Role of Ultrasound Methods for the Assessment of NAFLD
Abstract
:1. Introduction
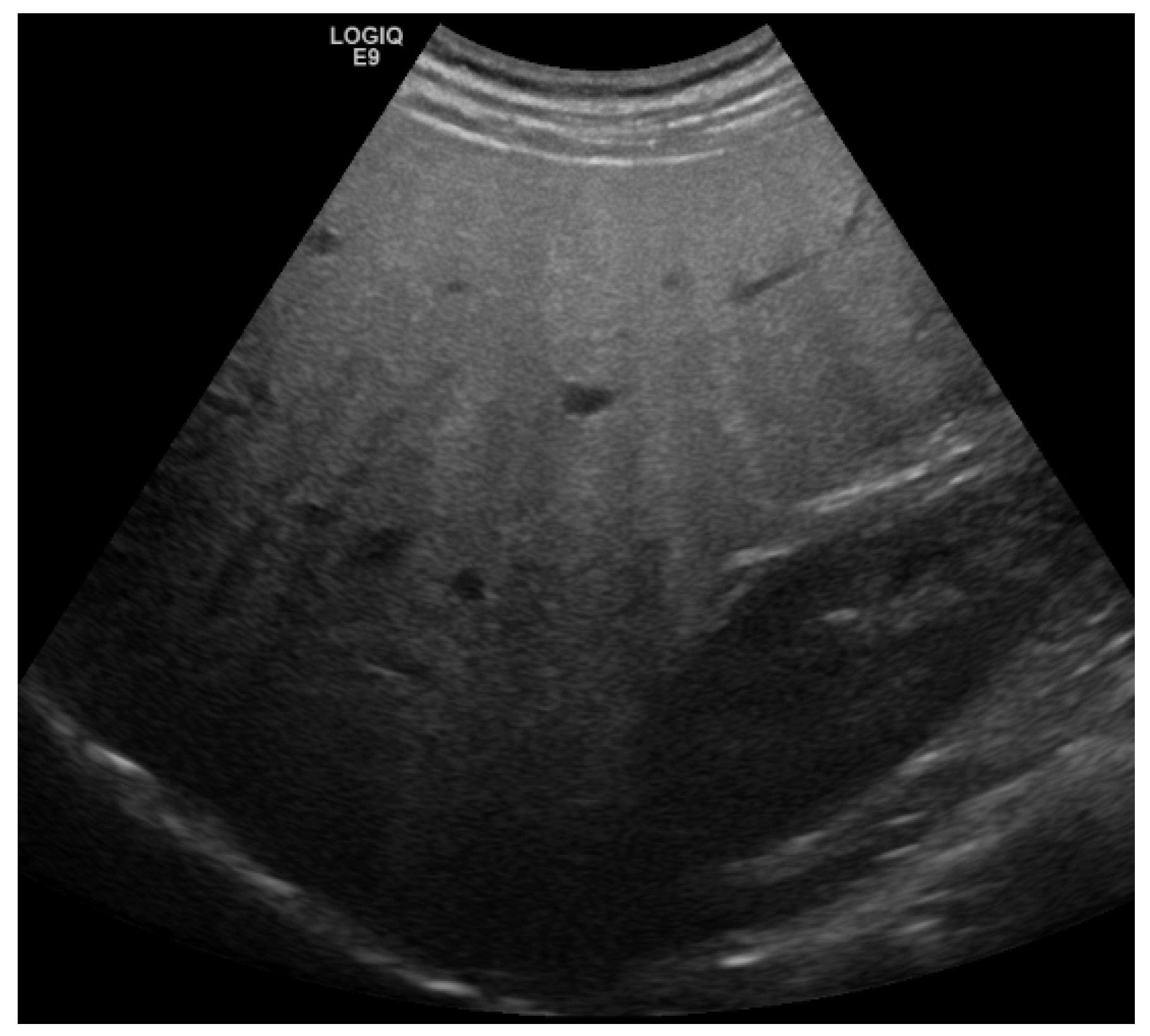
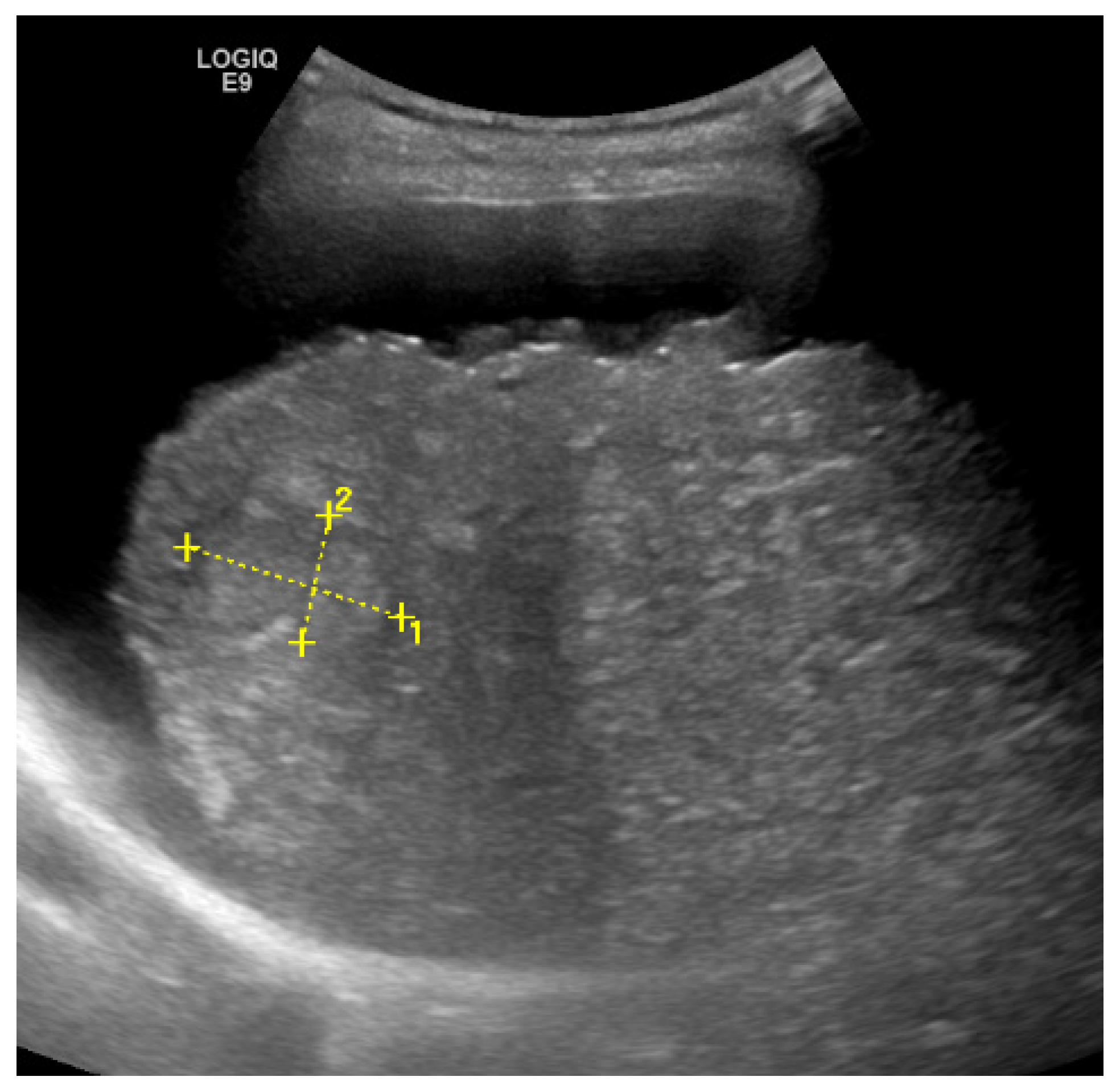
2. Detection and Graduation of Hepatic Steatosis
3. Liver Fibrosis
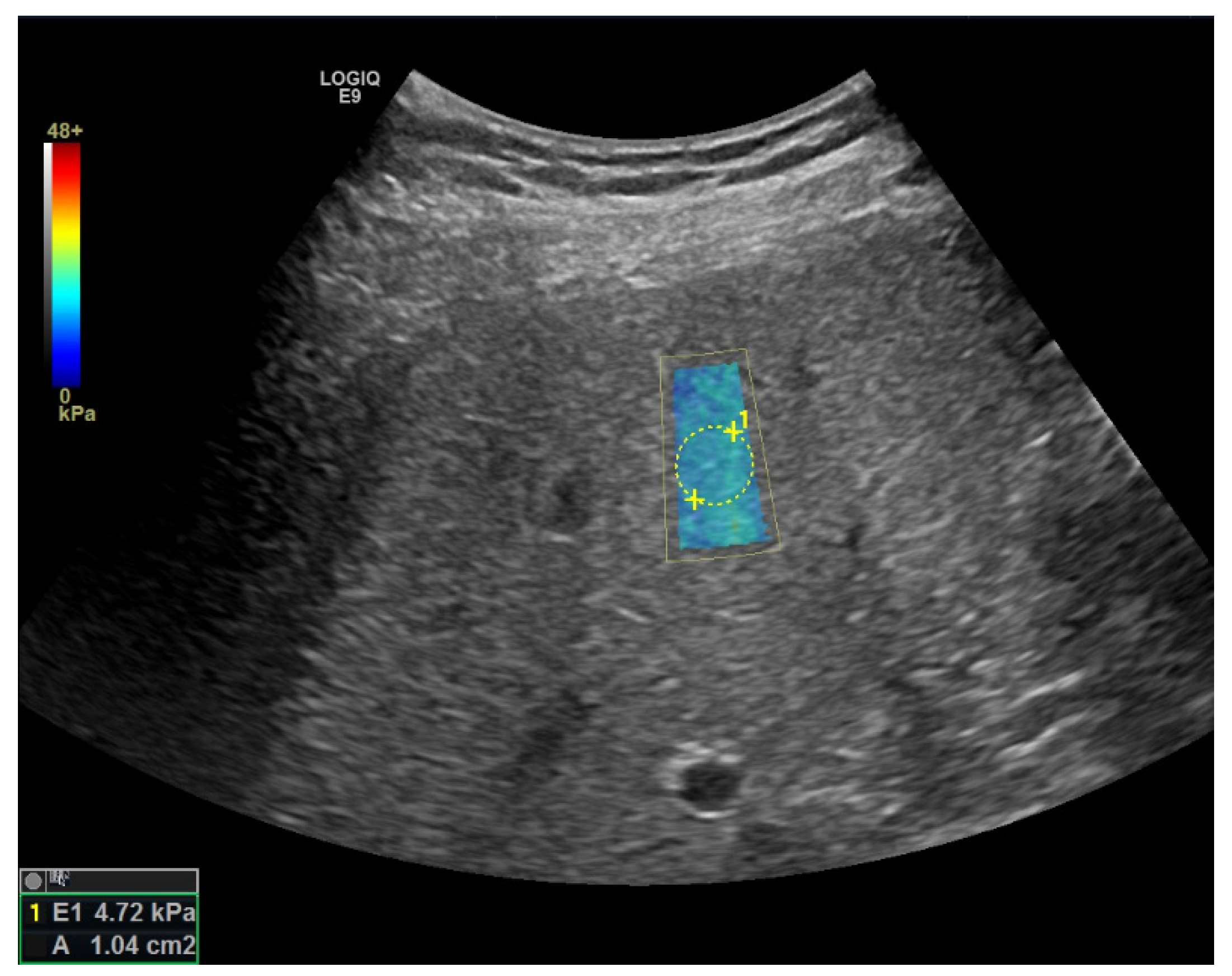
Elastography
4. Screening for Hepatocellular Carcinoma
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Younossi, Z.M. Non-Alcoholic Fatty Liver Disease—A Global Public Health Perspective. J. Hepatol. 2019, 70, 531–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanwal, F.; Shubrook, J.H.; Adams, L.A.; Pfotenhauer, K.; Wai-Sun Wong, V.; Wright, E.; Abdelmalek, M.F.; Harrison, S.A.; Loomba, R.; Mantzoros, C.S.; et al. Clinical Care Pathway for the Risk Stratification and Management of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2021, 161, 1657–1669. [Google Scholar] [CrossRef] [PubMed]
- EASL–EASD–EASO Clinical Practice Guidelines for the Management of Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2016, 64, 1388–1402. [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.-C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and Validation of a Histological Scoring System for Nonalcoholic Fatty Liver Disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Simon, T.G.; Roelstraete, B.; Khalili, H.; Hagström, H.; Ludvigsson, J.F. Mortality in Biopsy-Confirmed Nonalcoholic Fatty Liver Disease: Results from a Nationwide Cohort. Gut 2021, 70, 1375–1382. [Google Scholar] [CrossRef]
- Roeb, E. Diagnostic and Therapy of Nonalcoholic Fatty Liver Disease: A Narrative Review. Visc. Med. 2022, 38, 126–132. [Google Scholar] [CrossRef]
- Rockey, D.C.; Caldwell, S.H.; Goodman, Z.D.; Nelson, R.C.; Smith, A.D.; American Association for the Study of Liver Diseases. Liver Biopsy. Hepatology 2009, 49, 1017–1044. [Google Scholar] [CrossRef]
- Khalifa, A.; Rockey, D.C. The Utility of Liver Biopsy in 2020. Curr. Opin. Gastroenterol. 2020, 36, 184–191. [Google Scholar] [CrossRef]
- Westin, J.; Lagging, L.M.; Wejstål, R.; Norkrans, G.; Dhillon, A.P. Interobserver Study of Liver Histopathology Using the Ishak Score in Patients with Chronic Hepatitis C Virus Infection. Liver 1999, 19, 183–187. [Google Scholar] [CrossRef]
- Regev, A.; Berho, M.; Jeffers, L.J.; Milikowski, C.; Molina, E.G.; Pyrsopoulos, N.T.; Feng, Z.-Z.; Reddy, K.R.; Schiff, E.R. Sampling Error and Intraobserver Variation in Liver Biopsy in Patients with Chronic HCV Infection. Am. J. Gastroenterol. 2002, 97, 2614–2618. [Google Scholar] [CrossRef]
