally advanced flap: A systematic review and network me-ta-analysis. Clin. Exp. Dent. Res. 2022, 8, 421–438. [Google Scholar] [CrossRef]

| Authors | Year | Design | Setting | N° Patients | N° Sites | Test Surgery | Control Surgery | Type of Defect | Follow-Up (Months) |
|---|---|---|---|---|---|---|---|---|---|
| Matoh et al. [23] | 2019 | RCT (split mouth) | University | 10 | 20 | CAF + XCM | CAF + CTG | single GRs | 12 |
| Pietruska et al. [24] | 2019 | RCT (split mouth) | University | 20 | 91 | tunnel + XCM | tunnel + CTG | multiple GRs | 12 |
| Tonetti et al. [25] | 2018 | RCT (parallel groups) | University | 187 | 485 | CAF + XCM | CAF + CTG | multiple GRs | 6 |
| Jepsen et al. [26] | 2017 | RCT (split mouth) | University | 18 | 36 | CAF + XCM | CAF | single GRs | 36 |
| Tatarakis et al. [35] | 2017 | RCT (parallel groups) | University | 8 | 8 | CAF + XCM | CAF + CTG | single GRs | 6 |
| Cieslik-Wegwmund et al. [27] | 2016 | RCT (parallel groups) | University | 28 | 106 | tunnel + XCM | tunnel + CTG | multiple GRs | 6 |
| McGuire et al. [28] | 2016 | RCT (split mouth) | Private practice | 17 | 34 | CAF + XCM | CAF + CTG | single GRs | 60 |
| Moreira et al. [34] | 2016 | RCT (parallel groups) | University | 40 | 40 | CAF + XCM | CAF | single GRs | 6 |
| Stefanini et al. [29] | 2016 | RCT (split mouth) | University and private practice | 45 | 90 | CAF + XCM | CAF | single GRs | 12 |
| Cardaropoli et al. [30] | 2014 | RCT (parallel groups) | Not specified | 32 | 113 | CAF + XCM | CAF | multiple GRs | 12 |
| Aroca et al. [31] | 2013 | RCT (split mouth) | University | 22 | 156 | tunnel + XCM | tunnel + CTG | multiple GRs | 12 |
| Jepsen et al. [36] | 2013 | RCT (split mouth) | University and private practice | 45 | 90 | CAF + XCM | CAF | single GRs | 6 |
| Cardaropoli et al. [32] | 2012 | RCT (parallel groups) | Private practice | 18 | 22 | CAF + XCM | CAF + CTG | multiple GRs | 12 |
| McGuire et al. [33] | 2010 | RCT (split mouth) | Private practice | 25 | 50 | CAF + XCM | CAF + CTG | single GRs | 12 |
| Authors | Year | Surgery Test VS Control | CRC (%) | MRC (%) | RecRed (mm) | ΔCAL (mm) | ΔKTW (mm) | ΔGT (mm) | Follow-Up (Months) |
|---|---|---|---|---|---|---|---|---|---|
| Matoh et al. [23] | 2019 | CAF + XCM | 70 | 85 ± 24 | - | - | - | 0.3 ± 0.2 | 12 |
| CAF + CTG | 100 | 100 | - | - | - | 0.9 ± 0.2 | |||
| Pietruska et al. [24] | 2019 | Tunnel + XCM | 19.6 | 53.20 ± 32.17 | 1.00 ± 0.69 | - | 0.52 ± 0.65 | 0.27 ± 0.40 | 12 |
| Tunnel + CTG | 68.8 | 83.10 ± 27.63 | 1.54 ± 0.58 | - | 2.78 ± 1.53 | 1.1 ± 0.54 | |||
