Antimicrobial Stewardship Interventions in Pediatric Oncology: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
3. Results
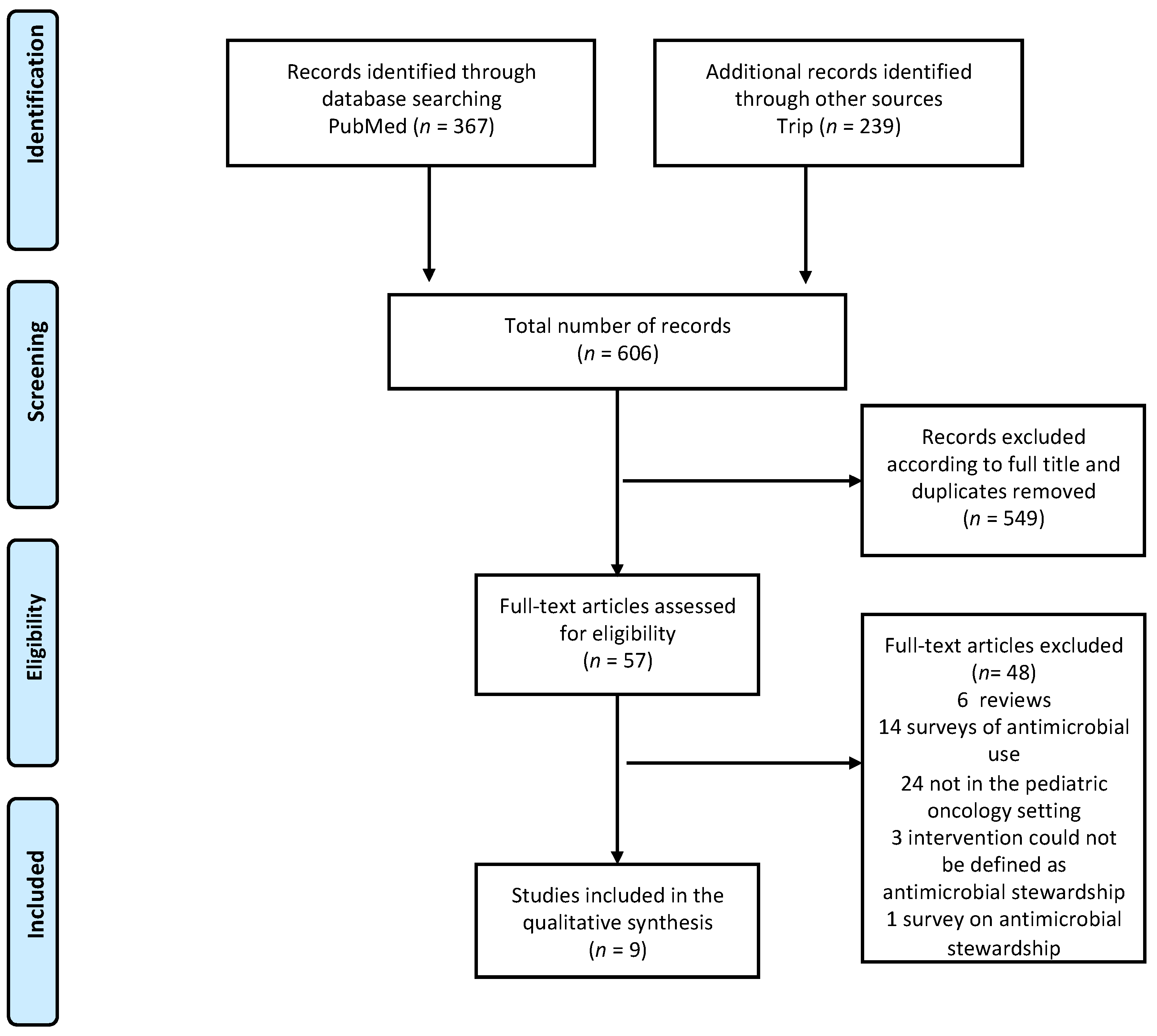
3.1. Literature Search
3.2. Antibacterial Stewardship
3.3. Antifungal Stewardship
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lehrnbecher, T.; Averbuch, D.; Castagnola, E.; Cesaro, S.; Ammann, R.A.; Garcia-Vidal, C.; Kanerva, J.; Lanternier, F.; Mesini, A.; Mikulska, M.; et al. 8th European Conference on Infections in Leukaemia: 2020 guidelines for the use of antibiotics in paediatric patients with cancer or post-haematopoietic cell transplantation. Lancet Oncol. 2021, 22, e270–e280. [Google Scholar] [CrossRef]
- Sung, L.; Phillips, R.; Lehrnbecher, T. Time for paediatric febrile neutropenia guidelines–children are not little adults. Eur. J. Cancer 2011, 47, 811–813. [Google Scholar] [CrossRef]
- Tu, Q.; Cotta, M.; Raman, S.; Graham, N.; Schlapbach, L.; Roberts, J.A. Individualized precision dosing approaches to optimize antimicrobial therapy in pediatric populations. Expert Rev. Clin. Pharmacol. 2021, 14, 1383–1399. [Google Scholar] [CrossRef]
- Basco, S.A.; Girotto, J.E. Contemporary Treatment of Resistant Gram-Negative Infections in Pediatric Patients. Infect. Dis. Clin. North Am. 2022, 36, 147–171. [Google Scholar] [CrossRef]
- Kearns, G.L.; Abdel-Rahman, S.M.; Alander, S.W.; Blowey, D.L.; Leeder, J.S.; Kauffman, R.E. Developmental pharmacology-drug disposition, action, and therapy in infants and children. N. Engl. J. Med. 2003, 349, 1157–1167. [Google Scholar] [CrossRef]
- Lehrnbecher, T.; Robinson, P.; Fisher, B.; Alexander, S.; Ammann, R.A.; Beauchemin, M.; Carlesse, F.; Groll, A.H.; Haeusler, G.; Santolaya, M.; et al. Guideline for the Management of Fever and Neutropenia in Children With Cancer and Hematopoietic Stem-Cell Transplantation Recipients: 2017 Update. J. Clin. Oncol. 2017, 35, 2082–2094. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Shaklee, J.F.; Smathers, S.; Prasad, P.; Asti, L.; Zoltanski, J.; Dul, M.; Nerandzic, M.; Coffin, S.E.; Toltzis, P.; et al. Risk Factors and Outcomes Associated With Severe Clostridium difficile Infection in Children. Pediatr. Infect. Dis. J. 2012, 31, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Trubiano, J.A.; Worth, L.J.; Thursky, K.A.; Slavin, M.A. The prevention and management of infections due to multidrug resistant organisms in haematology patients. Br. J. Clin. Pharmacol. 2015, 79, 195–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masetti, R.; Muratore, E.; Leardini, D.; Zama, D.; Turroni, S.; Brigidi, P.; Esposito, S.; Pession, A. Gut microbiome in pediatric acute leukemia: From predisposition to cure. Blood Adv. 2021, 5, 4619–4629. [Google Scholar] [CrossRef]
- Masetti, R.; Zama, D.; Leardini, D.; Muratore, E.; Turroni, S.; Brigidi, P.; Pession, A. Microbiome-Derived Metabolites in Allogeneic Hematopoietic Stem Cell Transplantation. Int. J. Mol. Sci. 2021, 22, 1197. [Google Scholar] [CrossRef] [PubMed]
- Science, M.; Timberlake, K. Antifungal stewardship: A budding branch of antimicrobial stewardship. Pediatr. Blood Cancer 2020, 67, 2019–2020. [Google Scholar] [CrossRef] [PubMed]
