Immediate and Quantitative Changes in Tear Film Parameters and Meibomian Gland Structures after Warm Compression and Meibomian Gland Squeezing in Meibomian Gland Dysfunction Patients and Normal Subjects
Abstract
:1. Introduction
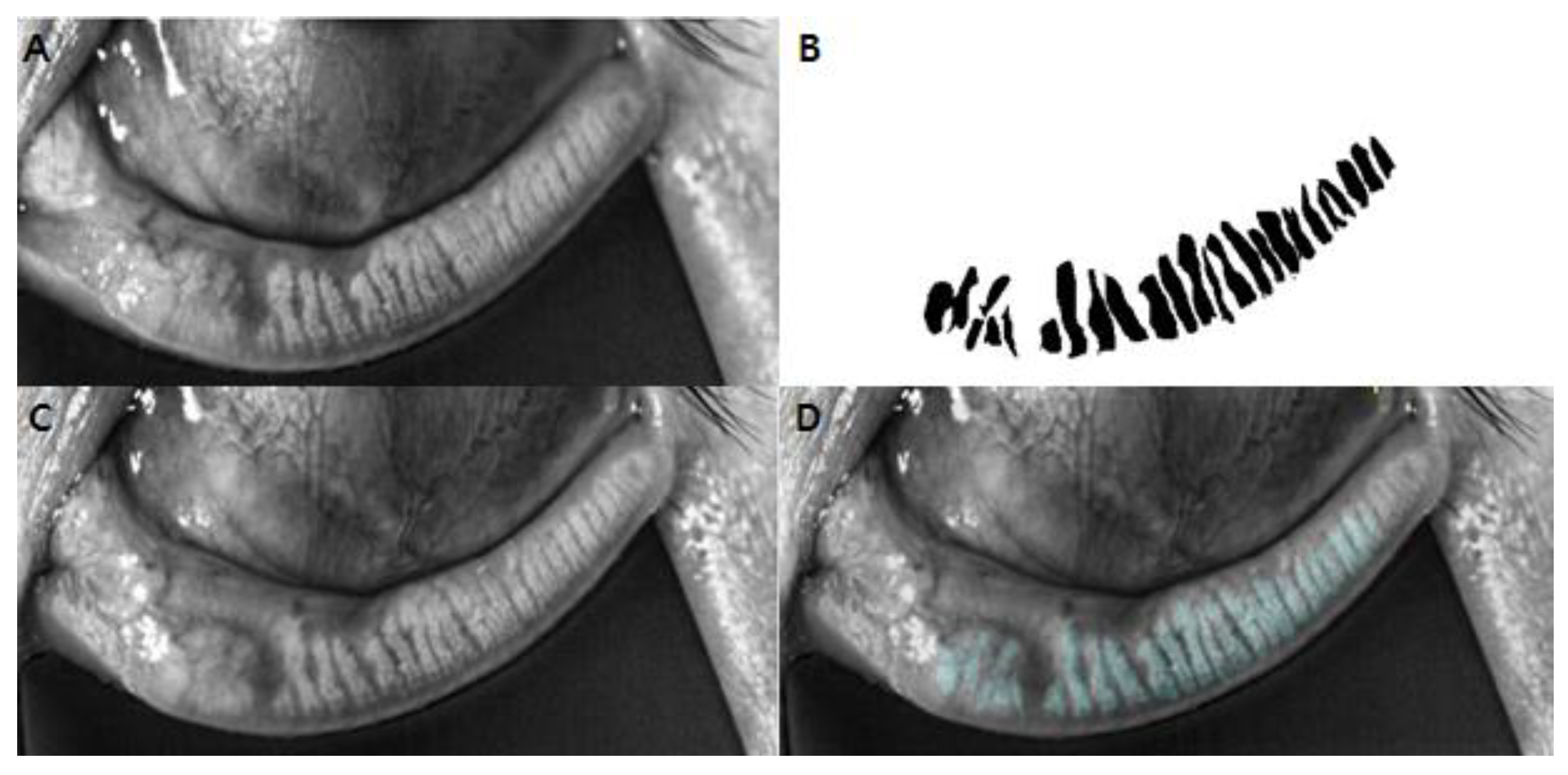
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tomlinson, A.; Bron, A.J.; Korb, D.R.; Amano, S.; Paugh, J.R.; Pearce, E.I.; Yee, R.; Yokoi, N.; Arita, R.; Dogru, M. The international workshop on meibomian gland dysfunction: Report of the diagnosis subcommittee. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2006–2049. [Google Scholar] [CrossRef] [PubMed]
- Gutgesell, V.J.; Stern, G.A.; Hood, C.I. Histopathology of meibomian gland dysfunction. Am. J. Ophthalmol. 1982, 94, 383–387. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Sato, E.A.; Ibrahim, O.M.A.; Dogru, M.; Tsubota, K. The application of in vivo laser confocal microscopy to the diagnosis and evaluation of meibomian gland dysfunction. Mol. Vis. 2008, 14, 1263–1271. [Google Scholar]
- Thode, A.R.; Latkany, R.A. Current and Emerging Therapeutic Strategies for the Treatment of Meibomian Gland Dysfunction (MGD). Drugs 2015, 75, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Geerling, G.; Tauber, J.; Baudouin, C.; Goto, E.; Matsumoto, Y.; O’Brien, T.; Rolando, M.; Tsubota, K.; Nichols, K.K. The international workshop on meibomian gland dysfunction: Report of the subcommittee on management and treatment of meibomian gland dysfunction. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2050–2064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bitton, E.; Lacroix, Z.; Leger, S. In-vivo heat retention comparison of eyelid warming masks. Cont. Lens Anterior Eye 2016, 39, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Bilkhu, P.S.; Naroo, S.A.; Wolffsohn, J.S. Effect of a commercially available warm compress on eyelid temperature and tear film in healthy eyes. Optom. Vis. Sci. 2014, 91, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.; Ho, L.; Wong, K.; La, A.; Lee, S.; Park, S.; Tran, L.; Stapleton, F. The effects of a hydrating mask compared to traditional warm compresses on tear film properties in meibomian gland dysfunction. Cont. Lens Anterior Eye 2018, 41, 83–87. [Google Scholar] [CrossRef]
- Terada, O.; Chiba, K.; Senoo, T.; Obara, Y. Ocular surface temperature of meibomia gland dysfunction patients and the melting point of meibomian gland secretions. Nippon Ganka Gakkai Zasshi 2004, 108, 690–693. [Google Scholar]
