Ocular Manifestations in Patients with Inflammatory Bowel Disease in the Biologics Era
Abstract
1. Introduction
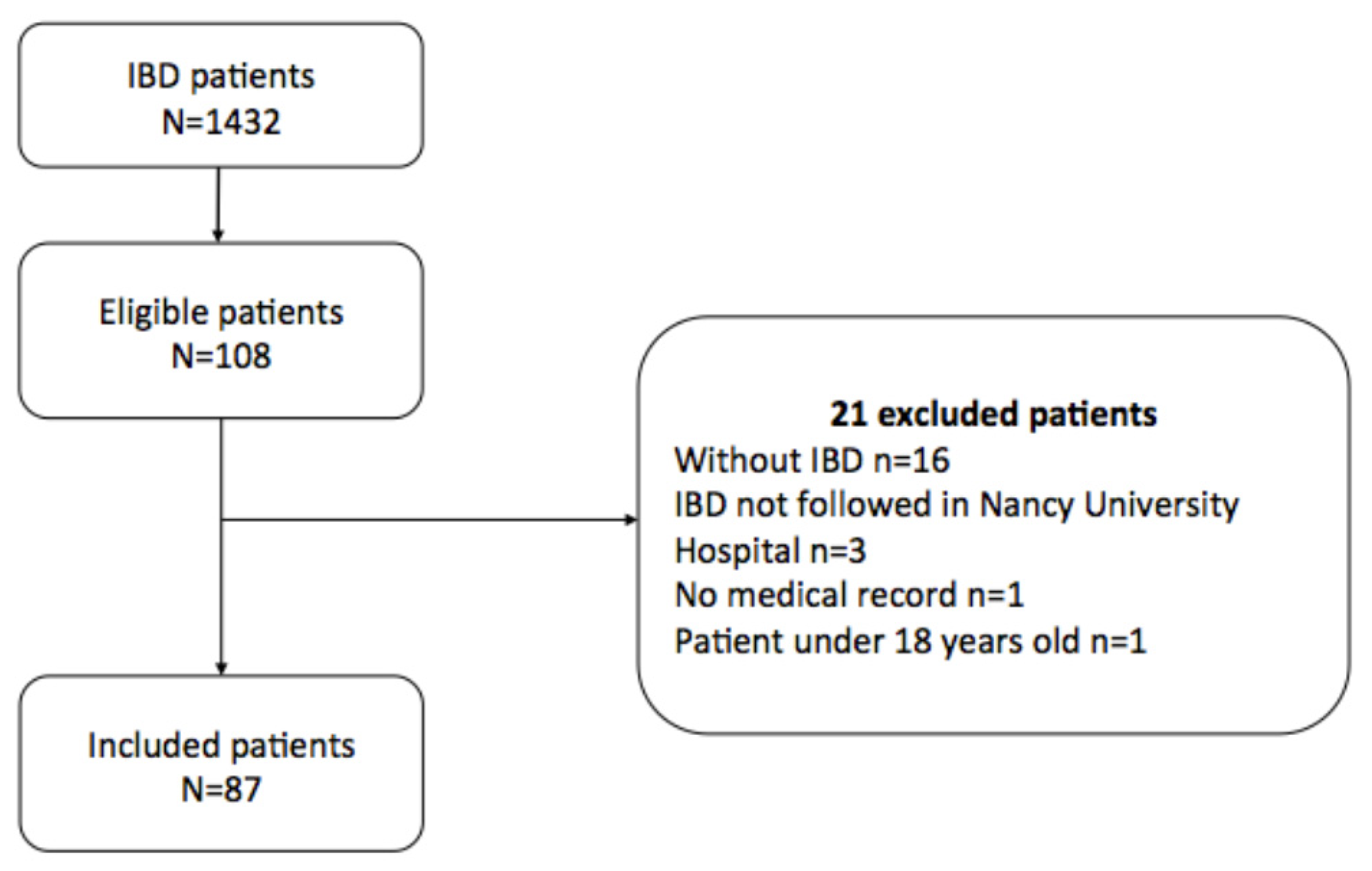
2. Methods
2.1. Study Design and Study Population
2.2. Data Collection
2.3. Definition of Groups
2.4. Statistical Analyses
3. Results
3.1. Patients’ Characteristics
3.2. Ocular Symptoms
3.3. Risk Factors for Ocular Manifestations
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.-F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Torres, J.; Mehandru, S.; Colombel, J.-F.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef]
- Vavricka, S.R.; Schoepfer, A.; Scharl, M.; Lakatos, P.L.; Navarini, A.; Rogler, G. Extraintestinal Manifestations of Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2015, 21, 1982–1992. [Google Scholar] [CrossRef] [PubMed]
- Zippi, M.; Corrado, C.; Pica, R.; Avallone, E.V.; Cassieri, C.; de Nitto, D.; Paoluzi, P.; Vernia, P. Extraintestinal manifestations in a large series of Italian inflammatory bowel disease patients. World J. Gastroenterol. 2014, 20, 17463–17467. [Google Scholar] [CrossRef]
- Veloso, F.T.; Carvalho, J.; Magro, F. Immune-Related Systemic Manifestations of Inflammatory Bowel Disease: A Prospective Study of 792 Patients. J. Clin. Gastroenterol. 1996, 23, 29–34. [Google Scholar] [CrossRef]
- Algaba, A.; Guerra, I.; Ricart, E.; Iglesias, E.; Mañosa, M.; Gisbert, J.P.; Guardiola, J.; Mínguez, M.; Castro, B.; de Francisco, R.; et al. Extraintestinal Manifestations in Patients with Inflammatory Bowel Disease: Study Based on the ENEIDA Registry. Dig. Dis. Sci. 2021, 66, 2014–2023. [Google Scholar] [CrossRef]
- Toutée, A.; Bodaghi, B. Atteintes de la surface oculaire des maladies de système: Behçet, sarcoïdose et MICI. Reflex. Ophtalmol. 2019, 26–29. [Google Scholar]
- Mintz, R.; Feller, E.R.; Bahr, R.L.; Shah, S.A. Ocular manifestations of inflammatory bowel disease. Inflamm. Bowel. Dis. 2004, 10, 135–139. [Google Scholar] [CrossRef]
- Troncoso, L.L.; Biancardi, A.L.; de Moraes, H.V., Jr.; Zaltman, C. Ophthalmic manifestations in patients with inflammatory bowel disease: A review. World J. Gastroenterol. 2017, 23, 5836. [Google Scholar] [CrossRef]
- Evans, P.E.; Pardi, D.S. Extraintestinal Manifestations of Inflammatory Bowel Disease: Focus on the Musculoskeletal, Dermatologic, and Ocular Manifestations. Medscape Gen. Med. 2007, 9, 55. [Google Scholar]
- Mady, R.; Grover, W.; Butrus, S. Ocular Complications of Inflammatory Bowel Disease. Sci. World J. 2015, 2015, 438402. [Google Scholar] [CrossRef] [PubMed]
