Sleep and Psychosocial Characteristics of Children with Narcolepsy According to Their Intellectual Profile: A Case–Control Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population
2.1.1. Control Children
2.1.2. Children with Narcolepsy
2.2. Procedure and Material
2.2.1. Psychometric Evaluation
2.2.2. Questionnaires
2.2.3. Polysomnography
2.3. Statistical Analysis
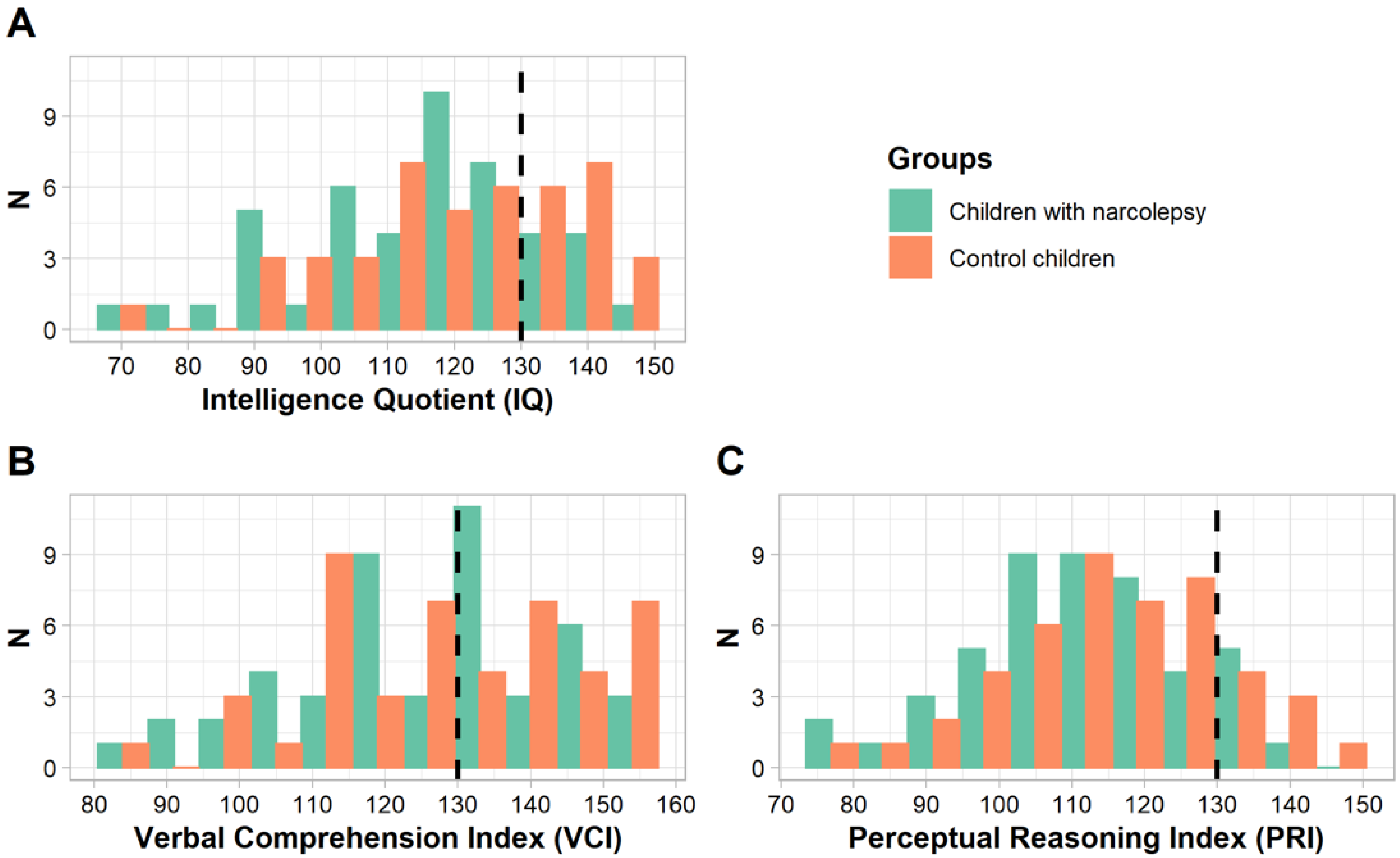
3. Results
3.1. Children with HIQ
3.2. Children with NIQ
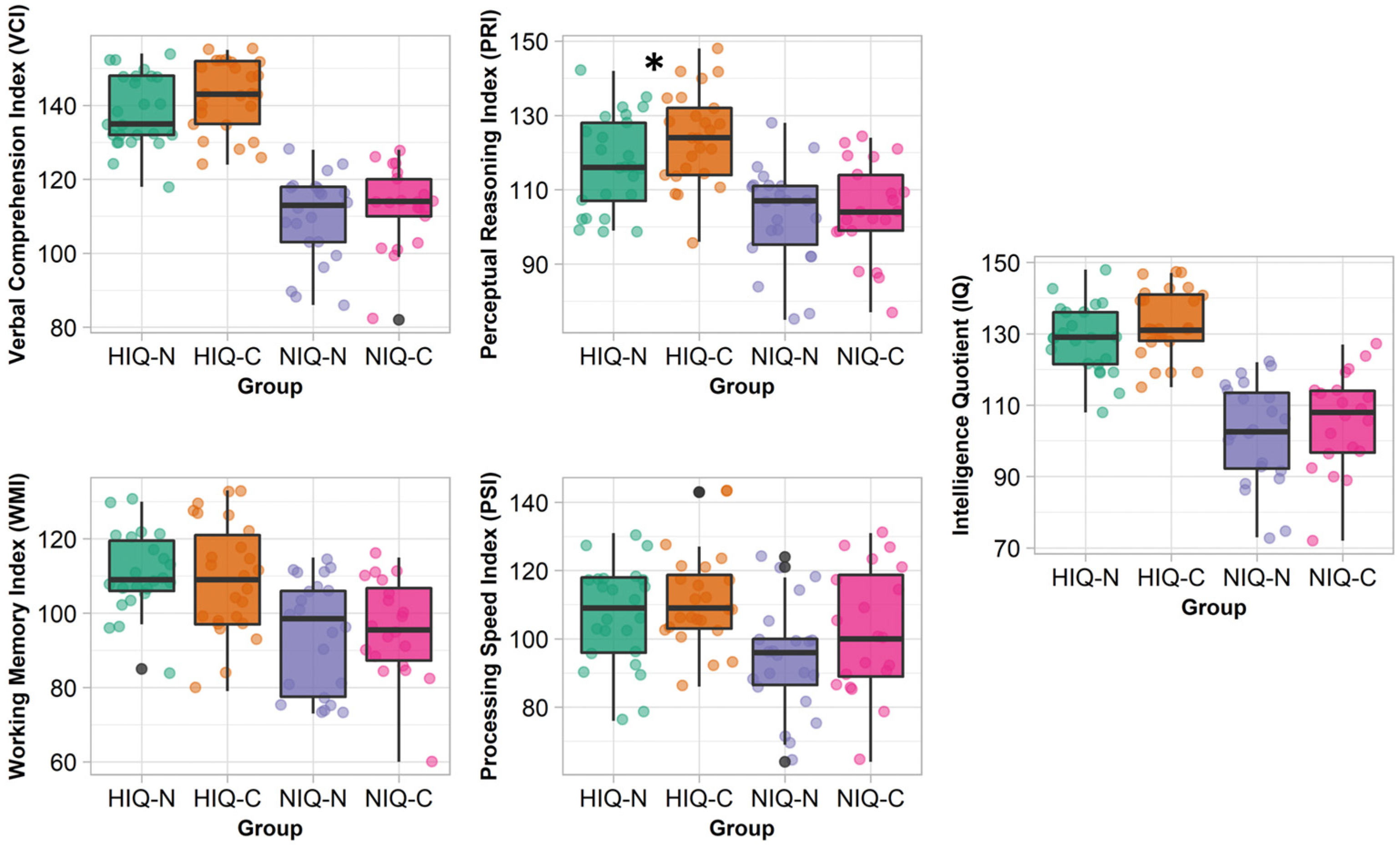
3.3. Children with HIQ Versus NIQ According to Their Pathological Status
3.3.1. Control Children
3.3.2. Children with Narcolepsy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bassetti, C.L.A.; Adamantidis, A.; Burdakov, D.; Han, F.; Gay, S.; Kallweit, U.; Khatami, R.; Koning, F.; Kornum, B.R.; Lammers, G.J.; et al. Narcolepsy—Clinical spectrum, aetiopathophysiology, diagnosis and treatment. Nat. Rev. Neurol. 2019, 15, 519–539. [Google Scholar] [CrossRef] [PubMed]
- Plazzi, G.; Clawges, H.M.; Owens, J.A. Clinical Characteristics and Burden of Illness in Pediatric Patients with Narcolepsy. Pediatr. Neurol. 2018, 85, 21–32. [Google Scholar] [CrossRef]
- Nevsimalova, S.; Buskova, J.; Kemlink, D.; Sonka, K.; Skibova, J. Does age at the onset of narcolepsy influence the course and severity of the disease? Sleep Med. 2009, 10, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Inocente, C.O.; Villanueva, C.; Lecendreux, M.; Dauvilliers, Y.; Lin, J.S.; Arnulf, I.; Gustin, M.P.; Thieux, M.; Franco, P. Narcolepsy with cataplexy: Does age at diagnosis change the clinical picture? CNS Neurosci. Ther. 2020, 26, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, J.E.; Alammar, H.A.; Weighall, A.R.; Kellar, I.; Nash, H.M. A systematic review of cognitive function and psychosocial well-being in school-age children with narcolepsy. Sleep Med. Rev. 2017, 34, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Morrish, E.; King, M.A.; Smith, I.E.; Shneerson, J.M. Factors associated with a delay in the diagnosis of narcolepsy. Sleep Med. 2004, 5, 37–41. [Google Scholar] [CrossRef]