| Tonetti et al. [25] | 2018 | CAF + XCM | 48 | - | 1.7 ± 1.1 | - | −0.1 ± 1.1 | - | 6 |
| CAF + CTG | 70 | - | 2.1 ± 1 | - | 0.5 ± 1.2 | - | |||
| Jepsen et al. [26] | 2017 | CAF + XCM | 61.1 | 91.70 ± 12.05 | 2.92 ± 0.71 | 3.17 ± 1.11 | 1.92 ± 1 | 0.59 ± 0.39 | 36 |
| CAF | 38.9 | 82.77 ± 17.03 | 2.53 ± 0.72 | 2.67 ± 1.14 | 1.03 ± 1.1 | 0.16 ± 0.40 | |||
| Cieslik-Wegwmund et al. [27] | 2016 | Tunnel + XCM | 14.3 | 91 ± 13 | - | - | - | - | 6 |
| Tunnel + CTG | 71.4 | 95 ± 11 | - | - | - | - | |||
| McGuire et al. [28] | 2016 | CAF + XCM | 52.9 | 77.6 ± 29.2 | - | - | - | - | 60 |
| CAF + CTG | 88.2 | 95.5 ± 12.8 | - | - | - | - | |||
| Moreira et al. [34] | 2016 | CAF + XCM | 40 | 77 ± 21.2 | 2.41 ± 0.73 | - | - | 0.40 ± 0.19 | 6 |
| CAF | 35 | 72 ± 14.4 | 2.25 ± 0.50 | - | - | 0.14 ± 0.29 | |||
| Stefanini et al. [29] | 2016 | CAF + XCM | 93.3 | 76.28 ± 28.07 | 2.48 ± 1.46 | - | 1.06 ± 1.07 | 0.52 ± 0.46 | 12 |
| CAF | 84.4 | 75.05 ± 25.24 | 2.26 ± 1.17 | - | 0.64 ± 1.05 | 0.27 ± 0.43 | |||
| Cardaropoli et al. [30] | 2014 | CAF + XCM | 72.4 | 93.25 ± 10.01 | 2.28 ± 0.82 | - | - | - | 12 |
| CAF | 48.1 | 81.49 ± 23.45 | 1.85 ± 0.99 | - | - | - | |||
| Aroca et al. [31] | 2013 | Tunnel + XCM | 23 | 71 ± 21 | - | - | - | - | 12 |
| Tunnel + CTG | 59 | 90 ± 18 | - | - | - | - | |||
| Cardaropoli et al. [32] | 2012 | CAF + XCM | 72 | 94.32 ± 11.68 | 2.86 ± 0.39 | 2.41 ± 0.83 | 1.23 ± 0.61 | 1 ± 0.32 | 12 |
| CAF + CTG | 81 | 96.97 ± 6.74 | 2.95 ± 0.69 | 2.95 ± 0.82 | 1.27 ± 0.65 | 1.23 ± 0.47 | |||
| McGuire et al. [33] | 2010 | CAF + XCM | - | 88.5 ± 21.08 | 2.17 ± 0.67 | 2.26 ± 1.21 | 1.11 ± 0.82 | - | 12 |
| CAF + CTG | - | 99.3 ± 2.54 | 3.17 ± 0.38 | 2.85 ± 0.63 | 1.09 ± 1.6 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Santis, D.; Luciano, U.; Pancera, P.; Castegnaro, G.; Alberti, C.; Gelpi, F. A New Matrix for Soft Tissue Management. J. Clin. Med. 2022, 11, 4486. https://doi.org/10.3390/jcm11154486
De Santis D, Luciano U, Pancera P, Castegnaro G, Alberti C, Gelpi F. A New Matrix for Soft Tissue Management. Journal of Clinical Medicine. 2022; 11(15):4486. https://doi.org/10.3390/jcm11154486
Chicago/Turabian StyleDe Santis, Daniele, Umberto Luciano, Paola Pancera, Giacomo Castegnaro, Christian Alberti, and Federico Gelpi. 2022. "A New Matrix for Soft Tissue Management" Journal of Clinical Medicine 11, no. 15: 4486. https://doi.org/10.3390/jcm11154486
APA StyleDe Santis, D., Luciano, U., Pancera, P., Castegnaro, G., Alberti, C., & Gelpi, F. (2022). A New Matrix for Soft Tissue Management. Journal of Clinical Medicine, 11(15), 4486. https://doi.org/10.3390/jcm11154486