- Perlin, D.S.; Rautemaa-Richardson, R.; Alastruey-Izquierdo, A. The global problem of antifungal resistance: Prevalence, mechanisms, and management. Lancet Infect. Dis. 2017, 17, e383–e392. [Google Scholar] [CrossRef]
- Society for Healthcare Epidemiology of America; Infectious Diseases Society of America; Pediatric Infectious Diseases Society. Policy statement on antimicrobial stewardship by the Society for Healthcare Epidemiology of America (SHEA), the Infectious Diseases Society of America (IDSA), and the Pediatric Infectious Diseases Society (PIDS). Infect. Control. Hosp. Epidemiol. 2012, 33, 322–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerber, J.S.; Jackson, M.A.; Tamma, P.D.; Zaoutis, T.E.; Maldonado, Y.A.; O’Leary, S.T.; Banerjee, R.; Barnett, E.D.; Campbell, J.D.; Caserta, M.T.; et al. Antibiotic Stewardship in Pediatrics. Pediatrics 2021, 147, e2020040295. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.J.; Gerber, J.S.; Hersh, A.L. Inpatient Antimicrobial Stewardship in Pediatrics: A Systematic Review. J. Pediatr. Infect. Dis. Soc. 2015, 4, e127–e135. [Google Scholar] [CrossRef] [Green Version]
- Webb, B.J.; Majers, J.; Healy, R.; Jones, P.B.; Butler, A.M.; Snow, G.; Forsyth, S.; Lopansri, B.K.; Ford, C.D.; Hoda, D. Antimicrobial Stewardship in a Hematological Malignancy Unit: Carbapenem Reduction and Decreased Vancomycin-Resistant Enterococcus Infection. Clin. Infect. Dis. 2019, 71, 960–967. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [Green Version]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Innitiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef] [Green Version]
- Zama, D.; Gori, D.; Muratore, E.; Leardini, D.; Rallo, F.; Turroni, S.; Prete, A.; Brigidi, P.; Pession, A.; Masetti, R. Enteral versus Parenteral Nutrition as Nutritional Support after Allogeneic Hematopoietic Stem Cell Transplantation: A Systematic Review and Meta-Analysis. Transplant. Cell. Ther. 2020, 27, 180.e1–180.e8. [Google Scholar] [CrossRef]
- Wolf, J.; Sun, Y.; Tang, L.; Newland, J.G.; Gerber, J.S.; Van Dyke, C.J.; Hymes, S.R.; Yu, D.; Carias, D.C.; Bryant, P.A. Antimicrobial Stewardship Barriers and Goals in Pediatric Oncology and Bone Marrow Transplantation: A Survey of Antimicrobial Stewardship Practitioners. Infect. Control Hosp. Epidemiol. 2015, 37, 343–347. [Google Scholar] [CrossRef] [Green Version]
- Dhanya, R.; Agarwal, R.K.; Ramprakash, S.; Trivedi, D.; Shah, V.; Bhat, N.; Reddy, M.; Elizabeth, S.; Batool, A.; Khalid, S.; et al. Do weekly surveillance cultures contribute to antibiotic stewardship and correlate with outcome of HSCT in children–a multicentre real-world experience of 5 years from Indian subcontinent? Transplant. Cell. Ther. 2021, 28, 170.e1–170.e7. [Google Scholar] [PubMed]
