Factors Associated with and Prognosis Impact of Perceived Sleep Quality and Estimated Quantity in Patients Receiving Non-Invasive Ventilation for Acute Respiratory Failure
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Data Quality
2.4. Statistical Analysis
3. Results
3.1. Study Population and Quality of Sleep
3.2. Factors Associated with Poor Sleep Quality and Short Sleep Duration
3.3. Factors Associated with NIV Failure
3.4. Association between Sleep Quality or Duration and Outcome and Post ICU Burden
4. Discussion
4.1. Sleep Quality and Duration
4.2. Factors Associated with Low Sleep Duration and Quality
4.3. Relationship between Sleep and NIV Failure
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rittayamai, N.; Wilcox, E.; Drouot, X.; Mehta, S.; Goffi, A.; Brochard, L. Positive and negative effects of mechanical ventilation on sleep in the ICU: A review with clinical recommendations. Intensive Care Med. 2016, 42, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Carreira, S.; Lavault, S.; Demoule, A. Sommeil en réanimation et impact de la ventilation mécanique. Réanimation 2016, 25, 85–93. [Google Scholar] [CrossRef]
- Thille, A.W.; Reynaud, F.; Marie, D.; Barrau, S.; Rousseau, L.; Rault, C.; Diaz, V.; Meurice, J.-C.; Coudroy, R.; Frat, J.-P.; et al. Impact of sleep alterations on weaning duration in mechanically ventilated patients: A prospective study. Eur. Respir. J. 2018, 51, 1702465. [Google Scholar] [CrossRef] [PubMed]
- Orwelius, L.; Nordlund, A.; Nordlund, P.; Edéll-Gustafsson, U.; Sjöberg, F. Prevalence of sleep disturbances and long-term reduced health-related quality of life after critical care: A prospective multicenter cohort study. Crit. Care Lond. Engl. 2008, 12, R97. [Google Scholar] [CrossRef] [PubMed]
- Pisani, M.A.; D’Ambrosio, C. Sleep and Delirium in Adults Who Are Critically Ill: A Contemporary Review. Chest 2019. [Google Scholar] [CrossRef]
- Demoule, A.; Chevret, S.; Carlucci, A.; Kouatchet, A.; Jaber, S.; Meziani, F.; Schmidt, M.; Schnell, D.; Clergue, C.; Aboab, J.; et al. Changing use of noninvasive ventilation in critically ill patients: Trends over 15 years in francophone countries. Intensive Care Med. 2016, 42, 82–92. [Google Scholar] [CrossRef]
- Fanfulla, F.; Delmastro, M.; Berardinelli, A.; Lupo, N.D.; Nava, S. Effects of different ventilator settings on sleep and inspiratory effort in patients with neuromuscular disease. Am. J. Respir. Crit. Care Med. 2005, 172, 619–624. [Google Scholar] [CrossRef]
- Vrijsen, B.; Buyse, B.; Belge, C.; Robberecht, W.; Van Damme, P.; Decramer, M.; Testelmans, D. Noninvasive ventilation improves sleep in amyotrophic lateral sclerosis: A prospective polysomnographic study. J. Clin. Sleep Med. JCSM 2015, 11, 559–566. [Google Scholar] [CrossRef]
