The Immunohistochemical Assessment of Neoangiogenesis Factors in Squamous Cell Carcinomas and Their Precursors in the Skin
Abstract
:1. Introduction
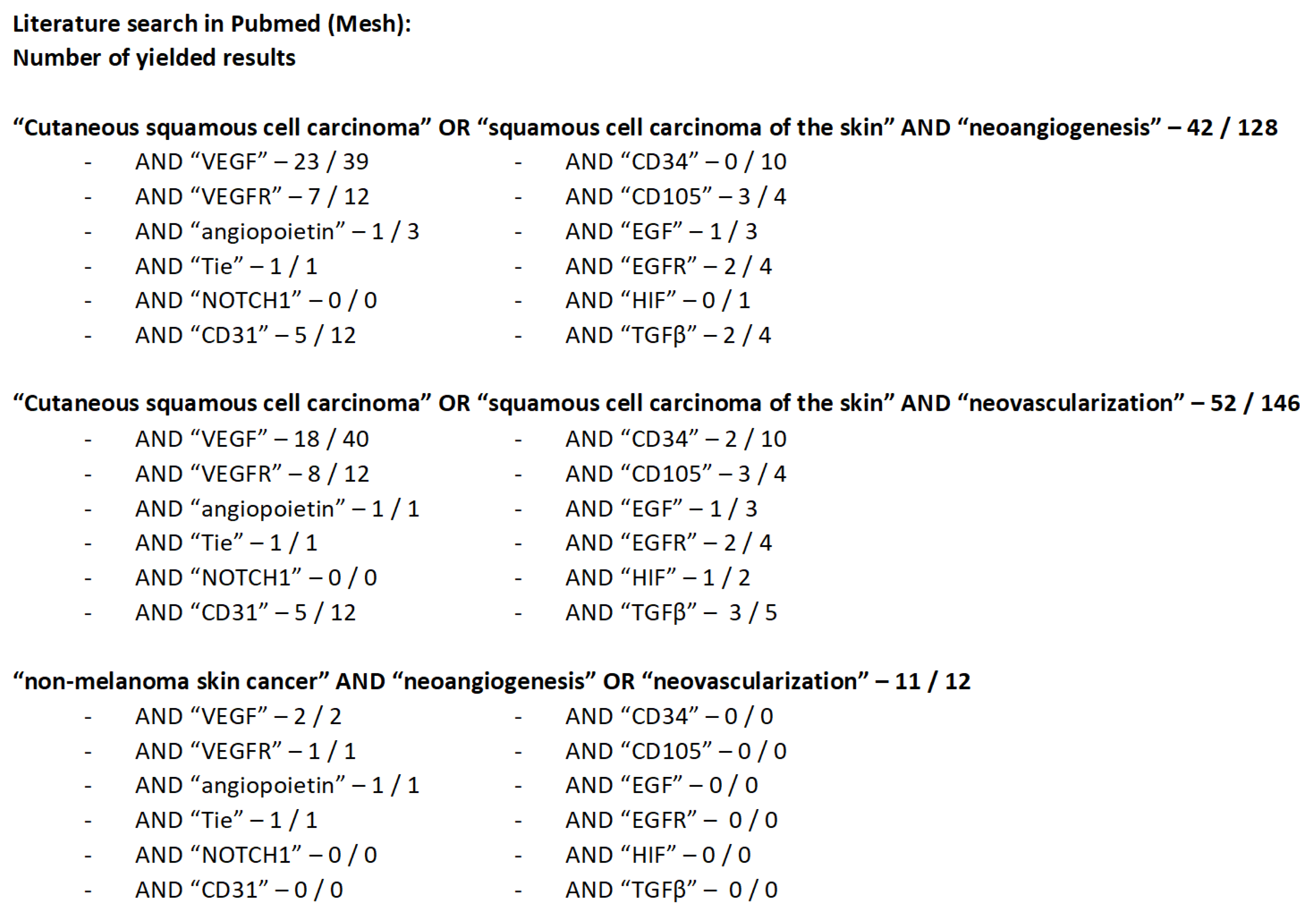
2. Literature Search Strategy and Yielded Results
2.1. VEGF and VEGFR
2.2. Angiopoietin 1,2 and Tie 2
2.3. Notch1
2.4. CD31, CD34, and CD105
2.5. EGF and EGFR
2.6. Other Factors
3. Discussion
- A potential diagnostic role was found for Ang2, which is expressed only by the endothelium of tumor vessels but is probably not specific for SCC, and Notch1, which is decreased in sun-exposed areas. Autofluorescence should also be considered in the future as a noninvasive diagnostic tool complementary to the current gold standard.