- Merriman, R.B.; Ferrell, L.D.; Patti, M.G.; Weston, S.R.; Pabst, M.S.; Aouizerat, B.E.; Bass, N.M. Correlation of Paired Liver Biopsies in Morbidly Obese Patients with Suspected Nonalcoholic Fatty Liver Disease. Hepatology 2006, 44, 874–880. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on Non-Invasive Tests for Evaluation of Liver Disease Severity and Prognosis—2021 Update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef]
- Saadeh, S.; Younossi, Z.M.; Remer, E.M.; Gramlich, T.; Ong, J.P.; Hurley, M.; Mullen, K.D.; Cooper, J.N.; Sheridan, M.J. The Utility of Radiological Imaging in Nonalcoholic Fatty Liver Disease. Gastroenterology 2002, 123, 745–750. [Google Scholar] [CrossRef]
- van Werven, J.R.; Marsman, H.A.; Nederveen, A.J.; Smits, N.J.; ten Kate, F.J.; van Gulik, T.M.; Stoker, J. Assessment of Hepatic Steatosis in Patients Undergoing Liver Resection: Comparison of US, CT, T1-Weighted Dual-Echo MR Imaging, and Point-Resolved 1H MR Spectroscopy. Radiology 2010, 256, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Petzold, G.; Lasser, J.; Rühl, J.; Bremer, S.C.B.; Knoop, R.F.; Ellenrieder, V.; Kunsch, S.; Neesse, A. Diagnostic Accuracy of B-Mode Ultrasound and Hepatorenal Index for Graduation of Hepatic Steatosis in Patients with Chronic Liver Disease. PLoS ONE 2020, 15, e0231044. [Google Scholar] [CrossRef]
- de Moura Almeida, A.; Cotrim, H.P.; Barbosa, D.B.V.; de Athayde, L.G.M.; Santos, A.S.; Bitencourt, A.G.V.; de Freitas, L.A.R.; Rios, A.; Alves, E. Fatty Liver Disease in Severe Obese Patients: Diagnostic Value of Abdominal Ultrasound. World J. Gastroenterol. 2008, 14, 1415–1418. [Google Scholar] [CrossRef]
- Lee, S.S.; Park, S.H.; Kim, H.J.; Kim, S.Y.; Kim, M.-Y.; Kim, D.Y.; Suh, D.J.; Kim, K.M.; Bae, M.H.; Lee, J.Y.; et al. Non-Invasive Assessment of Hepatic Steatosis: Prospective Comparison of the Accuracy of Imaging Examinations. J. Hepatol. 2010, 52, 579–585. [Google Scholar] [CrossRef]
- Palmentieri, B.; de Sio, I.; La Mura, V.; Masarone, M.; Vecchione, R.; Bruno, S.; Torella, R.; Persico, M. The Role of Bright Liver Echo Pattern on Ultrasound B-Mode Examination in the Diagnosis of Liver Steatosis. Dig. Liver Dis. 2006, 38, 485–489. [Google Scholar] [CrossRef]
