Lip Lifting Efficacy of Hyaluronic Acid Filler Injections: A Quantitative Assessment Using 3-Dimensional Photography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Randomization
2.3. Injection Protocol
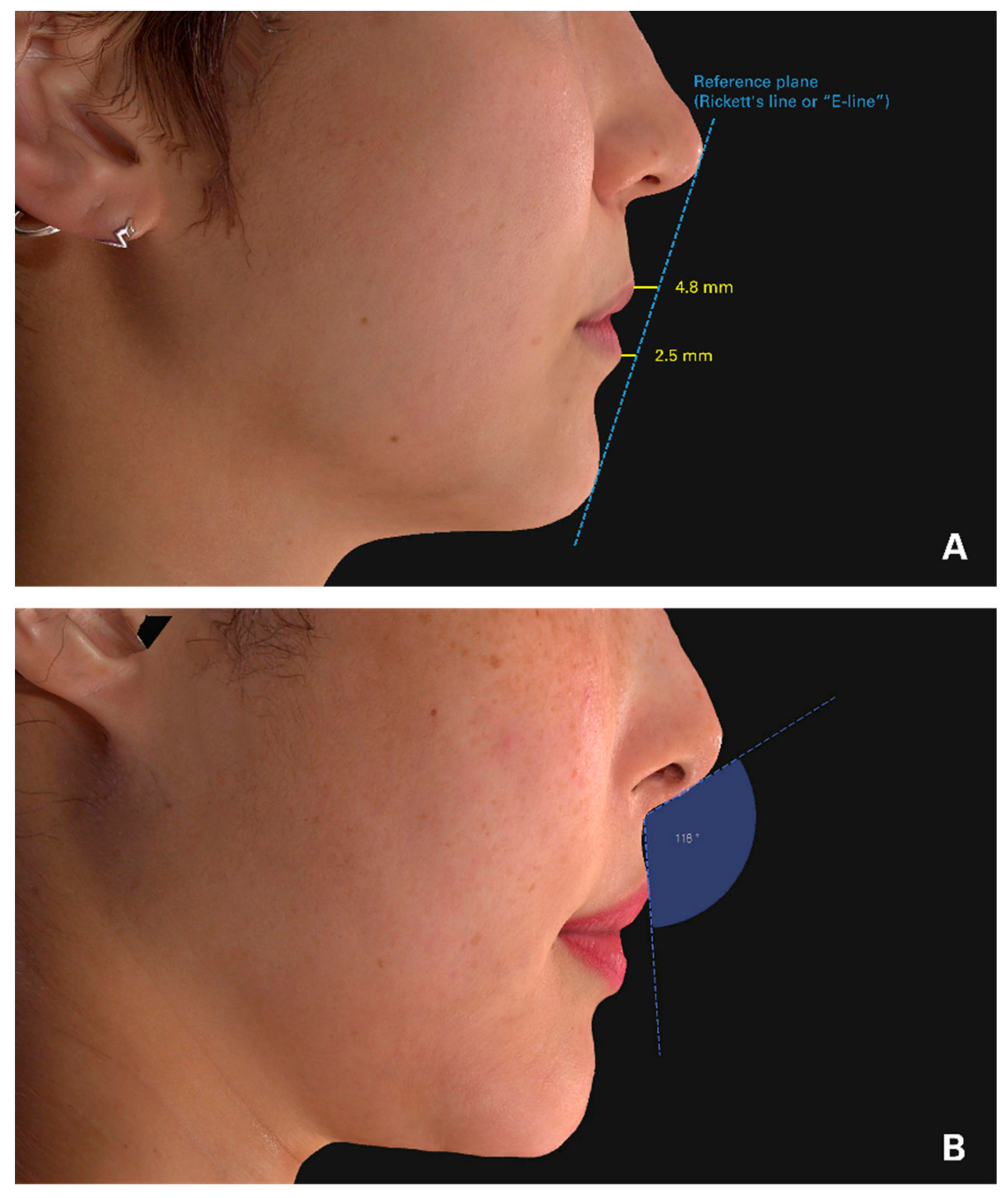
2.4. Follow-Up and Evaluation
3. Results
3.1. Non-Quantitative Evaluations
3.2. Lip Volume Changes
3.3. Lip Projection
3.4. Columella-Labial Angle Changes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wollina, U. Perioral rejuvenation: Restoration of attractiveness in aging females by minimally invasive procedures. Clin. Interv. Aging 2013, 8, 1149–1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forte, A.J.; Andrew, T.W.; Colasante, C.; Persing, J.A. Perception of age, attractiveness, and tiredness after isolated and combined facial subunit aging. Aesthetic Plast. Surg. 2015, 39, 856–869. [Google Scholar] [CrossRef] [PubMed]
- Kablik, J.; Monheit, G.D.; Yu, L.P.; Chang, G.; Gershkovich, J. Comparative physical properties of hyaluronic acid dermal fillers. Dermatol. Surg. 2009, 35, 302–312. [Google Scholar] [CrossRef]
- Jeon, O.; Song, S.J.; Lee, K.J.; Park, M.H.; Lee, S.H.; Hahn, S.K.; Kim, S.; Kim, B.S. Mechanical properties and degradation behaviors of hyaluronic acid hydrogels cross-linked at various cross-linking densities. Carbohydr. Polym. 2007, 70, 251–257. [Google Scholar] [CrossRef]
- Chopra, R.; Graivier, M.; Fabi, S.; Nestor, M.; Meuse, P.; Mashburn, J. A multicenter, open-label, prospective study of cannula injection of small-particle hyaluronic acid plus lidocaine (SPHAL) for lip augmentation. J. Drugs Dermatol. 2018, 17, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Flowers, R.S.; Smith, E.M. Technique for correction of the retracted columella, acute columellar-labial angle, and long upper lip. Aesthetic Plast. Surg. 1999, 23, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Jones, I.T.; Fabi, S.G.; Carruthers, J. Lip Augmentation. In Soft Tissue Augmentation; Carruthers, J., Carruthers, A., Eds.; Elsevier Inc.: London, UK, 2018; pp. 141–147. [Google Scholar]
- Stella, E.; Petrillo, A. Di Standard evaluation of the patient: The Merz Scale. In Injections in Aesthetic Medicine; Goisis, M., Ed.; Springer: Milan, Italy, 2014; pp. 33–50. [Google Scholar]
- Chong, Y.; Dong, R.; Liu, X.; Wang, X.; Yu, N.; Long, X. Stereophotogrammetry to reveal age-related changes of labial morphology among Chinese women aging from 20 to 60. Skin Res. Technol. 2021, 27, 41–48. [Google Scholar] [CrossRef]
