A Study on the Pathogenesis of Vascular Cognitive Impairment and Dementia: The Chronic Cerebral Hypoperfusion Hypothesis
Abstract
:1. Introduction
2. CBF Regulation and VCID
3. Experimental Animal Models of CCH
4. The Multiple Mechanisms of CCH-Induced VCID
4.1. CCH-Induced Aβ Accumulation in VCID
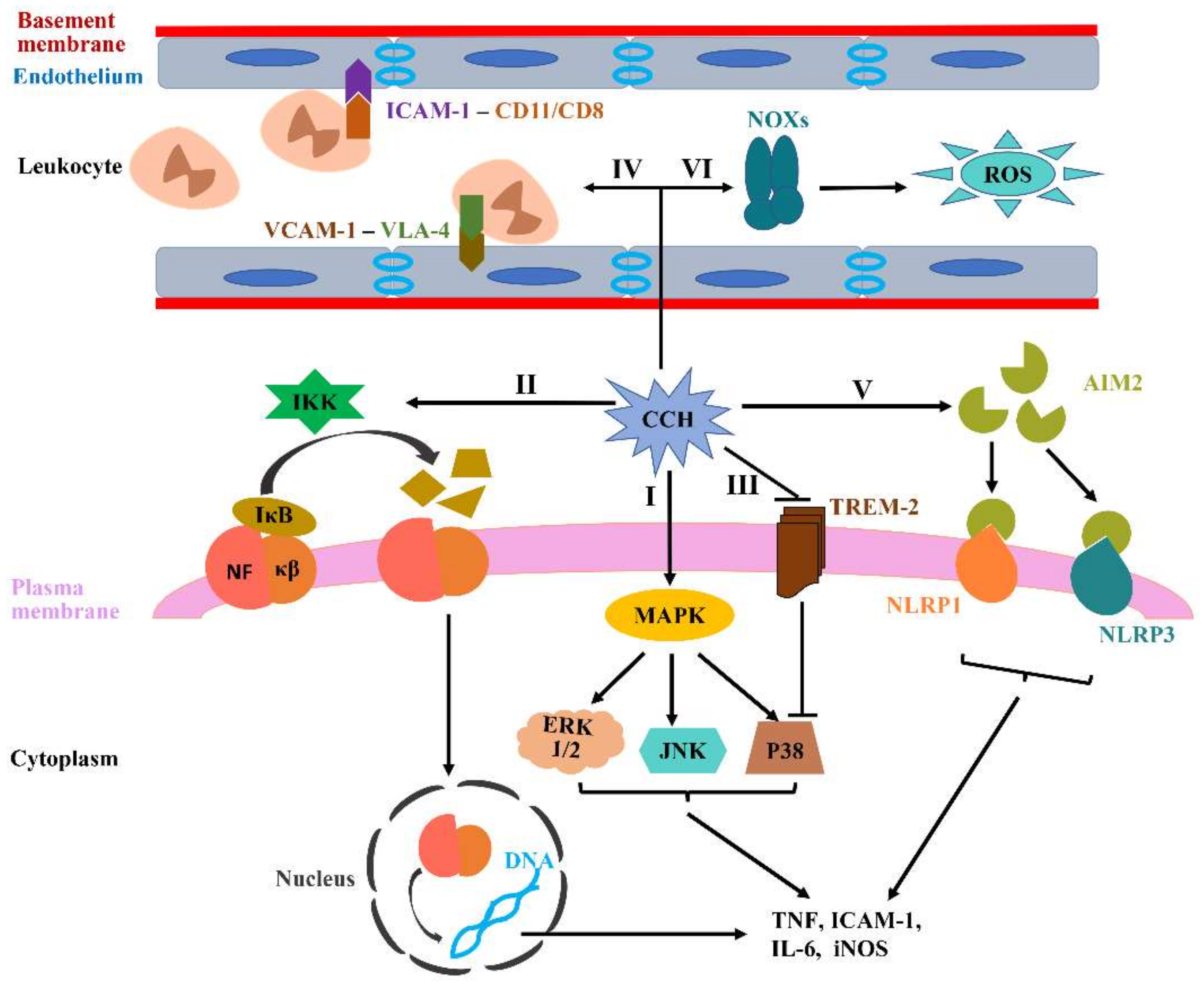
4.2. CCH-Induced Neuroinflammation in VCID
4.3. CCH-Induced Oxidative Stress in VCID
4.4. CCH-Induced Trophic Uncoupling in the NVU
4.5. CCH-Induced BBB Breakdown in VCID
4.6. CCH-Induced Demyelination and Failure of Remyelination in VCID
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jellinger, K.A. Pathology and pathogenesis of vascular cognitive impairment—A critical update. Front. Aging Neurosci. 2013, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C. The Pathobiology of Vascular Dementia. Neuron 2013, 80, 844–866. [Google Scholar] [CrossRef] [PubMed]
- Frantellizzi, V.; Pani, A.; Ricci, M.; Locuratolo, N.; Fattapposta, F.; De Vincentis, G. Neuroimaging in Vascular Cognitive Impairment and Dementia: A Systematic Review. J. Alzheimers Dis. 2020, 73, 1279–1294. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Sun, Y.; Lu, Z.; Leak, R.; Zhang, F. The impact of cerebrovascular aging on vascular cognitive impairment and dementia. Ageing Res. Rev. 2016, 34, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Mijajlović, M.D.; Pavlović, A.; Brainin, M.; Heiss, W.D.; Quinn, T.J.; Ihle-Hansen, H.B.; Hermann, D.M.; Assayag, E.B.; Richard, E.; Thiel, A.; et al. Post-stroke dementia—A comprehensive review. BMC Med. 2017, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Kalaria, R.N. Neuropathological diagnosis of vascular cognitive impairment and vascular dementia with implications for Alzheimer’s disease. Acta Neuropathol. 2016, 131, 659–685. [Google Scholar] [CrossRef] [PubMed]
- Attems, J.; Jellinger, K.A. The overlap between vascular disease and Alzheimer’s disease—Lessons from pathology. BMC Med. 2014, 12, 206. [Google Scholar] [CrossRef]
- Fitzpatrick, A.L.; Kuller, L.H.; Lopez, O.L.; Kawas, C.H.; Jagust, W. Survival following dementia onset: Alzheimer’s disease and vascular dementia. J. Neurol. Sci. 2005, 229, 43–49. [Google Scholar] [CrossRef]
- Gorelick, P.B.; Scuteri, A.; Black, S.E.; DeCarli, C.; Greenberg, S.M.; Iadecola, C.; Launer, L.J.; Laurent, S.; Lopez, O.L.; Seshadri, S. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the american heart association/american stroke association. Stroke 2011, 42, 2672–2713. [Google Scholar] [CrossRef]
- Asif, M.; Louis Soiza, R.; McEvoy, M.; Mangoni, A.A. Asymmetric dimethylarginine: A possible link between vascular disease and dementia. Curr. Alzheimer Res. 2013, 10, 347–356. [Google Scholar] [CrossRef]