- Miller, E.; Schmidberger, J.; Kratzer, W. Focal Fatty Sparing as an Indicator of Higher-Grade Fatty Liver Assessed by Attenuation Imaging: A Prospective Clinical Study in NAFLD Population. Z. Gastroenterol. 2021. [Google Scholar] [CrossRef]
- Webb, M.; Yeshua, H.; Zelber-Sagi, S.; Santo, E.; Brazowski, E.; Halpern, Z.; Oren, R. Diagnostic Value of a Computerized Hepatorenal Index for Sonographic Quantification of Liver Steatosis. AJR Am. J. Roentgenol. 2009, 192, 909–914. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, A.; Sultan, L.R.; Furth, E.E.; Jones, L.P.; Khungar, V.; Sehgal, C.M. Diagnostic Accuracy of Hepatorenal Index in the Detection and Grading of Hepatic Steatosis. J. Clin. Ultrasound 2016, 44, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Marshall, R.H.; Eissa, M.; Bluth, E.I.; Gulotta, P.M.; Davis, N.K. Hepatorenal Index as an Accurate, Simple, and Effective Tool in Screening for Steatosis. AJR Am. J. Roentgenol. 2012, 199, 997–1002. [Google Scholar] [CrossRef]
- Wang, J.-H.; Hung, C.-H.; Kuo, F.-Y.; Eng, H.-L.; Chen, C.-H.; Lee, C.-M.; Lu, S.-N.; Hu, T.-H. Ultrasonographic Quantification of Hepatic-Renal Echogenicity Difference in Hepatic Steatosis Diagnosis. Dig. Dis. Sci. 2013, 58, 2993–3000. [Google Scholar] [CrossRef] [PubMed]
- Karlas, T.; Petroff, D.; Sasso, M.; Fan, J.-G.; Mi, Y.-Q.; de Lédinghen, V.; Kumar, M.; Lupsor-Platon, M.; Han, K.-H.; Cardoso, A.C.; et al. Individual Patient Data Meta-Analysis of Controlled Attenuation Parameter (CAP) Technology for Assessing Steatosis. J. Hepatol. 2017, 66, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Petroff, D.; Blank, V.; Newsome, P.N.; Shalimar; Voican, C.S.; Thiele, M.; de Lédinghen, V.; Baumeler, S.; Chan, W.K.; Perlemuter, G.; et al. Assessment of Hepatic Steatosis by Controlled Attenuation Parameter Using the M and XL Probes: An Individual Patient Data Meta-Analysis. Lancet Gastroenterol. Hepatol. 2021, 6, 185–198. [Google Scholar] [CrossRef]
- Ferraioli, G.; Berzigotti, A.; Barr, R.G.; Choi, B.I.; Cui, X.W.; Dong, Y.; Gilja, O.H.; Lee, J.Y.; Lee, D.H.; Moriyasu, F.; et al. Quantification of Liver Fat Content with Ultrasound: A WFUMB Position Paper. Ultrasound Med. Biol. 2021, 47, 2803–2820. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Iijima, H.; Kobayashi, N.; Yoshida, M.; Nishimura, T.; Kumada, T.; Kondo, R.; Yano, H.; Kage, M.; Nakano, C.; et al. Usefulness of Attenuation Imaging with an Ultrasound Scanner for the Evaluation of Hepatic Steatosis. Ultrasound Med. Biol. 2019, 45, 2679–2687. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Moriyasu, F.; Oshiro, H.; Takeuchi, H.; Abe, M.; Yoshimasu, Y.; Kasai, Y.; Sakamaki, K.; Hara, T.; Itoi, T. The Role of Multiparametric US of the Liver for the Evaluation of Nonalcoholic Steatohepatitis. Radiology 2020, 296, 532–540. [Google Scholar] [CrossRef]