- Hansson, S.; Östlund, E.; Bazargani, F. The Vectra M3 3-dimensional digital stereophotogrammetry system: A reliable technique for detecting chin asymmetry. Imaging Sci. Dent. 2022, 52, 43–51. [Google Scholar] [CrossRef]
- Andrade, L.M.; Rodrigues Da Silva, A.M.B.; Magri, L.V.; Rodrigues Da Silva, M.A.M. Repeatability study of angular and linear measurements on facial morphology analysis by means of stereophotogrammetry. J. Craniofac. Surg. 2017, 28, 1107–1111. [Google Scholar] [CrossRef]
- Li, Z.; Liang, Y.; Schenck, T.L.; Frank, K.; Giunta, R.E.; Koban, K.C. Investigating the reliability of novel nasal anthropometry using advanced three-dimensional digital stereophotogrammetry. J. Pers. Med. 2022, 12, 60. [Google Scholar] [CrossRef]
- Downie, J.; Mao, Z.; Rachel Lo, T.W.; Barry, S.; Bock, M.; Siebert, J.P.; Bowman, A.; Ayoub, A. A double-blind, clinical evaluation of facial augmentation treatments: A comparison of PRI 1, PRI 2, Zyplast® and Perlane®. J. Plast. Reconstr. Aesthetic Surg. 2009, 62, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Nikolis, A.; Bertucci, V.; Solish, N.; Lane, V.; Nogueira, A. An objective, quantitative assessment of flexible hyaluronic acid fillers in lip and perioral enhancement. Dermatol. Surg. 2021, 47, e168–e173. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, J.P.; Nanda, R.S.; Currier, G.F. An evaluation of the nasolabial angle and the relative inclinations of the nose and upper lip. Am. J. Orthod. Dentofac. Orthop. 1992, 102, 328–334. [Google Scholar] [CrossRef]
- Harris, R.; Nagarkar, P.; Amirlak, B. Varied definitions of nasolabial angle: Searching for consensus among rhinoplasty surgeons and an algorithm for selecting the ideal method. Plast. Reconstr. Surg. Glob. Open 2016, 4, e752. [Google Scholar] [CrossRef] [PubMed]
- Sutton, D.N.; Lewis, B.R.K.; Patel, M.; Cawood, J.I. Changes in facial form relative to progressive atrophy of the edentulous jaws. Int. J. Oral Maxillofac. Surg. 2004, 33, 676–682. [Google Scholar] [CrossRef]
- Ghannam, S.; Bageorgou, F. The Lip Flip for beautification and rejuvenation of the lips. J. Drugs Dermatol. 2022, 21, 71–76. [Google Scholar] [CrossRef]
- Droubi, M.; Al-Moudallal, Y.; Mouhamad, M.; Al-Nerabieah, Z. Changes in nasolabial angle and mentolabialangle after lips augmentation with hyaluronic acid: Clinical study. Int. J. Dent. Oral Sci. 2020, 7, 912–916. [Google Scholar] [CrossRef]