- Foulks, G.N. The correlation between the tear film lipid layer and dry eye disease. Surv. Ophthalmol. 2007, 52, 369–374. [Google Scholar] [CrossRef]
- Borchman, D.; Foulks, G.N.; Yappert, M.C.; Bell, J.; Wells, E.; Neravetla, S.; Greenstone, V. Human meibum lipid conformation and thermodynamic changes with meibomian-gland dysfunction. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3805–3817. [Google Scholar] [CrossRef] [PubMed]
- Olson, M.C.; Korb, D.R.; Greiner, J.V. Increase in tear film lipid layer thickness following treatment with warm compresses in patients with meibomian gland dysfunction. Eye Contact Lens 2003, 29, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.W.; Eom, Y.S.; Rhim, J.W.; Kang, S.Y.; Kim, H.M.; Song, J.S. The Effects of Warm Compression on Eyelid Temperature and Lipid Layer Thickness of Tear Film. J. Korean Ophthalmol. Soc. 2016, 57, 876. [Google Scholar] [CrossRef] [Green Version]
- Korb, D.R.; Blackie, C.A. Meibomian gland therapeutic expression: Quantifying the applied pressure and the limitation of resulting pain. Eye Contact Lens 2011, 37, 298–301. [Google Scholar] [CrossRef]
- Arita, R.; Minoura, I.; Morishige, N.; Shirakawa, R.; Fukuoka, S.; Asai, K.; Goto, T.; Imanaka, T.; Nakamura, M. Development of Definitive and Reliable Grading Scales for Meibomian Gland Dysfunction. Am. J. Ophthalmol. 2016, 169, 125–137. [Google Scholar] [CrossRef] [Green Version]
- Setu, M.A.K.; Horstmann, J.; Schmidt, S.; Stern, M.E.; Steven, P. Deep learning-based automatic meibomian gland segmentation and morphology assessment in infrared meibography. Sci. Rep. 2021, 11, 7649. [Google Scholar] [CrossRef]
- Nichols, K.K.; Foulks, G.N.; Bron, A.J.; Glasgow, B.J.; Dogru, M.; Tsubota, K.; Lemp, M.A.; Sullivan, D.A. The international workshop on meibomian gland dysfunction: Executive summary. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1922–1929. [Google Scholar] [CrossRef] [Green Version]
- Han, D.; Kim, H.; Kim, S.; Park, Y.; Cho, K.J. Comparative study on the effect of hyperthermic massage and mechanical squeezing in the patients with mild and severe meibomian gland dysfunction: An interventional case series. PLoS ONE 2021, 16, e0247365. [Google Scholar] [CrossRef]
- Lee, H.; Kim, M.; Park, S.Y.; Kim, E.K.; Seo, K.Y.; Kim, T.I. Mechanical meibomian gland squeezing combined with eyelid scrubs and warm compresses for the treatment of meibomian gland dysfunction. Clin. Exp. Optom. 2017, 100, 598–602. [Google Scholar] [CrossRef] [Green Version]
- Mathers, W.D.; Lane, J.A. Meibomian gland lipids, evaporation, and tear film stability. Lacrimal Gland. Tear Film. Dry Eye Syndr. 1998, 438, 349–360. [Google Scholar] [CrossRef]