- VEGF, Notch1, CD105, p53, EGFR, HIF-1α, p21, and TGFβ could be prognostically important factors. All of these factors are highly elevated in the advanced stages of cSCC.
- Possible therapeutic strategies include the use of β2 adrenergic receptor antagonist, Ang1 analogs, anti-EGFR, anti-TRAF6, anti-EGFR combined with anti-IGF-IR, anti-HIF-1α, and collagen VII.
4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A12 | Human monoclonal antibody against IGF-IR |
| AK | Actinic keratosis |
| Ang1/2 | Angiopoietin 1/2 |
| BCC | Basal cell carcinoma/Basalioma |
| CD31 | Cluster of differentiation 31 = Platelet endothelial cell adhesion molecule |
| CD34 | Cluster of differentiation 34 |
| CD105 | Cluster of differentiation 105 = Endoglin |
| CT | Computed tomography |
| cSCC | Spinocellular carcinoma of the skin/squamous cell carcinoma/spinalioma |
| EGF | Epidermal growth factor |
| EGFR | Epidermal growth factor receptor |
| HIF-1α | Hypoxia-inducible factor 1α |
| HPV | Human papillomavirus |
| IGF-IR | Insulin-like growth factor 1 receptor |
| IgG1 | Immunoglobulin G1 |
| Ki67 | Antigen Ki67 |
| LRIG2 | leucine-rich repeats and immunoglobulin-like domains 2 |
| mRNA | Messenger ribonucleic acid |
| NF-κB | Nuclear factor kappa B |
| p21 | CDK-Inhibitor 1 = cyclin-dependent kinase inhibitor 1 |
| PD1 | Programmed cell death protein 1 |
| RAS | Rat sarcoma |
| RDBE | recessive dystrophic epidermolysis bullosa |
| RNA | Ribonucleic acid |
| αSMA | Smooth muscle actin |
| TGFβ | Transforming growth factor β |
| TP53/P53 | Tumorprotein p53 |
| TPA | 12-O-tetradecanoylphorbol-13-acetate |
| TRAF6 | Tumor necrosis factor receptor-associated factor 6 |
| UV | Ultraviolet |
| VEGF | Vascular endothelial growth factor |
| VEGFR | Vascular endothelial growth factor receptor |
References
- Morteza Seyed Jafari, S.; Bossart, S.; Houriet, C.; Hunger, R.E. Hautkrebs–Vorbeugung und Therapie. Therapeutische Umsch. 2019, 76, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.; Otley, C.C. Skin cancer in organ transplant recipients: Epidemiology, pathogenesis, and management. J. Am. Acad. Dermatol. 2002, 47, 1–20. [Google Scholar] [CrossRef]
- Dummer, R.; Ludwig, S.; Kaufmann, C.; Binkert, A.; Mangana, J.; Goldinger, S.M.; Braun, R. Modernes Management von Hauttumoren. Schweiz. Med.-Forum 2017, 17, 678–685. [Google Scholar] [CrossRef] [Green Version]
- Kempf, W.; Hantschke, M.; Kutzner, H.; Burgdorf, W. Dermatopathologie, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2011; ISBN 978-3-642-12896-7. Available online: https://www.springer.com/de/book/9783642128967 (accessed on 24 April 2020).