- Jeon, S.K.; Lee, J.M.; Joo, I.; Yoon, J.H.; Lee, D.H.; Lee, J.Y.; Han, J.K. Prospective Evaluation of Hepatic Steatosis Using Ultrasound Attenuation Imaging in Patients with Chronic Liver Disease with Magnetic Resonance Imaging Proton Density Fat Fraction as the Reference Standard. Ultrasound Med. Biol. 2019, 45, 1407–1416. [Google Scholar] [CrossRef]
- Labyed, Y.; Milkowski, A. Novel Method for Ultrasound-Derived Fat Fraction Using an Integrated Phantom. J. Ultrasound Med. 2020, 39, 2427–2438. [Google Scholar] [CrossRef]
- Ajmera, V.; Park, C.C.; Caussy, C.; Singh, S.; Hernandez, C.; Bettencourt, R.; Hooker, J.; Sy, E.; Behling, C.; Xu, R.; et al. Magnetic Resonance Imaging Proton Density Fat Fraction Associates With Progression of Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2018, 155, 307–310.e2. [Google Scholar] [CrossRef]
- Hagström, H.; Nasr, P.; Ekstedt, M.; Hammar, U.; Stål, P.; Hultcrantz, R.; Kechagias, S. Fibrosis Stage but Not NASH Predicts Mortality and Time to Development of Severe Liver Disease in Biopsy-Proven NAFLD. J. Hepatol. 2017, 67, 1265–1273. [Google Scholar] [CrossRef]
- Vilar-Gomez, E.; Calzadilla-Bertot, L.; Wai-Sun Wong, V.; Castellanos, M.; Aller-de la Fuente, R.; Metwally, M.; Eslam, M.; Gonzalez-Fabian, L.; Alvarez-Quiñones Sanz, M.; Conde-Martin, A.F.; et al. Fibrosis Severity as a Determinant of Cause-Specific Mortality in Patients With Advanced Nonalcoholic Fatty Liver Disease: A Multi-National Cohort Study. Gastroenterology 2018, 155, 443–457.e17. [Google Scholar] [CrossRef]
- Dulai, P.S.; Singh, S.; Patel, J.; Soni, M.; Prokop, L.J.; Younossi, Z.; Sebastiani, G.; Ekstedt, M.; Hagstrom, H.; Nasr, P.; et al. Increased Risk of Mortality by Fibrosis Stage in Nonalcoholic Fatty Liver Disease: Systematic Review and Meta-Analysis. Hepatology 2017, 65, 1557–1565. [Google Scholar] [CrossRef]
- Poynard, T.; Bedossa, P.; Opolon, P. Natural History of Liver Fibrosis Progression in Patients with Chronic Hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC Groups. Lancet 1997, 349, 825–832. [Google Scholar] [CrossRef]