- Taleban, S.; Li, D.; Targan, S.R.; Ippoliti, A.; Brant, S.R.; Cho, J.H.; Duerr, R.H.; Rioux, J.D.; Silverberg, M.S.; Vasiliauskas, E.A.; et al. Ocular Manifestations in Inflammatory Bowel Disease Are Associated with Other Extra-intestinal Manifestations, Gender, and Genes Implicated in Other Immune-related Traits. J. Crohn’s Colitis 2016, 10, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Peyrin-Biroulet, L.; Van Assche, G.; Gómez-Ulloa, D.; García-Álvarez, L.; Lara, N.; Black, C.M.; Kachroo, S. Systematic Review of Tumor Necrosis Factor Antagonists in Extraintestinal Manifestations in Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2017, 15, 25–36.e27. [Google Scholar] [CrossRef] [PubMed]
- Jabs, D.A.; Rosenbaum, J.T.; Foster, C.S.; Holland, G.N.; Jaffe, G.J.; Louie, J.S.; Nussenblatt, R.B.; Stiehm, E.R.; Tessler, H.; Van Gelder, R.N.; et al. Guidelines for the Use of Immunosuppressive Drugs in Patients with Ocular Inflammatory Disorders: Recommendations of an Expert Panel. Am. J. Ophthalmol. 2000, 130, 492–513. [Google Scholar] [CrossRef]
- Dick, A.D.; Rosenbaum, J.T.; Al-Dhibi, H.A.; Belfort, R., Jr.; Brézin, A.P.; Chee, S.P.; Davis, J.L.; Ramanan, A.V.; Sonoda, K.H.; Carreño, E.; et al. Guidance on Noncorticosteroid Systemic Immunomodulatory Therapy in Noninfectious Uveitis. Ophthalmology 2018, 125, 757–773. [Google Scholar] [CrossRef]
- Susanna, F.N.; Pavesio, C. A review of ocular adverse events of biological anti-TNF drugs. J. Ophthalmic Inflamm. Infect. 2020, 10, 11. [Google Scholar] [CrossRef]
- Guillo, L.; D’Amico, F.; Serrero, M.; Angioi, K.; Loeuille, D.; Costanzo, A.; Danese, S.; Peyrin-Biroulet, L. Assessment of extraintestinal manifestations in inflammatory bowel diseases: A systematic review and a proposed guide for clinical trials. United Eur. Gastroenterol. J. 2020, 8, 1013–1030. [Google Scholar] [CrossRef] [PubMed]
- Maaser, C.; Sturm, A.; Vavricka, S.R.; Kucharzik, T.; Fiorino, G.; Annese, V.; Calabrese, E.; Baumgart, D.C.; Bettenworth, D.; Borralho Nunes, P.; et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J. Crohn’s Colitis 2019, 13, 144–164K. [Google Scholar] [CrossRef]
- Satsangi, J. The Montreal classification of inflammatory bowel disease: Controversies, consensus, and implications. Gut 2006, 55, 749–753. [Google Scholar] [CrossRef]
- Harvey, R.F.; Bradshaw, J.M. A simple index of Crohn’s-disease activity. Lancet 1980, 1, 514. [Google Scholar] [CrossRef]
- Rutgeerts, P.; Sandborn, W.J.; Feagan, B.G.; Reinisch, W.; Olson, A.; Johanns, J.; Travers, S.; Rachmilewitz, D.; Hanauer, S.B.; Lichtenstein, G.R.; et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2005, 353, 2462–2476. [Google Scholar] [CrossRef]