- Jenni, D.; Hofbauer, G.F. Spinozelluläre Karzinome der Haut–ein weisser Hautkrebs kommt selten allein. Swiss Med. Forum 2013, 13, 814–817. [Google Scholar] [CrossRef]
- Ribero, S.; Stucci, L.S.; Daniels, G.A.; Borradori, L. Drug therapy of advanced cutaneous squamous cell carcinoma: Is there any evidence? Curr. Opin. Oncol. 2017, 29, 129–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, A.; Duggan, S.; Deeks, E.D. Cemiplimab: A Review in Advanced Cutaneous Squamous Cell Carcinoma. Drugs 2020, 80, 813–819. [Google Scholar] [CrossRef]
- Seyed Jafari, S.M.; Wiedmer, C.; Cazzaniga, S.; Frangež, Ž.; Shafighi, M.; Beltraminelli, H.; Weber, B.; Simon, H.-U.; Hunger, R.E. Correlation of Vascular Endothelial Growth Factor subtypes and their receptors with melanoma progression: A next-generation Tissue Microarray (ngTMA) automated analysis. PLoS ONE 2018, 13, e0207019. [Google Scholar] [CrossRef] [Green Version]
- Strieth, S.; Hartschuh, W.; Pilz, L.; Fusenig, N.E. Angiogenic switch occurs late in squamous cell carcinomas of human skin. Br. J. Cancer 2000, 82, 591–600. [Google Scholar] [CrossRef]
- Bowden, J.; Brennan, P.A.; Umar, T.; Cronin, A. Expression of vascular endothelial growth factor in basal cell carcinoma and cutaneous squamous cell carcinoma of the head and neck. J. Cutan. Pathol. 2002, 29, 585–589. [Google Scholar] [CrossRef]
- Kanitz, A.; Imig, J.; Dziunycz, P.J.; Primorac, A.; Galgano, A.; Hofbauer, G.F.L.; Gerber, A.P.; Detmar, M. The expression levels of microRNA-361-5p and its target VEGFA are inversely correlated in human cutaneous squamous cell carcinoma. PLoS ONE 2012, 7, e49568. [Google Scholar] [CrossRef] [Green Version]
- Tzoutzos, K.; Batistatou, A.; Kitsos, G.; Liasko, R.; Stefanou, D. Study of microvascular density and expression of vascular endothelial growth factor and its receptors in cancerous and precancerous lesions of the eyelids. Anticancer Res. 2014, 34, 4977–4983. [Google Scholar] [PubMed]
- Ciortea, C.D.; Jung, I.; Gurzu, S.; Kövecsi, A.; Turdean, S.G.; Bara, T. Correlation of angiogenesis with other immunohistochemical markers in cutaneous basal and squamous cell carcinomas. Rom. J. Morphol. Embryol. 2015, 56, 665–670. [Google Scholar] [PubMed]
- Bălăşoiu, A.T.; Ciurea, R.N.; Mănescu, M.R.; Mocanu, C.L.; Stepan, A.E.; Bălăşoiu, M.; Niculescu, M. Assessment of VEGF and EGFR in the study of angiogenesis of eyelid carcinomas. Rom. J. Morphol. Embryol. 2016, 57, 1229–1234. [Google Scholar] [PubMed]
- Nie, X.-J.; Liu, W.-M.; Zhang, L. Association of VEGF Gene Polymorphisms with the Risk and Prognosis of Cutaneous Squamous Cell Carcinoma. Med. Sci. Monit. 2016, 22, 3658–3665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pradeep, C.R.; Sunila, E.S.; Kuttan, G. Expression of vascular endothelial growth factor (VEGF) and VEGF receptors in tumor angiogenesis and malignancies. Integr. Cancer Ther. 2005, 4, 315–321. [Google Scholar] [CrossRef]
- Lu, K.; Baht, M.; Peters, S.; Mitra, R.; Oberyszyn, T. Suppression of beta 2 adrenergic receptor actions prevent UVB mediated cutaneous squamous cell tumorigenesis through inhibition of VEGF-A induced angiogenesis. Mol. Carcinog. 2021, 60, 172–178. [Google Scholar] [CrossRef]
- Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group; Martin, D.F.; Maguire, M.G.; Fine, S.L.; Ying, G.; Jaffe, G.J.; Grunwald, J.E.; Toth, C.; Redford, M.; Ferris, F.L. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: Two-year results. Ophthalmology 2012, 119, 1388–1398. [Google Scholar] [CrossRef] [Green Version]
- Diabetic Retinopathy Clinical Research Network; Wells, J.A.; Glassman, A.R.; Ayala, A.R.; Jampol, L.M.; Aiello, L.P.; Antoszyk, A.N.; Arnold-Bush, B.; Baker, C.W.; Bressler, N.M.; et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N. Engl. J. Med. 2015, 372, 1193–1203. [Google Scholar] [CrossRef] [Green Version]
- Hawighorst, T.; Skobe, M.; Streit, M.; Hong, Y.-K.; Velasco, P.; Brown, L.F.; Riccardi, L.; Lange-Asschenfeldt, B.; Detmar, M. Activation of the tie2 receptor by angiopoietin-1 enhances tumor vessel maturation and impairs squamous cell carcinoma growth. Am. J. Pathol. 2002, 160, 1381–1392. [Google Scholar] [CrossRef] [Green Version]
- El-Nabarawy, E.A.; El-Hanafy, G.M.; Rashed, L.A.; Yasin, F.S. Expression of angiopoietin-1, angiopoietin-2, and their receptor Tie2 in verruca vulgaris (common skin warts). Int. J. Dermatol. 2016, 55, e327–e331. [Google Scholar] [CrossRef]
- Tampa, M.; Mitran, C.I.; Mitran, M.I.; Nicolae, I.; Dumitru, A.; Matei, C.; Manolescu, L.; Popa, G.L.; Caruntu, C.; Georgescu, S.R. The Role of Beta HPV Types and HPV-Associated Inflammatory Processes in Cutaneous Squamous Cell Carcinoma. J. Immunol. Res. 2020, 2020, 5701639. [Google Scholar] [CrossRef] [PubMed]
- Panelos, J.; Tarantini, F.; Paglierani, M.; Di Serio, C.; Maio, V.; Pellerito, S.; Pimpinelli, N.; Santucci, M.; Massi, D. Photoexposition discriminates Notch 1 expression in human cutaneous squamous cell carcinoma. Mod. Pathol. 2008, 21, 316–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, N.J.; Sanborn, Z.; Arnett, K.L.; Bayston, L.J.; Liao, W.; Proby, C.M.; Leigh, I.M.; Collisson, E.A.; Gordon, P.B.; Jakkula, L.; et al. Loss-of-function mutations in Notch receptors in cutaneous and lung squamous cell carcinoma. Proc. Natl. Acad. Sci. USA 2011, 108, 17761–17766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- South, A.P.; Purdie, K.J.; Watt, S.A.; Haldenby, S.; den Breems, N.; Dimon, M.; Arron, S.T.; Kluk, M.J.; Aster, J.C.; McHugh, A.; et al. NOTCH1 mutations occur early during cutaneous squamous cell carcinogenesis. J. Investig. Dermatol. 2014, 134, 2630–2638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demehri, S.; Turkoz, A.; Kopan, R. Epidermal Notch1 loss promotes skin tumorigenesis by impacting the stromal microenvironment. Cancer Cell 2009, 16, 55–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Biswas, S.; Qin, X.; Gong, W.; Deng, W.; Yu, H. Does Notch play a tumor suppressor role across diverse squamous cell carcinomas? Cancer Med. 2016, 5, 2048–2060. [Google Scholar] [CrossRef] [PubMed]