- Córdoba-Izquierdo, A.; Drouot, X.; Thille, A.W.; Galia, F.; Roche-Campo, F.; Schortgen, F.; Prats-Soro, E.; Brochard, L. Sleep in hypercapnic critical care patients under noninvasive ventilation: Conventional versus dedicated ventilators. Crit. Care Med. 2013, 41, 60–68. [Google Scholar] [CrossRef]
- Contal, O.; Janssens, J.-P.; Dury, M.; Delguste, P.; Aubert, G.; Rodenstein, D. Sleep in ventilatory failure in restrictive thoracic disorders. Effects of treatment with non invasive ventilation. Sleep Med. 2011, 12, 373–377. [Google Scholar] [CrossRef]
- Roche Campo, F.; Drouot, X.; Thille, A.W.; Galia, F.; Cabello, B.; d’Ortho, M.-P.; Brochard, L. Poor sleep quality is associated with late noninvasive ventilation failure in patients with acute hypercapnic respiratory failure. Crit. Care Med. 2010, 38, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Azoulay, E.; Kouatchet, A.; Jaber, S.; Lambert, J.; Meziani, F.; Schmidt, M.; Schnell, D.; Mortaza, S.; Conseil, M.; Tchenio, X.; et al. Noninvasive mechanical ventilation in patients having declined tracheal intubation. Intensive Care Med. 2013, 39, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Dangers, L.; Montlahuc, C.; Kouatchet, A.; Jaber, S.; Meziani, F.; Perbet, S.; Similowski, T.; Resche-Rigon, M.; Azoulay, E.; Demoule, A.; et al. Dyspnoea in patients receiving noninvasive ventilation for acute respiratory failure: Prevalence, risk factors and prognostic impact: A prospective observational study. Eur. Respir. J. 2018, 52, 1702637. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, J.R.; Lemeshow, S.; Saulnier, F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 1993, 270, 2957–2963. [Google Scholar] [CrossRef]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef]
- Devlin, J.W.; Skrobik, Y.; Gélinas, C.; Needham, D.M.; Slooter, A.J.C.; Pandharipande, P.P.; Watson, P.L.; Weinhouse, G.L.; Nunnally, M.E.; Rochwerg, B.; et al. Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit. Care Med. 2018, 46, e825–e873. [Google Scholar] [CrossRef]
- Brunet, A.; St-Hilaire, A.; Jehel, L.; King, S. Validation of a French version of the impact of event scale-revised. Can. J. Psychiatry Rev. Can. Psychiatr. 2003, 48, 56–61. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Pisani, M.A.; Friese, R.S.; Gehlbach, B.K.; Schwab, R.J.; Weinhouse, G.L.; Jones, S.F. Sleep in the intensive care unit. Am. J. Respir. Crit. Care Med. 2015, 191, 731–738. [Google Scholar] [CrossRef]
- Beltrami, F.G.; Nguyen, X.-L.; Pichereau, C.; Maury, E.; Fleury, B.; Fagondes, S. Sleep in the intensive care unit. J. Bras. Pneumol. Publicacao Soc. Bras. Pneumol. E Tisilogia 2015, 41, 539–546. [Google Scholar] [CrossRef]