- Orchard, T.R.; Chua, C.; Ahmad, T.; Cheng, H.; Welsh, K.I.; Jewell, D.P. Uveitis and erythema nodosum in inflammatory bowel disease: Clinical features and the role of HLA genes. Gastroenterology 2002, 123, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Ott, C.; Schölmerich, J. Extraintestinal manifestations and complications in IBD. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Lanna, C.C.; Ferrari, M.D.; Rocha, S.L.; Nascimento, E.; de Carvalho, M.A.; da Cunha, A.S. A cross-sectional study of 130 Brazilian patients with Crohn’s disease and ulcerative colitis: Analysis of articular and ophthalmologic manifestations. Clin. Rheumatol. 2008, 27, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, D.; Bandyopadhyay, S.; Ghosh, P.; De, A.; Bhattacharya, A.; Dhali, G.K.; Das, K. Extraintestinal manifestations in inflammatory bowel disease: Prevalence and predictors in Indian patients. Indian J. Gastroenterol. 2015, 34, 387–394. [Google Scholar] [CrossRef]
- Mendoza, J.L.; Lana, R.; Taxonera, C.; Alba, C.; Izquierdo, S.; Díaz-Rubio, M. Extraintestinal manifestations in inflammatory bowel disease: Differences between Crohn’s disease and ulcerative colitis. Med. Clin. 2005, 125, 297–300. [Google Scholar]
- Lee, H.J.; Song, H.J.; Jeong, J.H.; Kim, H.U.; Boo, S.-J.; Na, S.-Y. Ophthalmologic manifestations in patients with inflammatory bowel disease. Intest. Res. 2017, 15, 380. [Google Scholar] [CrossRef] [PubMed]
- The Epidemiology of Dry Eye Disease: Report of the Epidemiology Subcommittee of the International Dry Eye WorkShop. Ocul. Surf. 2007, 5, 93–107. [CrossRef]
- Schaumberg, D.A.; Dana, R.; Buring, J.E.; Sullivan, D.A. Prevalence of dry eye disease among US men: Estimates from the Physicians’ Health Studies. Arch. Ophthalmol. 2009, 127, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Cury, D.B.; Moss, A.C. Ocular manifestations in a community-based cohort of patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2010, 16, 1393–1396. [Google Scholar] [CrossRef]
- Barta, Z.; Czompa, L.; Rentka, A.; Zold, E.; Remenyik, J.; Biro, A.; Gesztelyi, R.; Zsuga, J.; Szodoray, P.; Kemeny-Beke, A. Evaluation of Objective Signs and Subjective Symptoms of Dry Eye Disease in Patients with Inflammatory Bowel Disease. BioMed Res. Int. 2019, 2019, 8310583. [Google Scholar] [CrossRef] [PubMed]
- Sankar, V.; Brennan, M.T.; Kok, M.R.; Leakan, R.A.; Smith, J.A.; Manny, J.; Baum, B.J.; Pillemer, S.R. Etanercept in Sjögren’s syndrome: A twelve-week randomized, double-blind, placebo-controlled pilot clinical trial: Randomized Controlled Pilot Study of Etanercept in SS. Arthritis Rheum. 2004, 50, 2240–2245. [Google Scholar] [CrossRef] [PubMed]
- Zandbelt, M.M.; de Wilde, P.; van Damme, P.; Hoyng, C.B.; van de Putte, L.; van den Hoogen, F. Etanercept in the Treatment of Patients with Primary Sjögren’s Syndrome: A Pilot Study. J. Rheumatol. 2004, 31, 96–101. [Google Scholar] [PubMed]