- Berzigotti, A.; Ashkenazi, E.; Reverter, E.; Abraldes, J.G.; Bosch, J. Non-Invasive Diagnostic and Prognostic Evaluation of Liver Cirrhosis and Portal Hypertension. Dis. Markers 2011, 31, 129–138. [Google Scholar] [CrossRef]
- Choong, C.-C.; Venkatesh, S.K.; Siew, E.P.Y. Accuracy of Routine Clinical Ultrasound for Staging of Liver Fibrosis. J. Clin. Imaging Sci. 2012, 2, 58. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Bamber, J.; Berzigotti, A.; Bota, S.; Cantisani, V.; Castera, L.; Cosgrove, D.; Ferraioli, G.; Friedrich-Rust, M.; Gilja, O.H.; et al. EFSUMB Guidelines and Recommendations on the Clinical Use of Liver Ultrasound Elastography, Update 2017 (Long Version). Ultraschall Med. 2017, 38, e16–e47. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Huang, Y.-S.; Wang, Z.-Z.; Yang, Z.-R.; Sun, F.; Zhan, S.-Y.; Liu, X.-E.; Zhuang, H. Systematic Review with Meta-Analysis: The Diagnostic Accuracy of Transient Elastography for the Staging of Liver Fibrosis in Patients with Chronic Hepatitis B. Aliment. Pharmacol. Ther. 2016, 43, 458–469. [Google Scholar] [CrossRef]
- Piscaglia, F.; Salvatore, V.; Mulazzani, L.; Cantisani, V.; Schiavone, C. Ultrasound Shear Wave Elastography for Liver Disease. A Critical Appraisal of the Many Actors on the Stage. Ultraschall Med. 2016, 37, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Selvaraj, E.A.; Mózes, F.E.; Jayaswal, A.N.A.; Zafarmand, M.H.; Vali, Y.; Lee, J.A.; Levick, C.K.; Young, L.A.J.; Palaniyappan, N.; Liu, C.-H.; et al. Diagnostic Accuracy of Elastography and Magnetic Resonance Imaging in Patients with NAFLD: A Systematic Review and Meta-Analysis. J. Hepatol. 2021, 75, 770–785. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Zhu, S.; Xiao, X.; Yan, L.; Yang, J.; Wu, G. Comparison of Laboratory Tests, Ultrasound, or Magnetic Resonance Elastography to Detect Fibrosis in Patients with Nonalcoholic Fatty Liver Disease: A Meta-Analysis. Hepatology 2017, 66, 1486–1501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendoza, Y.P.; Rodrigues, S.G.; Delgado, M.G.; Murgia, G.; Lange, N.F.; Schropp, J.; Montani, M.; Dufour, J.-F.; Berzigotti, A. Inflammatory Activity Affects the Accuracy of Liver Stiffness Measurement by Transient Elastography but Not by Two-Dimensional Shear Wave Elastography in Non-Alcoholic Fatty Liver Disease. Liver Int. 2022, 42, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Cassinotto, C.; Boursier, J.; de Lédinghen, V.; Lebigot, J.; Lapuyade, B.; Cales, P.; Hiriart, J.-B.; Michalak, S.; Bail, B.L.; Cartier, V.; et al. Liver Stiffness in Nonalcoholic Fatty Liver Disease: A Comparison of Supersonic Shear Imaging, FibroScan, and ARFI with Liver Biopsy. Hepatology 2016, 63, 1817–1827. [Google Scholar] [CrossRef]