- McCulley, J.P.; Shine, W.E. The lipid layer of tears: Dependent on meibomian gland function. Exp. Eye Res. 2004, 78, 361–365. [Google Scholar] [CrossRef]
- Goto, E.; Endo, K.; Suzuki, A.; Fujikura, Y.; Tsubota, K. Improvement of tear stability following warm compression in patients with meibomian gland dysfunction. Lacrimal Gland. Tear Film. Dry Eye Syndr. 2002, 506, 1149–1152. [Google Scholar]
- Wang, M.T.M.; Jaitley, Z.; Lord, S.M.; Craig, J.P. Comparison of self-applied heat therapy for meibomian gland dysfunction. Optom. Vis. Sci. 2015, 92, 321–326. [Google Scholar] [CrossRef] [PubMed]
- McCulley, J.P.; Shine, W.E. Meibomian secretions in chronic blepharitis. Lacrimal Gland. Tear Film. Dry Eye Syndr. 1998, 438, 319–326. [Google Scholar] [CrossRef]
- Knop, E.; Knop, N.; Millar, T.; Obata, H.; Sullivan, D.A. The international workshop on meibomian gland dysfunction: Report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1938–1978. [Google Scholar] [CrossRef] [Green Version]
| All (N = 26) | MGD (N = 19) | Normal (N = 7) | p-Value | |
|---|---|---|---|---|
| Age | 38.53 ± 14.02 | 42.08 ± 15.86 | 30.83 ± 0.41 | 0.480 |
| Sex (male:female) | 8:18 | 4:15 | 4:3 | 0.083 |
| Eyelid | ||||
| Vascularity | 2.78 ± 1.99 | 4.0 ± 1.34 | 0.86 ± 1.07 | <0.01 * |
| Plugging | 1.50 ± 1.2 | 2.27 ± 0.65 | 0.26 ± 0.76 | <0.01 * |
| Irregularity | 0.11 ± 0.32 | 0.18 ± 0.41 | 0.0 ± 0.0 | 0.245 |
| Thickening | 0.56 ± 0.92 | 0.91 ± 1.04 | 0.0 ± 0.0 | 0.041 |
| Meibum quality | 2.73 ± 1.42 | 3.07 ± 0.96 | 2.00 ± 2.00 | 0.055 |
| Meibum expressibility | 3.09 ± 1.90 | 3.87 ± 1.72 | 1.43 ± 0.98 | 0.005 * |
| Before Treatment | After Treatment | p-Value | ||
|---|---|---|---|---|
| Tear Film Lipid Layer Thickness (TFLLT) | ||||
| Average | 62.04 ± 22.68 | 82.27 ± 20.83 | 0.002 * | |
| Maximum | 78.12 ± 22.75 | 90.81 ± 13.71 | 0.016 * | |
| Minimum | 54.31 ± 21.20 | 76.38 ± 23.09 | 0.001 * | |
| Total blink frequency (TBF) | 5.31 ± 5.28 | 4.85 ± 4.90 | 0.407 | |
| Complete blink frequency (CBF) | 2.27 ± 4.51 | 2.19 ± 4.89 | 0.923 | |
| Partial blink frequency (PBF) | 3.04 ± 2.24 | 2.65 ± 2.42 | 0.613 | |
| Partial blink rate (PBR) | 0.67 ± 0.38 | 0.64 ± 0.39 | 0.943 | |
| MGD | Normal | ||||||
|---|---|---|---|---|---|---|---|
| Before Tx | After Tx | p-Value | Before Tx | After Tx | p-Value | ||
| TFLLT | |||||||
| Avr | 64.74 ± 23.43 | 77.16 ± 21.93 | 0.067 | 54.71 ± 20.25 | 96.14 ± 7.58 | 0.018 * | |
| Max | 79.42 ± 23.51 | 87.42 ± 14.71 | 0.164 | 74.57 ± 21.85 | 100 ± 0 | 0.028 * | |