- Meyer, T.J.; Pressman, M.R.; Benditt, J.; McCool, F.D.; Millman, R.P.; Natarajan, R.; Hill, N.S. Air leaking through the mouth during nocturnal nasal ventilation: Effect on sleep quality. Sleep 1997, 20, 561–569. [Google Scholar] [CrossRef] [PubMed][Green Version]
- O’Donoghue, F.J.; Catcheside, P.G.; Ellis, E.E.; Grunstein, R.R.; Pierce, R.J.; Rowland, L.S.; Collins, E.R.; Rochford, S.E.; McEvoy, R.D. Sleep hypoventilation in hypercapnic chronic obstructive pulmonary disease: Prevalence and associated factors. Eur. Respir. J. 2003, 21, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Broughton, R.; Baron, R. Sleep patterns in the intensive care unit and on the ward after acute myocardial infarction. Electroencephalogr. Clin. Neurophysiol. 1978, 45, 348–360. [Google Scholar] [CrossRef]
- Dohno, S.; Paskewitz, D.A.; Lynch, J.J.; Gimbel, K.S.; Thomas, S.A. Some aspects of sleep disturbance in coronary patients. Percept. Mot. Skills 1979, 48, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Fanfulla, F.; Ceriana, P.; D’Artavilla Lupo, N.; Trentin, R.; Frigerio, F.; Nava, S. Sleep disturbances in patients admitted to a step-down unit after ICU discharge: The role of mechanical ventilation. Sleep 2011, 34, 355–362. [Google Scholar] [CrossRef]
- Teschler, H.; Stampa, J.; Ragette, R.; Konietzko, N.; Berthon-Jones, M. Effect of mouth leak on effectiveness of nasal bilevel ventilatory assistance and sleep architecture. Eur. Respir. J. 1999, 14, 1251–1257. [Google Scholar] [CrossRef]
- Vignaux, L.; Vargas, F.; Roeseler, J.; Tassaux, D.; Thille, A.W.; Kossowsky, M.P.; Brochard, L.; Jolliet, P. Patient-ventilator asynchrony during non-invasive ventilation for acute respiratory failure: A multicenter study. Intensive Care Med. 2009, 35, 840–846. [Google Scholar] [CrossRef]
- Nava, S.; Gregoretti, C.; Fanfulla, F.; Squadrone, E.; Grassi, M.; Carlucci, A.; Beltrame, F.; Navalesi, P. Noninvasive ventilation to prevent respiratory failure after extubation in high-risk patients. Crit. Care Med. 2005, 33, 2465–2470. [Google Scholar] [CrossRef]
- Bosma, K.; Ferreyra, G.; Ambrogio, C.; Pasero, D.; Mirabella, L.; Braghiroli, A.; Appendini, L.; Mascia, L.; Ranieri, V.M. Patient-ventilator interaction and sleep in mechanically ventilated patients: Pressure support versus proportional assist ventilation. Crit. Care Med. 2007, 35, 1048–1054. [Google Scholar] [CrossRef]
- Papadimitriou, G.N.; Linkowski, P. Sleep disturbance in anxiety disorders. Int. Rev. Psychiatry Abingdon Engl. 2005, 17, 229–236. [Google Scholar] [CrossRef]