- Toth, P.; Tarantini, S.; Csiszar, A.; Ungvari, Z. Functional vascular contributions to cognitive impairment and dementia: Mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am. J. Physiol. Circ. Physiol. 2017, 312, H1–H20. [Google Scholar] [CrossRef]
- Gold, G.; Giannakopoulos, P.; Herrmann, F.R.; Bouras, C.; Kövari, E. Identification of Alzheimer and vascular lesion thresholds for mixed dementia. Brain 2007, 130, 2830–2836. [Google Scholar] [CrossRef]
- Hainsworth, A.H.; Oommen, A.T.; Bridges, L.R. Endothelial Cells and Human Cerebral Small Vessel Disease. Brain Pathol. 2014, 25, 44–50. [Google Scholar] [CrossRef]
- Williams, L.R.; Leggett, R.W. Reference values for resting blood flow to organs of man. Clin. Phys. Physiol. Meas. 1989, 10, 187–217. [Google Scholar] [CrossRef]
- Csiszar, A.; Tucsek, Z.; Toth, P.; Sosnowska, D.; Gautam, T.; Koller, A.; Deak, F.; Sonntag, W.E.; Ungvari, Z. Synergistic effects of hypertension and aging on cognitive function and hippocampal expression of genes involved in β-amyloid generation and Alzheimer’s disease. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1120–H1130. [Google Scholar] [CrossRef]
- Iadecola, C. The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 2017, 96, 17–42. [Google Scholar] [CrossRef]
- Buxton, R.B.; Uludağ, K.; Dubowitz, D.J.; Liu, T.T. Modeling the hemodynamic response to brain activation. NeuroImage 2004, 23, S220–S233. [Google Scholar] [CrossRef]
- Lassen, N.A. Autoregulation of cerebral blood flow. Circ. Res. 1964, 15, 201–204. [Google Scholar]
- Ainslie, P.N.; Ashmead, J.C.; Ide, K.; Morgan, B.J.; Poulin, M.J. Differential responses to CO2 and sympathetic stimulation in the cerebral and femoral circulations in humans. J. Physiology 2005, 566, 613–624. [Google Scholar] [CrossRef]
- Hall, C.N.; Reynell, C.; Gesslein, B.; Hamilton, N.B.; Mishra, A.; Sutherland, B.A.; O’Farrell, F.M.; Buchan, A.M.; Lauritzen, M.; Attwell, D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014, 508, 55–60. [Google Scholar] [CrossRef]
- Zhao, Y.; Gong, C.-X. From Chronic Cerebral Hypoperfusion to Alzheimer-Like Brain Pathology and Neurodegeneration. Cell. Mol. Neurobiol. 2014, 35, 101–110. [Google Scholar] [CrossRef]
- Sarti, C.; Pantoni, L.; Bartolini, L.; Inzitari, D. Cognitive impairment and chronic cerebral hypoperfusion: What can be learned from experimental models. J. Neurol. Sci. 2002, 203, 263–266. [Google Scholar] [CrossRef]
- De Silva, T.M.; Faraci, F.M. Microvascular Dysfunction and Cognitive Impairment. Cell. Mol. Neurobiol. 2016, 36, 241–258. [Google Scholar] [CrossRef]
- Cao, W.; Lin, J.; Xiang, W.; Liu, J.; Wang, B.; Liao, W.; Jiang, T. Physical Exercise-Induced Astrocytic Neuroprotection and Cognitive Improvement Through Primary Cilia and Mitogen-Activated Protein Kinases Pathway in Rats With Chronic Cerebral Hypoperfusion. Front. Aging Neurosci. 2022, 14, 866366. [Google Scholar] [CrossRef]