- Inocente, C.O.; Gustin, M.P.; Lavault, S.; Guignard-Perret, A.; Raoux, A.; Christol, N.; Gerard, D.; Dauvilliers, Y.; Reimão, R.; Bat-Pitault, F.; et al. Quality of Life in Children with Narcolepsy. CNS Neurosci. Ther. 2014, 20, 763–771. [Google Scholar] [CrossRef]
- Stores, G.; Montgomery, P.; Wiggs, L. The psychosocial problems of children with narcolepsy and those with excessive daytime sleepiness of uncertain origin. Pediatrics 2006, 118, e1116-23. [Google Scholar] [CrossRef] [Green Version]
- Lecendreux, M.; Lavault, S.; Lopez, R.; Inocente, C.O.; Konofal, E.; Cortese, S.; Franco, P.; Arnulf, I.; Dauvilliers, Y. Attention-Deficit/Hyperactivity Disorder (ADHD) Symptoms in Pediatric Narcolepsy: A Cross-Sectional Study. Sleep 2015, 38, 1285–1295. [Google Scholar] [CrossRef]
- Ryland, H.K.; Lundervold, A.J.; Elgen, I.; Hysing, M. Is there a protective effect of normal to high intellectual function on mental health in children with chronic illness? Child Adolesc. Psychiatry Ment. Health 2010, 4, 2–9. [Google Scholar] [CrossRef] [Green Version]
- Posar, A.; Pizza, F.; Parmeggiani, A.; Plazzi, G. Neuropsychological findings in childhood narcolepsy. J. Child Neurol. 2014, 29, 1370–1376. [Google Scholar] [CrossRef] [PubMed]
- Dorris, L.; Zuberi, S.M.; Scott, N.; Moffat, C.; McArthur, I. Psychosocial and intellectual functioning in childhood narcolepsy. Dev. Neurorehabil. 2008, 11, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Janssens, K.A.M.; Quaedackers, L.; Lammers, G.J.; Amesz, P.; Van Mierlo, P.; Aarts, L.; Peeters, E.; Hendriks, D.; Vandenbussche, N.; Overeem, S.; et al. Effect of treatment on cognitive and attention problems in children with narcolepsy type 1. Sleep 2020, 43, zsaa114. [Google Scholar] [CrossRef]
- Szakács, A.; Hallböök, T.; Tideman, P.; Darin, N.; Wentz, E. Psychiatric Comorbidity and Cognitive Profile in Children with Narcolepsy with or without Association to the H1N1 Influenza Vaccination. Sleep 2015, 38, 615–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, S.M.; Joo, E.Y.; Kim, J.Y.; Hwang, K.J.; Hong, S.B. Is high IQ protective against cognitive dysfunction in narcoleptic patients? J. Clin. Neurol. 2013, 9, 118–124. [Google Scholar] [CrossRef] [Green Version]