- Cassinotto, C.; Lapuyade, B.; Mouries, A.; Hiriart, J.-B.; Vergniol, J.; Gaye, D.; Castain, C.; Le Bail, B.; Chermak, F.; Foucher, J.; et al. Non-Invasive Assessment of Liver Fibrosis with Impulse Elastography: Comparison of Supersonic Shear Imaging with ARFI and FibroScan®. J. Hepatol. 2014, 61, 550–557. [Google Scholar] [CrossRef]
- Staugaard, B.; Christensen, P.B.; Mössner, B.; Hansen, J.F.; Madsen, B.S.; Søholm, J.; Krag, A.; Thiele, M. Feasibility of Transient Elastography versus Real-Time Two-Dimensional Shear Wave Elastography in Difficult-to-Scan Patients. Scand. J. Gastroenterol. 2016, 51, 1354–1359. [Google Scholar] [CrossRef]
- Sporea, I.; Șirli, R.; Mare, R.; Popescu, A.; Ivașcu, S.C. Feasibility of Transient Elastography with M and XL Probes in Real Life. Med. Ultrason. 2016, 18, 7–10. [Google Scholar] [CrossRef] [Green Version]
- Grgurević, I.; Bokun, T.; Mustapić, S.; Trkulja, V.; Heinzl, R.; Banić, M.; Puljiz, Ž.; Lukšić, B.; Kujundžić, M. Real-Time Two-Dimensional Shear Wave Ultrasound Elastography of the Liver Is a Reliable Predictor of Clinical Outcomes and the Presence of Esophageal Varices in Patients with Compensated Liver Cirrhosis. Croat. Med. J. 2015, 56, 470–481. [Google Scholar] [CrossRef] [Green Version]
- Khadka, S.; Pandit, R.; Dhital, S.; Baniya, J.B.; Tiwari, S.; Shrestha, B.; Pandit, S.; Sato, F.; Fujita, M.; Sharma, M.; et al. Evaluation of Five International HBV Treatment Guidelines: Recommendation for Resource-Limited Developing Countries Based on the National Study in Nepal. Pathophysiology 2020, 27, 2. [Google Scholar] [CrossRef]
- Mózes, F.E.; Lee, J.A.; Selvaraj, E.A.; Jayaswal, A.N.A.; Trauner, M.; Boursier, J.; Fournier, C.; Staufer, K.; Stauber, R.E.; Bugianesi, E.; et al. Diagnostic Accuracy of Non-Invasive Tests for Advanced Fibrosis in Patients with NAFLD: An Individual Patient Data Meta-Analysis. Gut 2022, 71, 1006–1019. [Google Scholar] [CrossRef]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular Carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- Singal, A.; Volk, M.L.; Waljee, A.; Salgia, R.; Higgins, P.; Rogers, M.a.M.; Marrero, J.A. Meta-Analysis: Surveillance with Ultrasound for Early-Stage Hepatocellular Carcinoma in Patients with Cirrhosis. Aliment. Pharmacol. Ther. 2009, 30, 37–47. [Google Scholar] [CrossRef]