- Wattier, R.L.; Levy, E.R.; Sabnis, A.J.; Dvorak, C.C.; Auerbach, A.D. Reducing Second Gram-Negative Antibiotic Therapy on Pediatric Oncology and Hematopoietic Stem Cell Transplantation Services. Infect. Control Hosp. Epidemiol. 2017, 38, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Horikoshi, Y.; Kaneko, T.; Morikawa, Y.; Isogai, M.; Suwa, J.; Higuchi, H.; Yuza, Y.; Shoji, T.; Ito, K. The North Wind and the Sun: Pediatric Antimicrobial Stewardship Program Combining Restrictive and Persuasive Approaches in Hematology-Oncology Ward and Hematopoietic Stem Cell Transplant Unit. Pediatr. Infect. Dis. J. 2018, 37, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Hennig, S.; Staatz, C.; Natanek, D.; Bialkowski, S.; Paez, C.C.L.; Lawson, R.; Clark, J. Antimicrobial stewardship in paediatric oncology: Impact on optimising gentamicin use in febrile neutropenia. Pediatr. Blood Cancer 2018, 65, 1–5. [Google Scholar] [CrossRef]
- Karandikar, M.V.; Milliren, C.E.; Zaboulian, R.; Peiris, P.; Sharma, T.; Place, A.E.; Sandora, T.J. Limiting Vancomycin Exposure in Pediatric Oncology Patients With Febrile Neutropenia May Be Associated With Decreased Vancomycin-Resistant Enterococcus Incidence. J. Pediatr. Infect. Dis. Soc. 2020, 9, 428–436. [Google Scholar] [CrossRef]
- Olson, J.; Mehra, S.; Hersh, A.L.; Thorell, E.A.; Stoddard, G.J.; Maese, L.; Barnette, P.E.; Lemons, R.S.; Pavia, A.T.; Knackstedt, E.D. Oral Step-Down Therapy With Levofloxacin for Febrile Neutropenia in Children With Cancer. J. Pediatr. Infect. Dis. Soc. 2021, 10, 27–33. [Google Scholar] [CrossRef]
- Mendoza-Palomar, N.; Garcia-Palop, B.; Melendo, S.; Martín, M.T.; Renedo-Miró, B.; Soler-Palacin, P.; Fernández-Polo, A. Antifungal stewardship in a tertiary care paediatric hospital: The PROAFUNGI study. BMC Infect. Dis. 2021, 21, 1–7. [Google Scholar]
- Santiago-García, B.; Rincón-López, E.M.; Salas, B.P.; de la Red, Y.A.; Colino, C.G.; Fernández-Llamazares, C.M.; Saavedra-Lozano, J.; Matos, T.H. The POPA Study Group Effect of an intervention to improve the prescription of antifungals in pediatric hematology-oncology. Pediatr. Blood Cancer 2020, 67, 1–8. [Google Scholar] [CrossRef]
- Amanati, A.; Badiee, P.; Jafarian, H.; Ghasemi, F.; Nematolahi, S.; Haghpanah, S.; Hamzavi, S.S. Impact of antifungal stewardship interventions on the susceptibility of colonized Candida species in pediatric patients with malignancy. Sci. Rep. 2021, 11, 1–12. [Google Scholar]
- Barlam, T.F.; Cosgrove, S.E.; Abbo, L.M.; MacDougall, C.; Schuetz, A.N.; Septimus, E.J.; Srinivasan, A.; Dellit, T.H.; Falck-Ytter, Y.T.; Fishman, N.O.; et al. Executive Summary: Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin. Infect. Dis. 2016, 62, 1197–1202. [Google Scholar] [CrossRef]
- Tamma, P.D.; Avdic, E.; Keenan, J.F.; Zhao, Y.; Anand, G.; Cooper, J.; Dezube, R.; Hsu, S.; Cosgrove, S.E. What is the More Effective Antibiotic Stewardship Intervention: Pre-Prescription Authorization or Post-Prescription Review with Feedback? Clin. Infect. Dis. 2017, 64, 537–543. [Google Scholar] [CrossRef]
- Ibrahim, O.M.; Polk, R.E. Antimicrobial use metrics and benchmarking to improve stewardship outcomes: Methodology, opportunities, and challenges. Infect. Dis. Clin. N. Am. 2014, 28, 195–214. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Klasa, A.; Piekarska, A.; Prejzner, W.; Bieniaszewska, M.; Hellmann, A. Colonization with multidrug-resistant bacteria increases the risk of complications and a fatal outcome after allogeneic hematopoietic cell transplantation. Ann. Hematol. 2018, 97, 509–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dandoy, C.; Ardura, M.I.; Papanicolaou, G.; Auletta, J.J. Bacterial bloodstream infections in the allogeneic hematopoietic cell transplant patient: New considerations for a persistent nemesis. Bone Marrow Transplant. 2017, 52, 1091–1106. [Google Scholar] [CrossRef]