- Thieux, M.; Zhang, M.; Marcastel, A.; Herbillon, V.; Guignard-Perret, A.; Seugnet, L.; Lin, J.-S.; Guyon, A.; Plancoulaine, S.; Franco, P. Intellectual Abilities of Children with Narcolepsy. J. Clin. Med. 2020, 9, 4075. [Google Scholar] [CrossRef]
- Guignard-Perret, A.; Thieux, M.; Guyon, A.; Mazza, S.; Zhang, M.; Revol, O.; Plancoulaine, S.; Franco, P. Sleep of Children with High Potentialities: A Polysomnographic Study. J. Clin. Med. 2020, 9, 3182. [Google Scholar] [CrossRef]
- American Academy of Sleep Medicine. The International Classification of Sleep Disorders [ICSD] Diagnostic and Coding Manual, 3rd ed.; American Academy of Sleep Medicine: Westchester, IL, USA, 2014. [Google Scholar]
- Iber, C.; Iber, C. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications; American Academy of Sleep Medicine: Westchester, IL, USA, 2007; Volume 1. [Google Scholar]
- Pizza, F.; Barateau, L.; Jaussent, I.; Vandi, S.; Antelmi, E.; Mignot, E.; Dauvilliers, Y.; Plazzi, G. Validation of Multiple Sleep Latency Test for the diagnosis of pediatric narcolepsy type 1. Neurology 2019, 93, e1034–e1044. [Google Scholar] [CrossRef]
- Mignot, E.; Lammers, G.J.; Ripley, B.; Okun, M.; Nevsimalova, S.; Overeem, S.; Vankova, J.; Black, J.; Harsh, J.; Bassetti, C.; et al. The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch. Neurol. 2002, 59, 1553–1562. [Google Scholar] [CrossRef]
- Cole, T.J.; Bellizzi, M.C.; Flegal, K.M.; Dietz, W.H. Establishing a standard definition for child overweight and obesity worldwide: International Survey. BMJ 2000, 320, 1240. [Google Scholar] [CrossRef] [Green Version]
- Wechsler, D. Wechsler Intelligence Scale for Children–Fourth Edition (WISC-IV); Pearson: San Antonio, TX, USA, 2003. [Google Scholar]