- Duncombe, J.; Kitamura, A.; Hase, Y.; Ihara, M.; Kalaria, R.N.; Horsburgh, K. Chronic cerebral hypoperfusion: A key mechanism leading to vascular cognitive impairment and dementia. Closing the translational gap between rodent models and human vascular cognitive impairment and dementia. Clin. Sci. 2017, 131, 2451–2468. [Google Scholar] [CrossRef]
- Jiwa, N.S.; Garrard, P.; Hainsworth, A.H. Experimental models of vascular dementia and vascular cognitive impairment: A systematic review. J. Neurochem. 2010, 115, 814–828. [Google Scholar] [CrossRef]
- Edrissi, H.; Schock, S.C.; Cadonic, R.; Hakim, A.M.; Thompson, C.S. Cilostazol reduces blood brain barrier dysfunction, white matter lesion formation and motor deficits following chronic cerebral hypoperfusion. Brain Res. 2016, 1646, 494–503. [Google Scholar] [CrossRef]
- Gooch, J.; Wilcock, D.M. Animal Models of Vascular Cognitive Impairment and Dementia (VCID). Cell. Mol. Neurobiol. 2016, 36, 233–239. [Google Scholar] [CrossRef]
- Gorelick, P.B.; Counts, S.E.; Nyenhuis, D. Vascular cognitive impairment and dementia. Biochim. Biophys. Acta 2016, 1862, 860–868. [Google Scholar] [CrossRef]
- Huang, Y.; Fan, S.; Li, J.; Wang, Y.-L. Bilateral Common Carotid Artery Occlusion in the Rat as a Model of Retinal Ischaemia. Neuro-Ophthalmology 2014, 38, 180–188. [Google Scholar] [CrossRef]
- Pulsinelli, W.A.; Brierley, J.B. A new model of bilateral hemispheric ischemia in the unanesthetized rat. Stroke 1979, 10, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Coltman, R.; Spain, A.; Tsenkina, Y.; Fowler, J.H.; Smith, J.; Scullion, G.; Allerhand, M.; Scott, F.; Kalaria, R.N.; Ihara, M.; et al. Selective white matter pathology induces a specific impairment in spatial working memory. Neurobiol. Aging 2011, 32, 2324.e7–2324.e12. [Google Scholar] [CrossRef] [PubMed]
- Shibata, M.; Ohtani, R.; Ihara, M.; Tomimoto, H. White Matter Lesions and Glial Activation in a Novel Mouse Model of Chronic Cerebral Hypoperfusion. Stroke 2004, 35, 2598–2603. [Google Scholar] [CrossRef]
- Nishio, K.; Ihara, M.; Yamasaki, N.; Kalaria, R.N.; Maki, T.; Fujita, Y.; Ito, H.; Oishi, N.; Fukuyama, H.; Miyakawa, T.; et al. A Mouse Model Characterizing Features of Vascular Dementia With Hippocampal Atrophy. Stroke 2010, 41, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- Fleischman, D.A.; Yang, J.; Arfanakis, K.; Arvanitakis, Z.; Leurgans, S.E.; Turner, A.D.; Barnes, L.L.; Bennett, D.A.; Buchman, A.S. Physical activity, motor function, and white matter hyperintensity burden in healthy older adults. Neurology 2015, 84, 1294–1300. [Google Scholar] [CrossRef]