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.-L.; Schirmacher, P.; Vilgrain, V. EASL Clinical Practice Guidelines: Management of Hepatocellular Carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [Green Version]
- Kanwal, F.; Kramer, J.R.; Mapakshi, S.; Natarajan, Y.; Chayanupatkul, M.; Richardson, P.A.; Li, L.; Desiderio, R.; Thrift, A.P.; Asch, S.M.; et al. Risk of Hepatocellular Cancer in Patients With Non-Alcoholic Fatty Liver Disease. Gastroenterology 2018, 155, 1828–1837.e2. [Google Scholar] [CrossRef] [Green Version]
- Karlas, T.; Wiegand, J.; Petroff, D. Do NAFLD-patients require HCC screening? Z. Gastroenterol. 2019, 57, 160–161. [Google Scholar] [CrossRef]
- Grgurevic, I.; Bozin, T.; Mikus, M.; Kukla, M.; O’Beirne, J. Hepatocellular Carcinoma in Non-Alcoholic Fatty Liver Disease: From Epidemiology to Diagnostic Approach. Cancers 2021, 13, 5844. [Google Scholar] [CrossRef]
- Loomba, R.; Lim, J.K.; Patton, H.; El-Serag, H.B. AGA Clinical Practice Update on Screening and Surveillance for Hepatocellular Carcinoma in Patients With Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology 2020, 158, 1822–1830. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Choi, J.-I. Current Status of Image-Based Surveillance in Hepatocellular Carcinoma. Ultrasonography 2021, 40, 45–56. [Google Scholar] [CrossRef]
| Sensitivity | Specificity | Advantage | Disadvantage | |
|---|---|---|---|---|
| B-Mode US [14,15,16,17,18] | 53–76% | 76–93% | -High availability -high-end-device not required | -semiquantitative -low sensitivity for mild steatosis (<30%) |
| HRI [20,21,22,23] | 62.5–100% | 54–95% | -quantitative -potentially better than US alone | -low sensitivity for mild steatosis (<30%) -additional program required to calculate HRI -lack of evidence, HRI-value depends on US device and cause of liver disease |
| CAP [24] | 68.8% | 82.2% | -widely validated, high evidence -defined cut-off values for grades of steatosis | -dedicated device required -lower accuracy in very obese patients |
| Newer fat-quantification techniques [27,28,29,30] | (68–100%) | (62–100%) | -integrated in high-end devices -potentially high sensitivity (more studies required) | -low evidence, lack of studies |