- Nusbaum, F.; Hannoun, S.; Kocevar, G.; Stamile, C.; Fourneret, P.; Revol, O.; Sappey-Marinier, D. Hemispheric differences in white matter microstructure between two profiles of children with high intelligence quotient vs. controls: A tract-based spatial statistics study. Front. Neurosci. 2017, 11, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuche, C.; Brasseur, S. Le Haut Potentiel en Questions: Psychologie Grand Public; Mardaga: Brussels, Belgium, 2017; ISBN 2804704157. [Google Scholar]
- Snow, A.; Gozal, E.; Malhotra, A.; Tiosano, D.; Perlman, R.; Vega, C.; Shahar, E.; Gozal, D.; Hochberg, Z.; Pillar, G. Severe hypersomnolence after pituitary/hypothalamic surgery in adolescents: Clinical characteristics and potential mechanisms. Pediatrics 2002, 110, e74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastien, C.H.; Vallières, A.; Morin, C.M. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. 2001, 2, 297–307. [Google Scholar] [CrossRef]
- Kovacs, M. The children’s depression inventory (CDI). Psychopharmacol. Bull. 1985, 21, 995–998. [Google Scholar]
- Goyette, C.H.; Conners, C.K.; Ulrich, R.F. Normative data on Revised Conners Parent and Teacher Rating Scales. J. Abnorm. Child Psychol. 1978, 6, 221–236. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Naumann, A.; Bellebaum, C.; Daum, I. Cognitive deficits in narcolepsy. J. Sleep Res. 2006, 15, 329–338. [Google Scholar] [CrossRef]
- Witt, S.T.; Drissi, N.M.; Tapper, S.; Wretman, A.; Szakács, A.; Hallböök, T.; Landtblom, A.M.; Karlsson, T.; Lundberg, P.; Engström, M. Evidence for cognitive resource imbalance in adolescents with narcolepsy. Brain Imaging Behav. 2018, 12, 411–424. [Google Scholar] [CrossRef] [Green Version]
- Ha, K.S.; Yoo, H.K.; Lyoo, I.K.; Jeong, D.U. Computerized assessment of cognitive impairment in narcoleptic patients. Acta Neurol. Scand. 2007, 116, 312–316. [Google Scholar] [CrossRef]
- Naumann, A.; Daum, I. Narcolepsy: Pathophysiology and neuropsychological changes. Behav. Neurol. 2003, 14, 89–98. [Google Scholar] [CrossRef] [Green Version]
- Fulda, S.; Schulz, H. Cognitive dysfunction in sleep disorders. Sleep Med. Rev. 2001, 5, 423–445. [Google Scholar] [CrossRef] [Green Version]