| Min | 57.26 ± 22.96 | 71.26 ± 24.57 | 0.042 * | 46.29 ± 13.76 | 90.29 ± 10.10 | 0.018 * | |
| TBF | 5.63 ± 6.05 | 5.16 ± 5.61 | 0.568 | 4.43 ± 2.30 | 4.00 ± 2.08 | 0.396 | |
| CBF | 3.00 ± 5.11 | 2.58 ± 5.57 | 0.575 | 0.29 ± 0.49 | 1.14 ± 2.19 | 0.18 | |
| PBF | 2.63 ± 2.22 | 2.58 ± 2.67 | 0.95 | 4.14 ± 2.04 | 2.86 ± 1.68 | 0.279 | |
| PBR | 0.56 ± 0.40 | 0.57 ± 0.40 | 0.285 | 0.95 ± 0.10 | 0.81 ± 0.32 | 0.592 | |
| Before Treatment | After Treatment | p-Value | |
|---|---|---|---|
| Partial glands | 2.27 ± 1.58 | 2.20 ± 1.57 | 0.317 |
| Gland dropout | 1.93 ± 0.96 | 1.93 ± 0.96 | 1 |
| Length (mm) | 4.45 ± 0.74 | 4.60 ± 0.90 | 0.047 * |
| Thickness (mm) | 0.74 ± 0.15 | 0.74 ± 0.15 | 0.814 |
| MGD | Normal | |||||
|---|---|---|---|---|---|---|
| Before Tx | After Tx | p-Value | Before Tx | After Tx | p-Value | |
| Partial glands | 3.38 ± 1.06 | 3.25 ± 1.17 | 0.317 | 1.0 ± 1.0 | 1.0 ± 1.0 | 1 |
| Glands dropout | 2.38 ± 0.74 | 2.38 ± 0.74 | 1 | 1.43 ± 0.98 | 1.43 ± 0.98 | 1 |
| Length (mm) | 4.08 ± 0.44 | 4.20 ± 0.78 | 0.401 | 4.87 ± 0.82 | 5.06 ± 0.86 | 0.028 * |
| Thickness (mm) | 0.71 ± 0.16 | 0.69 ± 0.19 | 0.31 | 0.79 ± 0.13 | 0.81 ± 0.11 | 0.345 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.M.; Lee, W.J.; Lim, H.W.; Kim, Y.J. Immediate and Quantitative Changes in Tear Film Parameters and Meibomian Gland Structures after Warm Compression and Meibomian Gland Squeezing in Meibomian Gland Dysfunction Patients and Normal Subjects. J. Clin. Med. 2022, 11, 4577. https://doi.org/10.3390/jcm11154577
Park HM, Lee WJ, Lim HW, Kim YJ. Immediate and Quantitative Changes in Tear Film Parameters and Meibomian Gland Structures after Warm Compression and Meibomian Gland Squeezing in Meibomian Gland Dysfunction Patients and Normal Subjects. Journal of Clinical Medicine. 2022; 11(15):4577. https://doi.org/10.3390/jcm11154577
Chicago/Turabian StylePark, Hae Min, Won June Lee, Han Woong Lim, and Yu Jeong Kim. 2022. "Immediate and Quantitative Changes in Tear Film Parameters and Meibomian Gland Structures after Warm Compression and Meibomian Gland Squeezing in Meibomian Gland Dysfunction Patients and Normal Subjects" Journal of Clinical Medicine 11, no. 15: 4577. https://doi.org/10.3390/jcm11154577
APA StylePark, H. M., Lee, W. J., Lim, H. W., & Kim, Y. J. (2022). Immediate and Quantitative Changes in Tear Film Parameters and Meibomian Gland Structures after Warm Compression and Meibomian Gland Squeezing in Meibomian Gland Dysfunction Patients and Normal Subjects. Journal of Clinical Medicine, 11(15), 4577. https://doi.org/10.3390/jcm11154577