- Eberhardt, M.; Rammohan, G. Blepharitis; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Yilmaz, S.; Aydemir, E.; Maden, A.; Unsal, B. The prevalence of ocular involvement in patients with inflammatory bowel disease. Int. J. Colorectal. Dis. 2007, 22, 1027–1030. [Google Scholar] [CrossRef] [PubMed]
- Felekis, T.; Katsanos, K.; Kitsanou, M.; Trakos, N.; Theopistos, V.; Christodoulou, D.; Asproudis, I.; Tsianos, E.V. Spectrum and frequency of ophthalmologic manifestations in patients with inflammatory bowel disease: A prospective single-center study. Inflamm. Bowel Dis. 2009, 15, 29–34. [Google Scholar] [CrossRef]
- Gritz, D.C.; Wong, I.G. Incidence and prevalence of uveitis in Northern California: The Northern California Epidemiology of Uveitis Study. Ophthalmology 2004, 111, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Van Tran, T.; Auer, C.; Guex-Crosier, Y.; Pittet, N.; Herbort, C.P. Epidemiological characteristics of uveitis in Switzerland. Int. Ophthalmol. 1994, 18, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Smit, R.L.M.J.; Seerp, G. Epidemiology of uveitis: Editorial review. Curr. Opin. Ophthalmol. 1995, 6, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Toussirot, É.; Aubin, F. Paradoxical reactions under TNF-α blocking agents and other biological agents given for chronic immune-mediated diseases: An analytical and comprehensive overview. RMD Open 2016, 2, e000239. [Google Scholar] [CrossRef]
- Isene, R.; Bernklev, T.; Høie, O.L.; Munkholm, P.I.; Tsianos, E.; Stockbrügger, R.; Odes, S.; Palm, Ø.; Småstuen, M.; Moum, B. Extraintestinal manifestations in Crohn’s disease and ulcerative colitis: Results from a prospective, population-based European inception cohort. Scand. J. Gastroenterol. 2015, 50, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Karmiris, K.; Avgerinos, A.; Tavernaraki, A.; Zeglinas, C.; Karatzas, P.; Koukouratos, T.; Oikonomou, K.A.; Kostas, A.; Zampeli, E.; Papadopoulos, V.; et al. Prevalence and Characteristics of Extra-intestinal Manifestations in a Large Cohort of Greek Patients with Inflammatory Bowel Disease. J. Crohn’s Colitis 2016, 10, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Cloché, V.; Buisson, A.; Tréchot, F.; Batta, B.; Locatelli, A.; Favel, C.; Premy, S.; Collet-Fenetrier, B.; Fréling, E.; Lopez, A.; et al. Ocular symptoms are not predictive of ophthalmologic inflammation in inflammatory bowel disease. Dig. Liver Dis. 2013, 45, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Harbord, M.; Annese, V.; Vavricka, S.R.; Allez, M.; Acosta, M.B.-D.; Boberg, K.M.; Burisch, J.; De Vos, M.; De Vries, A.-M.; Dick, A.D.; et al. The First European Evidence-based Consensus on Extra-intestinal Manifestations in Inflammatory Bowel Disease. J. Crohn’s Colitis 2016, 10, 239–254. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Blanchard, J.F.; Rawsthorne, P.; Yu, N. The prevalence of extraintestinal diseases in inflammatory bowel disease: A population-based study. Am. J. Gastroenterol. 2001, 96, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Tessler, H.H.; Goldstein, D.A. Family History of Inflammatory Bowel Disease in Patients with Idiopathic Ocular Inflammation. Investig. Ophthalmol. Vis. Sci. 2006, 47, 588. [Google Scholar] [CrossRef]
- Annese, V. A review of extraintestinal manifestations and complications of inflammatory bowel disease. Saudi J. Med. Med. Sci. 2019, 7, 66. [Google Scholar] [CrossRef]
- Caramoy, A.; Lappas, A.; Fauser, S.; Kirchhof, B. Central scotoma and blurred vision in a patient with Crohn’s disease. Ophthalmologe 2009, 106, 836–838. [Google Scholar] [CrossRef]
- Geyshis, B.; Katz, G.; Ben-Horin, S.; Kopylov, U. A patient with ulcerative colitis and central serous chorioretinopathy—A therapeutic dilemma. J. Crohn’s Colitis 2013, 7, e66–e68. [Google Scholar] [CrossRef]
- Zullow, S.; Fazelat, A.; Farraye, F.A. Central Serous Chorioretinopathy in a Patient with Ulcerative Colitis with Pouchitis on Budesonide-EC. Inflamm. Bowel Dis. 2017, 23, E19. [Google Scholar] [CrossRef][Green Version]
| Characteristics | Total n (%) | CD n (%) | UC n (%) | p * |
|---|---|---|---|---|
| Total number of patients | 87 | 61 (70.1) | 26 (29.9) | |
| Gender | 0.6871 | |||
| Female | 53 (60.9) | 38 (62.3) | 15 (57.7) | |
| Male | 34 (39.1) | 23 (37.7) | 11 (42.3) | |
| Smoking status | 0.3651 | |||
| Active smoker | 26 (29.9) | 20 (32.8) | 6 (23.1) | |
| Non smoker or former smoker | 61 (70.1) | 41 (67.2) | 20 (76.9) | |
| Median age at IBD diagnosis (years [Q1; Q3]) | 31 [21; 43] | 34 [20; 42] | 30 [23; 43] | 0.9926 |
| Montreal classification E (UC) | ||||
| E1 (rectitis) | - | - | 9 (34.6) | |
| E2 (left-sided colitis) | - | - | 7 (26.9) | |
| E3 (extensive colitis) | - | - | 10 (38.5) | |
| Montreal classification L (CD) | ||||
| L1 (ileitis) | - | 18 (29.5) | - | |
| L2 (colitis) | - | 15 (24.6) | - | |
| L3 (ileocolitis) | - | 28 (45.9) | - | |
| L4 (upper localization) | - | 1 (1.6) | - | |
| Montreal classification B (CD) | ||||
| B1 (non-stricturing and non-penetrating) | - | 41 (67.2) | - | |
| B2 (stricturing) | - | 10 (16.4) | - | |
| B3 (penetrating) | - | 10 (16.4) | - | |
| Perianal disease (CD) | - | 13 (21.3) | - | |
| Surgery for IBD | 30 (34.5) | 28 (45.9) | 2 (7.7) | 0.0006 |
| 0 intervention | 57 (65.5) | 33 (54.1) | 9 (34.6) | |
| 1 intervention | 15 (17.2) | 14 (23.0) | 1 (3.8) | |
| ≥2 interventions | 15 (17.2) | 14 (23.0) | 1 (3.8) | |
| IBD treatments | ||||
| Steroids | 1 (2.9) | 1 (1.6) | 0 | NR |