- Mellman, T.A. Sleep and anxiety disorders. Psychiatr. Clin. N. Am. 2006, 29, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Pires, G.N.; Bezerra, A.G.; Tufik, S.; Andersen, M.L. Effects of acute sleep deprivation on state anxiety levels: A systematic review and meta-analysis. Sleep Med. 2016, 24, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Currow, D.C.; Chang, S.; Ferreira, D.; Eckert, D.J.; Gonzalez-Chica, D.; Stocks, N.; Ekström, M.P. Chronic breathlessness and sleep problems: A population-based survey. BMJ Open 2021, 11, e046425. [Google Scholar] [CrossRef] [PubMed]
- Lou, V.W.Q.; Chen, E.J.; Jian, H.; Zhou, Z.; Zhu, J.; Li, G.; He, Y. Respiratory Symptoms, Sleep, and Quality of Life in Patients With Advanced Lung Cancer. J. Pain Symptom Manag. 2017, 53, 250–256.e1. [Google Scholar] [CrossRef]
- Schmidt, M.; Demoule, A.; Polito, A.; Porchet, R.; Aboab, J.; Siami, S.; Morelot-Panzini, C.; Similowski, T.; Sharshar, T. Dyspnea in mechanically ventilated critically ill patients. Crit. Care Med. 2011, 39, 2059–2065. [Google Scholar] [CrossRef]
- Redolfi, S.; Grassion, L.; Rivals, I.; Chavez, M.; Wattiez, N.; Arnulf, I.; Gonzalez-Bermejo, J.; Similowski, T. Abnormal Activity of Neck Inspiratory Muscles during Sleep as a Prognostic Indicator in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2020, 201, 414–422. [Google Scholar] [CrossRef]
- Rault, C.; Sangaré, A.; Diaz, V.; Ragot, S.; Frat, J.-P.; Raux, M.; Similowski, T.; Robert, R.; Thille, A.W.; Drouot, X. Impact of Sleep Deprivation on Respiratory Motor Output and Endurance. A Physiological Study. Am. J. Respir. Crit. Care Med. 2020, 201, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Kamdar, B.B.; Shah, P.A.; King, L.M.; Kho, M.E.; Zhou, X.; Colantuoni, E.; Collop, N.A.; Needham, D.M. Patient-nurse interrater reliability and agreement of the Richards-Campbell sleep questionnaire. Am. J. Crit. Care 2012, 21, 261–269. [Google Scholar] [CrossRef]
- Ritmala-Castren, M.; Virtanen, I.; Vahlberg, T.; Leivo, S.; Kaukonen, K.-M.; Leino-Kilpi, H. Evaluation of patients’ sleep by nurses in an ICU. J. Clin. Nurs. 2016, 25, 1606–1613. [Google Scholar] [CrossRef]
- Drouot, X.; Roche-Campo, F.; Thille, A.W.; Cabello, B.; Galia, F.; Margarit, L.; d’Ortho, M.-P.; Brochard, L. A new classification for sleep analysis in critically ill patients. Sleep Med. 2012, 13, 7–14. [Google Scholar] [CrossRef]

| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| All Patients (n = 389) | Poor Sleep Quality (n = 155) | Acceptable-to-Very Good Sleep Quality (n = 234) | p-Value | Odds Ratio (95% Confidence Interval) | p-Value | |
| Patient characteristics | ||||||
| Age, years | 69 (59–77) | 67 (59–76) | 69 (59–78) | 0.307 | ||
| Males, n (%) | 248 (64) | 99 (64) | 149 (64) | 1.000 | ||
| BMI, kg·m−2 | 26 (22–33) | 26 (22–31) | 26 (23–33) | 0.553 | ||
| Chronic respiratory disease, n (%) | 249 (64) | 98 (63) | 151 (65) | 0.830 | ||
| Chronic cardiac disease, n (%) | 86 (22) | 36 (23) | 50 (21) | 0.709 | ||