- Choi, S.Y.; Kim, S.J.; Lee, H.Y.; Chang, D.S.; Choi, M.S. Esthetic nasolabial angle according to the degree of upper lip protrusion in an Asian population. Am. J. Rhinol. Allergy 2018, 32, 66–70. [Google Scholar] [CrossRef]
- Marechek, A.; Perenack, J.; Christensen, B.J. Subnasal lip lift and its effect on nasal esthetics. J. Oral Maxillofac. Surg. 2021, 79, 895–901. [Google Scholar] [CrossRef]
- Edsman, K.; Nord, L.I.; Öhrlund, Å.; Lärkner, H.; Kenne, A.H. Gel properties of hyaluronic acid dermal fillers. Dermatol. Surg. 2012, 38, 1170–1179. [Google Scholar] [CrossRef]
- Hee, C.K.; Shumate, G.T.; Narurkar, V.; Bernardin, A.; Messina, D.J. Rheological properties and in vivo performance characteristics of soft tissue fillers. Dermatol. Surg. 2015, 41, S373–S381. [Google Scholar] [CrossRef] [PubMed]
- Shome, D.; Shah, R.A.; Gowda, D.; Vadera, S.; Kumar, V.; Raj, M.; Atif, A.; Doshi, K.; Vekaria, M.; Pathak, M.; et al. A prospective, open-label, multicentric, single-arm, post-marketing clinical study to evaluate effectiveness and safety of cross-linked sodium hyaluronate 24 mg with lidocaine 3 mg injection in subjects undergoing treatment for facial wrinkles and lip augmentation. J. Cosmet. Dermatol. 2021, 20, 2472–2479. [Google Scholar] [CrossRef] [PubMed]
- Fagien, S.; Maas, C.; Murphy, D.K.; Thomas, J.A.; Beddingfield, F.C. Juvéderm ultra for lip enhancement: An open-label, multicenter study. Aesthetic Surg. J. 2013, 33, 414–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raspaldo, H.; Chantrey, J.; Belhaouari, L.; Eccleston, D.; Saleh, R.; Acquilla, R.; Murphy, D.K. Lip and perioral enhancement: A 12-month prospective, randomized, controlled study. J. Drugs Dermatol. 2015, 14, 1444–1452. [Google Scholar]
- Boen, M.; Alhaddad, M.; Guiha, I.; Wu, D.P.; Goldman, M.P. A single site, open-label clinical trial, evaluating the duration, efficacy, and safety of a novel lip plumper. J. Drugs Dermatol. 2018, 17, 999–1004. [Google Scholar]





| L’ORIENT NO.2 | L’ORIENT NO.4 | |
|---|---|---|
| Designated group | Group A | Group B |
| Total HA 1 concentration (mg/mL) | 20 | 20 |
| Cross-linking agent | BDDE 2 | BDDE |
| Degree of cross-linking | 1–2% | 2–3% |
| Elastic modulus, G′ (Pa) | 249 | 436 |
| Viscous modulus, G′′ (Pa) | 34.67 | 43.70 |
| Tan δ (G′′/G′) | 0.14 | 0.10 |
| Cohesiveness (N) | −0.21 | −0.21 |