| Sensitivity | Specificity | Advantage | Disadvantage | |
|---|---|---|---|---|
| B-Mode US [36,37] | F ≥ 2: 32% F ≥ 3: 40% F = 4: 20–91% | F ≥ 2: 85% F ≥ 3: 85% F = 4: 82–100% | -high availability -high specificity for cirrhosis | -high US experience required, -low sensitivity, even for cirrhosis -very low accuracy for fibrosis stages |
| TE [41] | F ≥ 2: 80% F ≥ 3: 80% F = 4: 76% | F ≥ 2: 73% F ≥ 3: 77% F = 4: 88% | -widely validated, high evidence -application recommended in guidelines -defined quality criteria -XL-probe for obese patients | -dedicated device required (low availability outside of centers) |
| pSWE [41] | F ≥ 2: 69% F ≥ 3: 80% F = 4: 76% | F ≥ 2: 85% F ≥ 3: 86% F = 4: 88% | -integrated in high-end devices, performing in combination with regular US | -smaller ROI |
| 2D-SWE [41] | F ≥ 2: 71% F ≥ 3: 72% F = 4: 78% | F ≥ 2: 67% F ≥ 3: 72% F = 4: 84% | -integrated in high-end devices, performing in combination with regular US -larger ROI (potential more representative) | -high failure rate in obese patients -lack of studies for most devices -lack of defined quality criteria |
| FIB-4 [50] | F ≥ 3: 69% | F ≥ 3: 70% | -based on simple variables widely available in clinical practice -free online calculator -Optimized-Cut-off Value: High NPV for ruling out advanced fibrosis | -High risk of false positive results for advanced fibrosis |
| NFS [50] | F ≥ 3: 75% | F ≥ 3: 63% | -High risk of false positive results for advanced fibrosis -lower performance in obese and diabetic patients | |
| APRI [50] | F ≥ 3: 67% | F ≥ 3: 63% | -only 2 simple parameters required -free online calculator | -Lower performance than FIB-4 and NFS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petzold, G. Role of Ultrasound Methods for the Assessment of NAFLD. J. Clin. Med. 2022, 11, 4581. https://doi.org/10.3390/jcm11154581
Petzold G. Role of Ultrasound Methods for the Assessment of NAFLD. Journal of Clinical Medicine. 2022; 11(15):4581. https://doi.org/10.3390/jcm11154581
Chicago/Turabian StylePetzold, Golo. 2022. "Role of Ultrasound Methods for the Assessment of NAFLD" Journal of Clinical Medicine 11, no. 15: 4581. https://doi.org/10.3390/jcm11154581
APA StylePetzold, G. (2022). Role of Ultrasound Methods for the Assessment of NAFLD. Journal of Clinical Medicine, 11(15), 4581. https://doi.org/10.3390/jcm11154581