| 5-aminosalicylates | 9 (19.1) | 2 (3.2) | 7 | NR |
| Immunosuppressants | 9 (19.1) | 8 (13.1) | 1 (3.8) | NR |
| Other biologics (vedolizumab, ustekinumab) | 6 (15.6) | 3 (4.9) | 3 (11.5) | NR |
| Anti TNF (infliximab, adalimumab) | 45 (51.7) | 33 (54.1) | 12 (46.2) | 0.4973 |
| Other EIM | 34 (39.1) | 26 (42.6) | 8 (30.8) | 0.2996 |
| IMIDs (APS, RA, JA and/or Psoriasis) | 34 (39.1) | 27 (44.3) | 7 (26.9) | 0.1292 |
| IBD Activity at ophthalmologic visit | 0.9064 | |||
| Active | 23 (26.4) | 15 (24.6) | 8 (30.8) | |
| Inactive | 64 (73.6) | 46 (75.4) | 18 (69.2) |
| Characteristics | Total n (%) |
|---|---|
| Median age at ophthalmologic outpatient visit (years [Q1; Q3]) | 47 [35; 56] |
| Median time from IBD diagnosis to ophthalmologic outpatient visit (years [Q1; Q3]) | 13 [8; 22] |
| Date of ophthalmologic outpatient visit in comparison with IBD diagnosis | |
| Before | 3 (3.4) |
| After | 83 (95.4) |
| Simultaneous | 1 (1.2) |
| Number of visit | |
| 1 | 73 (83.9) |
| ≥2 | 14 (16.1) |
| Reasons for visit | |
| Redness | 29 (25) |
| Pain | 20 (17.2) |
| Visual acuity loss | 27 (23.2) |
| Others * | 40 (34.4) |
| Diagnosis | |
| Dry eyes | 15 (17.2) |
| Uveitis | 13 (1.9) |
| Episcleritis | 5 (5.7) |
| Scleritis | 2 (2.3) |
| PUK | 1 (1.1) |
| Blepharitis | 9 (10.3) |
| Chalazion | 5 (5.7) |
| Herpetic keratitis | 1 (1.1) |
| Corneal abscess | 1 (1.1) |
| CRAO | 1 (1.1) |
| Cataract | 9 (10.3) |
| Refractive disorder | 8 (9.2) |
| Systematic control | 5 (5.7) |
| AMD | 1 (1.1) |
| Conjunctivitis | 4 (4.6) |
| Subconjunctival hemorrhage | 1 (1.1) |
| Post-traumatic ulcer | 1 (1.1) |
| CSCR | 1 (1.1) |
| Ophthalmic migraine | 1 (1.1) |
| Valsalva retinopathy | 1 (1.1) |
| Floating bodies | 1 (1.1) |
| Relationship with IBD | |
| Probable | 21 (24.1) |
| Possible | 32 (36.8) |
| Unlikely | 34 (39.1) |
| Ophthalmological treatment | |
| Artificial tears | 32 (36.8) |
| Anti inflammatory (Steroid and non steroid, topic or systemic) | 26 (29.9) |
| Others (anti viral, eyes drops antiseptic, antibiotic) | 19 (21.8) |
| Ophthalmologic complications | 3 (3.4) |
| Switch of IBD treatment | 13 (14.9) |
| Related to IBD | Not Related to IBD | ||||
|---|---|---|---|---|---|
| n = 53 (60.9%) | n = 34 (39.1%) | ||||
| Characteristics | n | % | n | % | p * |
| Gender | 0.4406 | ||||
| Male | 19 | 35.8 | 15 | 44.1 | |
| Female | 34 | 64.2 | 19 | 55.9 | |
| Smoking | 0.0654 | ||||
| Active smoker | 12 | 22.6 | 14 | 41.2 | |
| Non-smoker or former smoker | 41 | 77.4 | 20 | 58.8 | |
| Median age at IBD diagnosis (years [Q1; Q3]) | 53 | 34 [23; 42] | 34 | 26.5 [20; 46] | 0.6334 |
| Median age at ophthalmologic outpatient visit (years [Q1; Q3]) | 53 | 47 [35; 44] | 34 | 47.5 [36; 49] | 0.401 |