| Pretreatment | 4 Weeks | 12 Weeks | p-Value | |
|---|---|---|---|---|
| Upper lip | ||||
| All patients | 2.1 ± 0.54 | 3.0 ± 0.51 | 2.8 ± 0.49 | <0.00001 * |
| Group A | 2.1 ± 0.66 | 2.9 ± 0.53 | 2.6 ± 0.45 | <0.00001 * |
| Group B | 2.2 ± 0.44 | 3.1 ± 0.47 | 3.0 ± 0.43 | <0.00001 * |
| Lower lip | ||||
| All patients | 2.0 ± 0.48 | 3.1 ± 0.47 | 2.8 ± 0.49 | <0.00001 * |
| Group A | 2.0 ± 0.58 | 3.0 ± 0.44 | 2.7 ± 0.48 | <0.00001 * |
| Group B | 2.0 ± 0.41 | 3.1 ± 0.49 | 3.0 ± 0.47 | <0.00001 * |
| Group A | Group B | p-Value | |
|---|---|---|---|
| Total lip volume change (mL) | |||
| 4 weeks | 0.8 ± 0.45 | 1.1 ± 0.81 | 0.1371 |
| 12 weeks | 0.6 ± 0.56 | 0.8 ± 0.56 | 0.3832 |
| Anterior displacement of the upper lip (mm) | |||
| 4 weeks | 0.7 ± 1.12 | 1.1 ± 0.81 | 0.2383 |
| 12 weeks | 0.3 ± 0.78 | 0.7 ± 1.09 | 0.2402 |
| Anterior displacement of the lower lip (mm) | |||
| 4 weeks | 0.9 ± 1.25 | 0.8 ± 0.89 | 0.8108 |
| 12 weeks | 0.8 ± 0.90 | 0.8 ± 0.90 | 1 |
| Pretreatment | 4 Weeks | 12 Weeks | p-Value | |
|---|---|---|---|---|
| All patients | 103.8 ± 9.50 | 100.1 ± 9.99 | 99.5 ± 9.33 | 0.00004 * |
| Group A | 104.4 ± 7.46 | 103.8 ± 7.35 | 102.8 ± 6.68 | 0.26035 |
| Group B | 103.2 ± 11.50 | 96.4 ± 11.19 | 96.3 ± 10.70 | 0.000013 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rho, N.-K.; Goo, B.L.; Youn, S.-J.; Won, C.-H.; Han, K.-H. Lip Lifting Efficacy of Hyaluronic Acid Filler Injections: A Quantitative Assessment Using 3-Dimensional Photography. J. Clin. Med. 2022, 11, 4554. https://doi.org/10.3390/jcm11154554
Rho N-K, Goo BL, Youn S-J, Won C-H, Han K-H. Lip Lifting Efficacy of Hyaluronic Acid Filler Injections: A Quantitative Assessment Using 3-Dimensional Photography. Journal of Clinical Medicine. 2022; 11(15):4554. https://doi.org/10.3390/jcm11154554
Chicago/Turabian StyleRho, Nark-Kyoung, Boncheol Leo Goo, Seong-Jae Youn, Chong-Hyun Won, and Kwang-Ho Han. 2022. "Lip Lifting Efficacy of Hyaluronic Acid Filler Injections: A Quantitative Assessment Using 3-Dimensional Photography" Journal of Clinical Medicine 11, no. 15: 4554. https://doi.org/10.3390/jcm11154554
APA StyleRho, N.-K., Goo, B. L., Youn, S.-J., Won, C.-H., & Han, K.-H. (2022). Lip Lifting Efficacy of Hyaluronic Acid Filler Injections: A Quantitative Assessment Using 3-Dimensional Photography. Journal of Clinical Medicine, 11(15), 4554. https://doi.org/10.3390/jcm11154554