- Florence, M.E.B.; Massuda, J.Y.; Bröcker, E.-B.; Metze, K.; Cintra, M.L.; de Souza, E.M. Angiogenesis in the progression of cutaneous squamous cell carcinoma: An immunohistochemical study of endothelial markers. Clinics (Sao Paulo) 2011, 66, 465–468. [Google Scholar] [CrossRef] [Green Version]
- Bossart, S.; Hartmeier, L.; Seyed Jafari, S.M.; Beltraminelli, H.; Hunger, R.E. Immunohistochemical Evaluation of Angiogenesis in Warts. Clin. Res. Trials 2020, 6, 1–3. [Google Scholar] [CrossRef]
- Kakasheva-Mazhenkovska, L.; Basheska, N.; Crvenkova, S.; Gordana, P.; Milenkova, L.; Janevska, V.; Serafimoski, V. Correlation between microvessel density and morphological features in skin squamous cell carcinoma. PRILOZI 2017, 38, 63–73. [Google Scholar] [CrossRef] [Green Version]
- Florence, M.E.B.; Massuda, J.Y.; Soares, T.C.B.; Stelini, R.F.; Poppe, L.M.; Bröcker, E.-B.; Metze, K.; Cintra, M.L.; de Souza, E.M. p53 immunoexpression in stepwise progression of cutaneous squamous cell carcinoma and correlation with angiogenesis and cellular proliferation. Pathol. Res. Pract. 2015, 211, 782–788. [Google Scholar] [CrossRef]
- Galer, C.E.; Corey, C.L.; Wang, Z.; Younes, M.N.; Gomez-Rivera, F.; Jasser, S.A.; Ludwig, D.L.; El-Naggar, A.K.; Weber, R.S.; Myers, J.N. Dual inhibition of epidermal growth factor receptor and insulin-like growth factor receptor I: Reduction of angiogenesis and tumor growth in cutaneous squamous cell carcinoma. Head. Neck 2011, 33, 189–198. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wu, L.; Xiao, T.; Tang, L.; Jia, X.; Guo, Y.; Zhang, J.; Li, J.; He, Y.; Su, J.; et al. TRAF6 regulates EGF-induced cell transformation and cSCC malignant phenotype through CD147/EGFR. Oncogenesis 2018, 7, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montaudié, H.; Viotti, J.; Combemale, P.; Dutriaux, C.; Dupin, N.; Robert, C.; Mortier, L.; Kaphan, R.; Duval-Modeste, A.; Dalle, S.; et al. Cetuximab is efficient and safe in patients with advanced cutaneous squamous cell carcinoma: A reprospective, multicentre study. Oncotarget 2020, 11, 378–385. [Google Scholar] [CrossRef] [Green Version]
- Cammareri, P.; Rose, A.M.; Vincent, D.F.; Wang, J.; Nagano, A.; Libertini, S.; Ridgway, R.A.; Athineos, D.; Coates, P.J.; McHugh, A.; et al. Inactivation of TGFβ receptors in stem cells drives cutaneous squamous cell carcinoma. Nat. Commun. 2016, 7, 12493. [Google Scholar] [CrossRef] [PubMed]
- Martins, V.L.; Caley, M.P.; Moore, K.; Szentpetery, Z.; Marsh, S.T.; Murrell, D.F.; Kim, M.H.; Avari, M.; McGrath, J.A.; Cerio, R.; et al. Suppression of TGβ and Angiogenesis by Type VII Collagen in Cutaneous SCC. J. Natl. Cancer Inst. 2016, 108, djv293. [Google Scholar] [CrossRef] [Green Version]
- Todd, C.; Reynolds, N.J. Up-regulation of p21WAF1 by phorbol ester and calcium in human keratinocytes through a protein kinase C-dependent pathway. Am. J. Pathol. 1998, 153, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.-S.; Bae, J.-M.; Chun, Y.-S.; Chung, J.-H.; Jeon, Y.-K.; Kim, I.-S.; Kim, M.-S.; Park, J.-W. HIF-1alpha controls keratinocyte proliferation by up-regulating p21(WAF1/Cip1). Biochim. Biophys. Acta 2008, 1783, 323–333. [Google Scholar] [CrossRef] [Green Version]