| Home oxygen therapy, n (%) | 80 (21) | 26 (17) | 54 (23) | 0.158 | ||
| NIV episode | ||||||
| SAPS 2 | 35 (27–44) | 36 (28–46) | 34 (26–43) | 0.065 | ||
| SOFA | 3 (2–5) | 3 (2–6) | 3 (2–5) | 0.278 | 1.11 (1.01–1.23) | 0.037 |
| Cause of ARF | ||||||
| Acute-on-chronic, n (%) | 234 (60) | 88 (57) | 146 (62) | 0.336 | ||
| Acute cardiogenic pulmonary edema, n (%) | 107 (28) | 49 (32) | 58 (25) | |||
| De novo ARF, n (%) | 48 (12) | 18 (12) | 30 (13) | |||
| On ICU admission, prior to NIV | ||||||
| Respiratory rate, cycle·min−1 | 32 (28–37) | 32 (28–37) | 31 (26-36) | 0.361 | ||
| Dyspnea Borg scale | 4 (3–5) | 4 (3–5) | 4 (3–5) | 0.194 | ||
| Blood gases | ||||||
| PaO2/FiO2, mmHg | 223 (161–287) | 222 (157–273) | 223 (163–298) | 0.356 | ||
| PaCO2, mmHg | 53 (40–71) | 53 (36–69) | 54 (42–72) | 0.170 | ||
| pH | 7.33 (7.27–7.40) | 7.34 (7.27–7.42) | 7.33 (7.27–7.40) | 0.737 | ||
| After the first NIV session | ||||||
| Air leaks, n (%) | 237 (61) | 107 (69) | 130 (56) | 0.008 | 1.92 (1.15–3.23) | 0.013 |
| Anxiety, n (%) | 216 (56) | 98 (63) | 118 (50) | <0.001 | 2.33 (1.39–3.85) | 0.001 |
| Respiratory rate, cycle·min−1 | 27 (23–33) | 29 (24–35) | 26 (23–30) | 0.005 | ||
| Dyspnea Borg scale | 3 (2–4) | 4 (3–5) | 3 (2–4) | <0.001 | ||
| Blood gases | ||||||
| PaO2/FiO2, mmHg | 213 (163–257) | 193 (150–240) | 220 (174–283) | 0.083 | ||
| PaCO2, mmHg | 54 (44–66) | 54 (41–65) | 54 (42–68) | 0.868 | ||
| pH | 7.35 (7.29–7.40) | 7.35 (7.29–7.40) | 7.35 (7.29–7.40) | 0.803 | ||
| NIV interface | 0.282 | |||||
| Oro-nasal mask | 245 (63) | 94 (61) | 151 (65) | |||
| Nasal mask | 4 (1) | 1 (1) | 3 (1) | |||
| Full face mask | 64 (16) | 31 (20) | 33 (14) | |||
| Type of ventilator | 0.192 | |||||
| NIV dedicated ventilator, n (%) | 51 (13) | 16 (10) | 35 (15) | |||
| ICU ventilator, n (%) | 167 (43) | 71 (46) | 96 (41) | |||
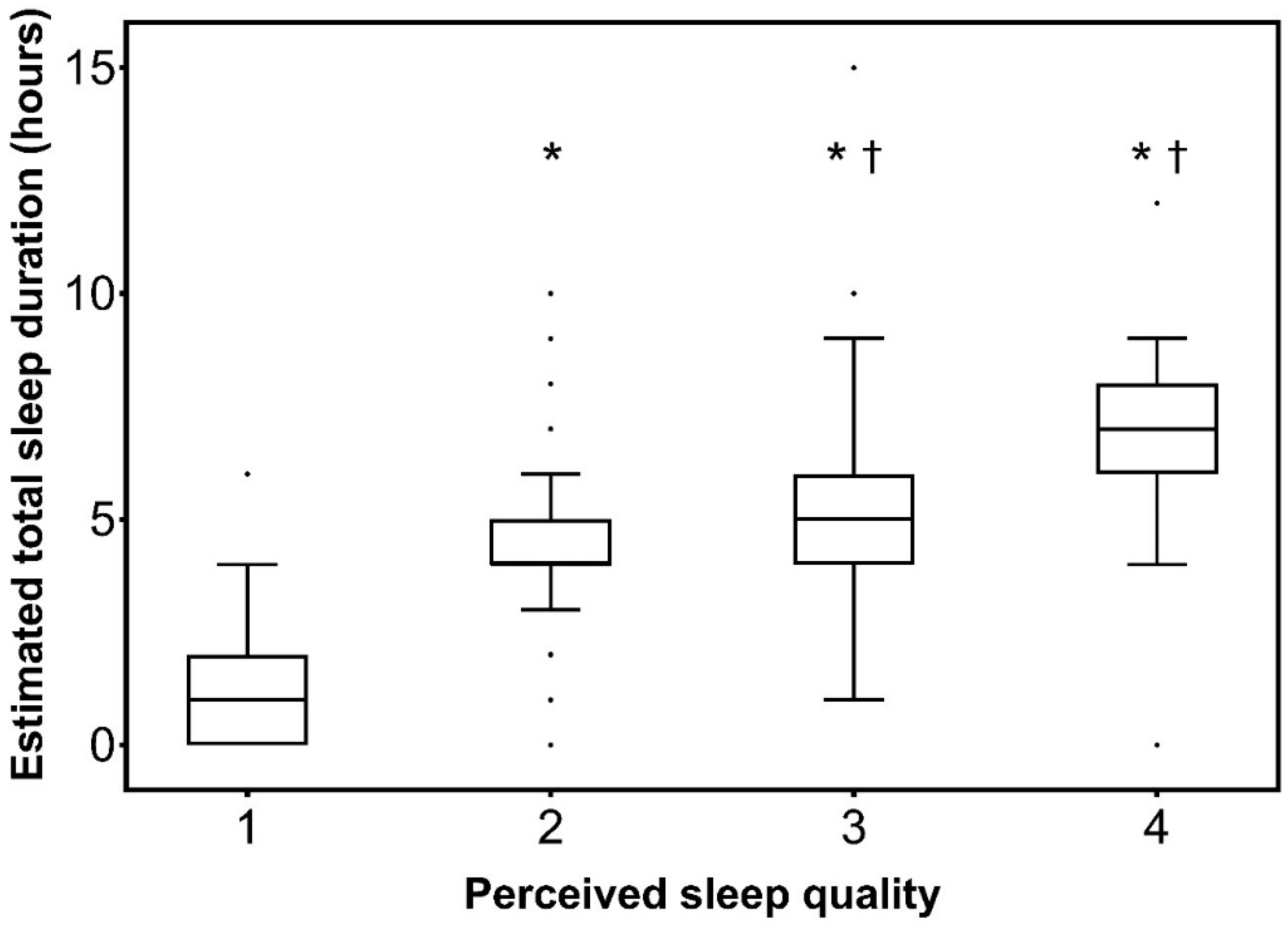
| Total sleep duration over the first 24 h after admission, h | 4 (2–5) | 1 (0–2) | 5 (4–6) | <0.001 | ||
| Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|
| Short Sleep Duration < 4 h (n = 148) | Acceptable Sleep Duration ≥ 4 h (n = 172) | p-Value | Odds Ratio (95% Confidence Interval) | p-Value | |
| Patient characteristics | |||||
| Age, years | 70 (57–7) | 70 (61–80) | 0.071 | ||
| Males, n (%) | 97 (66) | 108 (63) | 0.641 | ||
| BMI, kg.m−2 | 26 (22–31) | 26 (23–32) | 0.488 | ||
| Chronic respiratory disease, n (%) | 92 (62) | 121 (70) | 0.125 | ||
| Chronic cardiac disease, n (%) | 31 (21) | 40 (23) | 0.686 | ||
| Home oxygen therapy, n (%) | 20 (14) | 46 (27) | 0.004 | ||
| NIV episode | |||||
| SAPS 2 | 40 (29–49) | 34 (27–42) | 0.001 | ||
| SOFA | 4 (2–7) | 3 (2–5) | 0.011 | 1.13 (1.04–1.23) | 0.005 |
| Cause of ARF | |||||