- Ford, C.D.; Gazdik, M.A.; Lopansri, B.K.; Webb, B.; Mitchell, B.; Coombs, J.; Hoda, D.; Petersen, F.B. Vancomycin-Resistant Enterococcus Colonization and Bacteremia and Hematopoietic Stem Cell Transplantation Outcomes. Biol. Blood Marrow Transplant. 2017, 23, 340–346. [Google Scholar] [CrossRef] [Green Version]
- Candoni, A.; Klimko, N.; Busca, A.; Di Blasi, R.; Shadrivova, O.; Cesaro, S.; Zannia, M.E.; Verga, L.; Forghieri, F.; Calore, E.; et al. Fungal infections of the central nervous system and paranasal sinuses in onco-haematologic patients. Epidemiological study reporting the diagnostic-therapeutic approach and outcome in 89 cases. Mycoses 2019, 62, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Hamzavi, S.S.; Amanati, A.; Badiee, P.; Kadiva, M.R.; Jafarian, H.; Ghasemi, F.; Haghpanah, S.; Dehghani, M.; Baghani, A.N. Changing face of Candida colonization pattern in pediatric patients with hematological malignancy during repeated hospitalizations, results of a prospective observational study (2016–2017) in shiraz, Iran. BMC Infect. Dis. 2019, 19, 759. [Google Scholar] [CrossRef] [Green Version]
- Safdar, A.; Armstrong, D. Prospective evaluation of Candida species colonization in hospitalized cancer patients: Impact on short-term survival in recipients of marrow transplantation and patients with hematological malignancies. Bone Marrow Transplant. 2002, 30, 931–935. [Google Scholar] [CrossRef] [Green Version]
- Pfaller, M.A.; Andes, D.R.; Diekema, D.J.; Horn, D.L.; Reboli, A.C.; Rotstein, C.; Franks, B.; Azie, N.E. Epidemiology and outcomes of invasive candidiasis due to non-albicans species of Candida in 2496 patients: Data from the Prospective Antifungal Therapy (PATH) registry 2004–2008. PLoS ONE 2014, 9, e101510. [Google Scholar] [CrossRef] [Green Version]
- André, P.; Diezi, L.; Dao, K.; Crisinel, P.A.; Rothuizen, L.E.; Chtioui, H.; Decosterd, L.A.; Diezi, M.; Asner, S.; Buclin, T. Ensuring Sufficient Trough Plasma Concentrations for Broad-Spectrum Beta-Lactam Antibiotics in Children With Malignancies: Beware of Augmented Renal Clearance! Front. Pediatr. 2021, 9, 768438. [Google Scholar] [CrossRef]
- Dini, G.; Zecca, M.; Balduzzi, A.; Messina, C.; Masetti, R.; Fagioli, F.; Favre, C.; Rabusin, M.; Porta, F.; Biral, E.; et al. No difference in outcome between children and adolescents transplanted for acute lymphoblastic leukemia in second remission. Blood 2011, 118, 6683–6690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masetti, R.; Zama, D.; Leardini, D.; Muratore, E.; Turroni, S.; Prete, A.; Brigidi, P.; Pession, A. The gut microbiome in pediatric patients undergoing allogeneic hematopoietic stem cell transplantation. Pediatr. Blood Cancer 2020, 67, e28711. [Google Scholar] [CrossRef] [PubMed]