| Date of ophthalmologic outpatient visit in comparison with IBD diagnosis | 0.3843 | ||||
| Before | 3 | 5.7 | 0 | 0 | |
| After | 49 | 92.5 | 34 | 100 | |
| Concomitant | 1 | 1.9 | 0 | 0 | |
| Median time from IBD diagnosis to ophthalmologic outpatient visit (years) | 0.7966 | ||||
| <11 years | 25 | 47.2 | 17 | 50 | |
| Equal or >11 years | 28 | 52.8 | 17 | 50 | |
| Surgery for IBD | 17 | 32.1 | 13 | 38.2 | 0.5553 |
| Active IBD at ophthalmologic outpatient visit | 14 | 26.4 | 9 | 26.5 | 0.9954 |
| IBD type | 0.2996 | ||||
| CD | 35 | 66 | 26 | 76.5 | |
| UC | 18 | 34 | 8 | 23.5 | |
| IMIDs (APS, RA, JA and/or Psoriasis) | 22 | 41.5 | 12 | 35.3 | 0.5621 |
| Other EIM | 21 | 39.6 | 13 | 38.2 | 0.897 |
| IBD Treatment | |||||
| Steroids | 0 | 0 | 1 | 2.9 | 0.3908 |
| 5 amino-salicylates | 7 | 13.2 | 2 | 5.9 | 0.4727 |
| Immunosuppressants | 7 | 13.2 | 2 | 5.9 | 0.4727 |
| Other biologics | 2 | 3.8 | 4 | 11.8 | 0.1512 |
| Anti TNF | 28 | 52.8 | 17 | 50 | 0.8289 |
| Related to IBD | Not Related to IBD | ||||
|---|---|---|---|---|---|
| n = 35 (57.4%) | n = 26 (42.6%) | ||||
| Characteristics | n | % | n | % | p * |
| Gender | 0.5226 | ||||
| Male | 12 | 34.3 | 11 | 42.3 | |
| Female | 23 | 65.7 | 15 | 57.7 | |
| Smoking | 0.1722 | ||||
| Active smoker | 9 | 25,7 | 11 | 42.3 | |
| Non-smoker or former smoker | 26 | 74.3 | 15 | 57.7 | |
| Median age at IBD diagnosis (years [Q1; Q3]) | 35 | 35 [23; 42] | 26 | 26 [19; 43] | 0.5046 |
| Median age at ophthalmologic outpatient visit (years [Q1; Q3]) | 35 | 45 [35; 54] | 26 | 47.5 [34; 59] | 0.6135 |
| Date of ophthalmologic visit in comparison with IBD diagnosis | NR | ||||
| Before | 2 | 5.7 | 0 | 0 | |
| After | 32 | 91.4 | 26 | 100 | |
| Concomitant | 1 | 2.9 | 0 | 0 | |
| Median time from IBD diagnosis to ophthalmologic outpatient visit (years) | 0.6273 | ||||
| <11 years | 18 | 51.4 | 15 | 57.7 | |
| Equal or >11 years | 17 | 48.6 | 11 | 42.3 | |
| Surgery for IBD | 16 | 45.7 | 12 | 46.2 | 0.9728 |
| Active IBD at ophthalmologic outpatient visit | 9 | 25.7 | 4 | 15.4 | 0.7153 |
| IMIDs (APS, RA, JA and/or Psoriasis) | 16 | 45.7 | 11 | 42.3 | 0.7911 |
| Other EIM | 14 | 40 | 12 | 46.2 | 0.794 |
| IBD Treatment | |||||
| Steroids | 0 | 0 | 1 | 3.8 | NR |
| Salicylates | 1 | 2.9 | 0 | 0 | NR |
| Immunosuppressants | 5 | 14.3 | 1 | 3.8 | 0.2269 |
| Other biologics | 1 | 2.9 | 2 | 7.7 | NR |
| Anti TNF | 19 | 54.3 | 14 | 53.8 | 0.9728 |
| Montreal classification L | 0.5339 | ||||
| L1 | 11 | 31.4 | 7 | 26.9 | |
| L2 | 10 | 28.6 | 5 | 19.2 | |
| L3 | 14 | 40 | 14 | 53.8 | |
| L4 | NR | ||||
| Montreal classification B | 0.3706 | ||||
| B1 | 26 | 74.3 | 15 | 57.7 | |
| B2 | 5 | 14.3 | 5 | 19.2 | |
| B3 | 4 | 11.4 | 6 | 23.1 | |
| Perianal disease | 9 | 25.7 | 4 | 15.4 | 0.3299 |