| Acute-on-chronic, n (%) | 81 (55) | 112 (65) | 0.082 | ||
| Acute cardiogenic pulmonary edema, n (%) | 48 (32) | 37 (22) | |||
| De novo ARF, n (%) | 19 (13) | 23 (13) | |||
| On ICU admission, prior to NIV | |||||
| Respiratory rate, cycle·min−1 | 32 (28–40) | 30 (25–36) | 0.016 | ||
| Dyspnea Borg scale | 4 (3–5) | 3 (3–4) | 0.013 | 1.13 (1.01–1.27) | 0.031 |
| Blood gases | |||||
| PaO2/FiO2, mmHg | 209 (141–269) | 223 (163–292) | 0.129 | ||
| PaCO2, mmHg | 52 (36–70) | 56 (43–72) | 0.041 | ||
| pH | 7.33 (7.25–7.41) | 7.33 (7.28–7.40) | 0.649 | ||
| After the first NIV session | |||||
| Air leaks, n (%) | 103 (70) | 97 (56) | 0.016 | 1.92 (1.18–3.14) | 0.008 |
| Anxiety, n (%) | 92 (62) | 87 (51) | 0.022 | ||
| Respiratory rate, cycle·min−1 | 30 (24–35) | 25 (22–30) | <0.001 | ||
| Dyspnea Borg scale | 4 (3–5) | 3 (2–3) | <0.001 | ||
| Blood gases | |||||
| PaO2/FiO2, mmHg | 205 (150–250) | 222 (184–283) | 0.114 | ||
| PaCO2, mmHg | 53 (41–64) | 56 (43–68) | 0.400 | ||
| pH | 7.35 (7.27–7.40) | 7.36 (7.31–7.40) | 0.511 | ||
| NIV interface | 0.089 | ||||
| Oro-nasal mask | 106 (72) | 104 (60) | |||
| Nasal mask | 0 (0) | 2 (1) | |||
| Full face mask | 18 (12) | 31 (18) | |||
| Type of ventilator | 0.457 | ||||
| NIV dedicated ventilator, n (%) | 15 (10) | 21 (12) | |||
| ICU ventilator, n (%) | 72 (49) | 72 (42) | |||
| Very poor perceived quality of sleep over the first 24 h after admission, n (%) | 113 (76) | 10 (6) | <0.001 | ||
| NIV Failure (n = 77) | NIV Success (n = 312) | p-Value | |
|---|---|---|---|
| Patient characteristics | |||
| Age, years | 66 (57–76) | 69 (59–78) | 0.108 |
| Males, n (%) | 55 (71) | 193 (62) | 0.145 |
| BMI, kg.m−2 | 26 (23–30) | 26 (22–33) | 0.857 |
| Chronic respiratory disease, n (%) | 39 (51) | 210 (67) | 0.008 |
| Chronic cardiac disease, n (%) | 14 (18) | 72 (23) | 0.363 |
| Home oxygen therapy, n (%) | 9 (12) | 71 (23) | 0.028 |
| NIV episode | |||
| SAPS 2 | 44 (34–57) | 33 (26–42) | <0.001 |
| SOFA | 6 (3–9) | 3 (2–4) | <0.001 |
| Cause of ARF | <0.001 | ||
| Acute-on-chronic, n (%) | 32 (42) | 202 (65) | |
| Acute cardiogenic pulmonary edema, n (%) | 38 (49) | 69 (22) | |
| De novo ARF, n (%) | 7 (9) | 41 (13) | |
| On ICU admission, prior to NIV | |||
| Respiratory rate, cycle·min−1 | 34 (28–40) | 31 (27–36) | 0.042 |
| Dyspnea Borg scale | 4 (3–5) | 3 (2–5) | 0.001 |
| Blood gases | |||
| PaO2/FiO2, mmHg | 176 (118–230) | 232 (180–295) | <0.001 |
| PaCO2, mmHg | 43 (32–54) | 60 (43–72) | <0.001 |
| pH | 7.36 (7.28–7.44) | 7.33 (7.27–7.40) | 0.121 |
| After the firt NIV session | |||
| Leaks, n (%) | 48 (62) | 189 (61) | 0.796 |
| anxiety, n (%) | 51 (74) | 165 (59) | 0.027 |
| Respiratory rate, cycle·min−1 | 30 (24–36) | 27 (23–32) | 0.023 |
| Dyspnea Borg scale | 4 (3–5) | 3 (2–5) | 0.014 |