- Biagi, E.; Zama, D.; Rampelli, S.; Turroni, S.; Brigidi, P.; Consolandi, C.; Severgnini, M.; Picotti, E.; Gasperini, P.; Merli, P.; et al. Early gut microbiota signature of aGvHD in children given allogeneic hematopoietic cell transplantation for hematological disorders. BMC Med. Genom. 2019, 12, 49. [Google Scholar] [CrossRef] [PubMed]
| First Author | Year | Type of Study | Antimicrobial Stewardship Interventions Applied | Results | Quality Assessment |
|---|---|---|---|---|---|
| Wattier [22] | 2017 | Observational study before and after the introduction of febrile neutropenia guidelines and antibacterial stewardship program; single center |
|
| Good |
| Horikoshi [23] | 2018 | Observational study before and after the introduction of antibacterial stewardship program; single center |
|
| Poor |
| Hennig [24] | 2017 | Observational study before and after the introduction of febrile neutropenia guidelines and antibacterial stewardship program; single center |
|
| Intermediate |
| Karandikar [25] | 2019 | Observational study before and after the introduction of febrile neutropenia guidelines; single center |
|
| Good |
| Olson [26] | 2020 | Observational study before and after the implementation of guidelines concerning about home antibiotic use; single center |
|
| Intermediate |
| First Author | Year | Type of Study | Antimicrobial Stewardship Interventions Applied | Results | Quality Assessment |
|---|---|---|---|---|---|
| Horikoshi [23] | 2018 | Observational study before and after the introduction of single-center antifungal stewardship program |
|
| Poor |
| Santiago-García [28] | 2019 | Observational study before and after the introduction of antifungal stewardship program; single center |
|
| Intermediate |
| Amanati [29] | 2021 | Observational study before and after the introduction of antifungal stewardship program; single center |
|
| Intermediate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muratore, E.; Baccelli, F.; Leardini, D.; Campoli, C.; Belotti, T.; Viale, P.; Prete, A.; Pession, A.; Masetti, R.; Zama, D. Antimicrobial Stewardship Interventions in Pediatric Oncology: A Systematic Review. J. Clin. Med. 2022, 11, 4545. https://doi.org/10.3390/jcm11154545
Muratore E, Baccelli F, Leardini D, Campoli C, Belotti T, Viale P, Prete A, Pession A, Masetti R, Zama D. Antimicrobial Stewardship Interventions in Pediatric Oncology: A Systematic Review. Journal of Clinical Medicine. 2022; 11(15):4545. https://doi.org/10.3390/jcm11154545
Chicago/Turabian StyleMuratore, Edoardo, Francesco Baccelli, Davide Leardini, Caterina Campoli, Tamara Belotti, Pierluigi Viale, Arcangelo Prete, Andrea Pession, Riccardo Masetti, and Daniele Zama. 2022. "Antimicrobial Stewardship Interventions in Pediatric Oncology: A Systematic Review" Journal of Clinical Medicine 11, no. 15: 4545. https://doi.org/10.3390/jcm11154545
APA StyleMuratore, E., Baccelli, F., Leardini, D., Campoli, C., Belotti, T., Viale, P., Prete, A., Pession, A., Masetti, R., & Zama, D. (2022). Antimicrobial Stewardship Interventions in Pediatric Oncology: A Systematic Review. Journal of Clinical Medicine, 11(15), 4545. https://doi.org/10.3390/jcm11154545