- Ramm, M.; Boentert, M.; Lojewsky, N.; Jafarpour, A.; Young, P.; Heidbreder, A. Disease-specific attention impairment in disorders of chronic excessive daytime sleepiness. Sleep Med. 2019, 53, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Wise, M.S.; Lynch, J. Narcolepsy in children. Semin. Pediatr. Neurol. 2001, 8, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Northam, E.A. Psychosocial impact of chronic illness in children. J. Paediatr. Child Health 1997, 33, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Bastien, L.; Théoret, R.; Gagnon, K.; Chicoine, M.; Godbout, R. Sleep Characteristics and Socio-Emotional Functioning of Gifted Children. Behav. Sleep Med. 2021. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thieux, M.; Zhang, M.; Marcastel, A.; Poitrinal, A.; Vassias, F.; Guyon, A.; Revol, O.; Mazza, S.; Guignard-Perret, A.; Franco, P. Sleep and Psychosocial Characteristics of Children with Narcolepsy According to Their Intellectual Profile: A Case–Control Study. J. Clin. Med. 2022, 11, 4681. https://doi.org/10.3390/jcm11164681
Thieux M, Zhang M, Marcastel A, Poitrinal A, Vassias F, Guyon A, Revol O, Mazza S, Guignard-Perret A, Franco P. Sleep and Psychosocial Characteristics of Children with Narcolepsy According to Their Intellectual Profile: A Case–Control Study. Journal of Clinical Medicine. 2022; 11(16):4681. https://doi.org/10.3390/jcm11164681
Chicago/Turabian StyleThieux, Marine, Min Zhang, Agathe Marcastel, Alice Poitrinal, Fanny Vassias, Aurore Guyon, Olivier Revol, Stephanie Mazza, Anne Guignard-Perret, and Patricia Franco. 2022. "Sleep and Psychosocial Characteristics of Children with Narcolepsy According to Their Intellectual Profile: A Case–Control Study" Journal of Clinical Medicine 11, no. 16: 4681. https://doi.org/10.3390/jcm11164681
APA StyleThieux, M., Zhang, M., Marcastel, A., Poitrinal, A., Vassias, F., Guyon, A., Revol, O., Mazza, S., Guignard-Perret, A., & Franco, P. (2022). Sleep and Psychosocial Characteristics of Children with Narcolepsy According to Their Intellectual Profile: A Case–Control Study. Journal of Clinical Medicine, 11(16), 4681. https://doi.org/10.3390/jcm11164681