| Blood gases | |||
| PaO2/FiO2, mmHg | 170 (143–214) | 222 (177–283) | 0.002 |
| PaCO2, mmHg | 48 (34–57) | 57 (45–69) | <0.001 |
| pH | 7.35 (7.26–7.41) | 7.35 (7.29–7.40) | 0.516 |
| NIV interfaces | 0.178 | ||
| Oro-nasal mask | 52 (68) | 193 (62) | |
| Nasal mask | 0 (0) | 4 (1) | |
| Full face mask | 8 (10) | 56 (18) | |
| Type of ventilator | 0.003 | ||
| NIV dedicated ventilator, n (%) | 3 (4) | 48 (15) | |
| ICU ventilator, n (%) | 42 (55) | 125 (40) | |
| First 24 h after admission | |||
| Poor perceived quality of sleep, n (%) | 46 (60) | 109 (35) | <0.001 |
| Total sleep time, h | 2 (0–4) | 4 (2–6) | <0.001 |
| Sleep Quality | Sleep Quantity | |||
|---|---|---|---|---|
| Odds Ratio (95% Confidence Interval) | p-Value | Odds Ratio (95% Confidence Interval) | p-Value | |
| SOFA | 1.26 (1.14–1.41) | 0.0001 | 1.33 (1.20–1.49) | 0.0001 |
| Cause of ARF, de novo ARF, n (%) | 3.21 (1.11–10.6) | 0.039 | ||
| PaCO2 on ICU admission, prior to NIV, per mmHg | 0.98 (0.96–0.99) | 0.006 | 0.98 (0.96–0.99) | 0.003 |
| Poor perceived quality of sleep | 3.02 (1.26–8.49) | 0.021 | ||
| Total sleep time, per hour | 0.77 (0.66–0.88) | 0.001 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lê Dinh, M.; Darmon, M.; Kouatchet, A.; Jaber, S.; Meziani, F.; Perbet, S.; Chanques, G.; Azoulay, E.; Demoule, A. Factors Associated with and Prognosis Impact of Perceived Sleep Quality and Estimated Quantity in Patients Receiving Non-Invasive Ventilation for Acute Respiratory Failure. J. Clin. Med. 2022, 11, 4620. https://doi.org/10.3390/jcm11154620
Lê Dinh M, Darmon M, Kouatchet A, Jaber S, Meziani F, Perbet S, Chanques G, Azoulay E, Demoule A. Factors Associated with and Prognosis Impact of Perceived Sleep Quality and Estimated Quantity in Patients Receiving Non-Invasive Ventilation for Acute Respiratory Failure. Journal of Clinical Medicine. 2022; 11(15):4620. https://doi.org/10.3390/jcm11154620
Chicago/Turabian StyleLê Dinh, Matthieu, Michael Darmon, Achille Kouatchet, Samir Jaber, Ferhat Meziani, Sebastien Perbet, Gerald Chanques, Elie Azoulay, and Alexandre Demoule. 2022. "Factors Associated with and Prognosis Impact of Perceived Sleep Quality and Estimated Quantity in Patients Receiving Non-Invasive Ventilation for Acute Respiratory Failure" Journal of Clinical Medicine 11, no. 15: 4620. https://doi.org/10.3390/jcm11154620
APA StyleLê Dinh, M., Darmon, M., Kouatchet, A., Jaber, S., Meziani, F., Perbet, S., Chanques, G., Azoulay, E., & Demoule, A. (2022). Factors Associated with and Prognosis Impact of Perceived Sleep Quality and Estimated Quantity in Patients Receiving Non-Invasive Ventilation for Acute Respiratory Failure. Journal of Clinical Medicine, 11(15), 4620. https://doi.org/10.3390/jcm11154620