- Seleit, I.; Bakry, O.A.; Al-Sharaky, D.R.; Ragab, R.A.A.; Al-Shiemy, S.A. Evaluation of Hypoxia Inducible Factor-1α and Glucose Transporter-1 Expression in Non Melanoma Skin Cancer: An Immunohistochemical Study. J. Clin. Diagn. Res. 2017, 11, EC09–EC16. [Google Scholar] [CrossRef] [PubMed]
- Ornat, M.; Kobierzycki, C.; Grzegrzolka, J.; Pula, B.; Zamirska, A. SOX18 Expression in Non-Melanoma Skin Cancer. Anticancer. Res. 2016, 36, 2379–2383. [Google Scholar]
- Neinaa, Y.; El-Ashmawy, A.; Alshenawy, H.; Arakeeb, E. Significance of SOX18 expression in nonmelanoma skin cancers for prediction of high-risk patients: A preliminary study. Int. J. Dermatol. 2020, 59, 1117–1124. [Google Scholar] [CrossRef]
- Alameda, J.; García-García, V.; López, S.; Hernando, A.; Page, A.; Navarro, M.; Moreno-Maldonado, R.; Paramio, J.; Ramírez, Á.; García-Fernández, R.; et al. CYLD Inhibits the development of Skin Squamous Cell Tumors in Immunocompetent Mice. Int. J. Mol. Sci. 2021, 22, 6736. [Google Scholar] [CrossRef] [PubMed]
- Neinaa, Y.; El-Ashmawy, A.; Alshenawy, H.; Dorgham, W. The Prognostic Value of Podoplanin Expression in Non-melanoma Skin Cancers: Correlation With Lymphatic Vessel Density. Am. J. Dermatopathol. 2020, 42, 432–438. [Google Scholar] [CrossRef]
- Hoesl, C.; Fröhlich, T.; Hundt, J.E.; Kneitz, H.; Goebeler, M.; Wolf, R.; Schneider, M.R.; Dahlhoff, M. The transmembrane orotein LRIG2 increases tumor progression in skin carcinogensis. Mol. Oncol. 2019, 13, 2476–2492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giovannacci, I.; Meleti, M.; Garbarino, F.; Cesinaro, A.; Mataca, E.; Pedrazzi, G.; Reggiani, C.; Paganelli, A.; Truzzi, A.; Elia, F.; et al. Correlation between autofluorescence intensity and histopathological features in non-melanoma skin cancer: An ex vivo study. Cancers 2021, 13, 3974. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daneluzzi, C.; Seyed Jafari, S.M.; Hunger, R.; Bossart, S. The Immunohistochemical Assessment of Neoangiogenesis Factors in Squamous Cell Carcinomas and Their Precursors in the Skin. J. Clin. Med. 2022, 11, 4494. https://doi.org/10.3390/jcm11154494
Daneluzzi C, Seyed Jafari SM, Hunger R, Bossart S. The Immunohistochemical Assessment of Neoangiogenesis Factors in Squamous Cell Carcinomas and Their Precursors in the Skin. Journal of Clinical Medicine. 2022; 11(15):4494. https://doi.org/10.3390/jcm11154494
Chicago/Turabian StyleDaneluzzi, Cloé, Seyed Morteza Seyed Jafari, Robert Hunger, and Simon Bossart. 2022. "The Immunohistochemical Assessment of Neoangiogenesis Factors in Squamous Cell Carcinomas and Their Precursors in the Skin" Journal of Clinical Medicine 11, no. 15: 4494. https://doi.org/10.3390/jcm11154494
APA StyleDaneluzzi, C., Seyed Jafari, S. M., Hunger, R., & Bossart, S. (2022). The Immunohistochemical Assessment of Neoangiogenesis Factors in Squamous Cell Carcinomas and Their Precursors in the Skin. Journal of Clinical Medicine, 11(15), 4494. https://doi.org/10.3390/jcm11154494