| Related to IBD | Not Related to IBD | ||||
|---|---|---|---|---|---|
| n = 18 (69.2%) | n = 8 (30.8%) | ||||
| Characteristics | n | % | n | % | p * |
| Gender | 0.6828 | ||||
| Male | 7 | 38.9 | 4 | 50 | |
| Female | 11 | 61.1 | 4 | 50 | |
| Smoking | 0.3301 | ||||
| Active smoker | 3 | 16.7 | 3 | 37.5 | |
| Non-smoker or former smoker | 15 | 83.3 | 5 | 62.5 | |
| Median age at IBD diagnosis (years [Q1; Q3]) | 18 | 30 [23; 42] | 8 | 31.5 [21.5; 50] | 0.7414 |
| Median age at ophthalmologic outpatient visit (years [Q1; Q3]) | 18 | 47.5 [35; 53] | 8 | 46.5 [42.5; 63] | 0.4432 |
| Date of ophthalmologic outpatient visit in comparison with IBD diagnosis | NR | ||||
| Before | 1 | 5.6 | 0 | 0 | |
| After | 17 | 94.4 | 8 | 100 | |
| Median time from IBD diagnosis to ophthalmologic outpatient visit (years) | 0.6673 | ||||
| <11 years | 7 | 38.9 | 2 | 25 | |
| Equal or >11 years | 11 | 61.1 | 6 | 75 | |
| Surgery for IBD | 1 | 5.6 | 1 | 12.5 | NR |
| Active IBD at ophthalmologic visit | 6 | 33.3 | 2 | 25 | 1 |
| IMIDs (APS, RA, JA and/or Psoriasis) | 6 | 33.3 | 1 | 12.5 | 0.3748 |
| Other EIM | 7 | 38.9 | 1 | 12.5 | 0.3602 |
| IBD Treatment | |||||
| Steroids | 0 | 0 | 0 | 0 | NR |
| 5 amino-salicylates | 6 | 33.3 | 2 | 25 | 1 |
| Immunosuppressants | 2 | 11.1 | 1 | 12.5 | NR |
| Other biologics | 1 | 5.6 | 2 | 25 | NR |
| Anti TNF | 9 | 50 | 3 | 37.5 | 0.6828 |
| Montreal classification E | 0.5814 | ||||
| E1 | 6 | 33.3 | 3 | 37.5 | |
| E2 | 6 | 33.3 | 1 | 12.5 | |
| E3 | 6 | 33.3 | 4 | 50 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuny, A.; Guillo, L.; Baumann, C.; Netter, P.; Danese, S.; Caron, B.; Peyrin-Biroulet, L.; Angioi, K. Ocular Manifestations in Patients with Inflammatory Bowel Disease in the Biologics Era. J. Clin. Med. 2022, 11, 4538. https://doi.org/10.3390/jcm11154538
Cuny A, Guillo L, Baumann C, Netter P, Danese S, Caron B, Peyrin-Biroulet L, Angioi K. Ocular Manifestations in Patients with Inflammatory Bowel Disease in the Biologics Era. Journal of Clinical Medicine. 2022; 11(15):4538. https://doi.org/10.3390/jcm11154538
Chicago/Turabian StyleCuny, Alix, Lucas Guillo, Cédric Baumann, Patrick Netter, Silvio Danese, Bénédicte Caron, Laurent Peyrin-Biroulet, and Karine Angioi. 2022. "Ocular Manifestations in Patients with Inflammatory Bowel Disease in the Biologics Era" Journal of Clinical Medicine 11, no. 15: 4538. https://doi.org/10.3390/jcm11154538
APA StyleCuny, A., Guillo, L., Baumann, C., Netter, P., Danese, S., Caron, B., Peyrin-Biroulet, L., & Angioi, K. (2022). Ocular Manifestations in Patients with Inflammatory Bowel Disease in the Biologics Era. Journal of Clinical Medicine, 11(15), 4538. https://doi.org/10.3390/jcm11154538