- Hattori, Y.; Enmi, J.I.; Kitamura, A.; Yamamoto, Y.; Saito, S.; Takahashi, Y.; Iguchi, S.; Tsuji, M.; Yamahara, K.; Nagatsuka, K.; et al. A novel mouse model of subcortical infarcts with dementia. J. Neurosci. Off. J. Soc. Neurosci. 2015, 35, 3915–3928. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Enmi, J.; Iguchi, S.; Saito, S.; Yamamoto, Y.; Tsuji, M.; Nagatsuka, K.; Kalaria, R.N.; Iida, H.; Ihara, M. Gradual Carotid Artery Stenosis in Mice Closely Replicates Hypoperfusive Vascular Dementia in Humans. J. Am. Heart Assoc. 2016, 5, e002757. [Google Scholar] [CrossRef]
- Fullerton, S.M.; Shirman, G.A.; Strittmatter, W.J.; Matthew, W.D. Impairment of the Blood-Nerve and blood-brain barriers in Apolipoprotein E Knockout Mice. Exp. Neurol. 2001, 169, 13–22. [Google Scholar] [CrossRef]
- Grootendorst, J.; Oitzl, M.S.; Dalm, S.; Enthoven, L.; Schachner, M.; de Kloet, R.; Sandi, C. Stress alleviates reduced expression of cell adhesion molecules (NCAM, L1), and deficits in learning and corticosterone regulation of apolipoprotein E knockout mice. Eur. J. Neurosci. 2001, 14, 1505–1514. [Google Scholar] [CrossRef]
- Herzig, M.C.; Winkler, D.T.; Burgermeister, P.; Pfeifer, M.; Kohler, E.; Schmidt, S.D.; Danner, S.; Abramowski, D.; Stürchler-Pierrat, C.; Bürki, K.; et al. Abeta is targeted to the vasculature in a mouse model of hereditary cerebral hemorrhage with amyloidosis. Nat. Neurosci. 2004, 7, 954–960. [Google Scholar] [CrossRef]
- Fan, R.; Xu, F.; Previti, M.L.; Davis, J.; Grande, A.M.; Robinson, J.K.; Van Nostrand, W.E. Minocycline Reduces Microglial Activation and Improves Behavioral Deficits in a Transgenic Model of Cerebral Microvascular Amyloid. J. Neurosci. 2007, 27, 3057–3063. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Jin, H.; Huang, Y. Mitochondria-associated membranes (MAMs): A potential therapeutic target for treating Alzheimer’s disease. Clin. Sci. 2021, 135, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Boix, C.P.; Lopez-Font, I.; Cuchillo-Ibañez, I.; Sáez-Valero, J. Amyloid precursor protein glycosylation is altered in the brain of patients with Alzheimer’s disease. Alzheimer’s Res. Ther. 2020, 12, 96. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, L.; Annaert, W. Membrane trafficking pathways in Alzheimer’s disease. Traffic 2012, 13, 759–770. [Google Scholar] [CrossRef]
- Leissring, M.A.; Murphy, M.P.; Mead, T.R.; Akbari, Y.; Sugarman, M.C.; Jannatipour, M.; Anliker, B.; Müller, U.; Saftig, P.; Strooper, B.D.; et al. A physiologic signaling role for the gamma-secretase-derived intracellular fragment of APP. Proc. Natl. Acad. Sci. USA 2002, 99, 4697–4702. [Google Scholar] [CrossRef]
- Jarosz-Griffiths, H.H.; Noble, E.; Rushworth, J.V.; Hooper, N.M. Amyloid-β Receptors: The Good, the Bad, and the Prion Protein. J. Biol. Chem. 2016, 291, 3174–3183. [Google Scholar] [CrossRef]
- Welander, H.; Frånberg, J.; Graff, C.; Sundström, E.; Winblad, B.; Tjernberg, L.O. Abeta43 is more frequent than Abeta40 in amyloid plaque cores from Alzheimer disease brains. J. Neurochem. 2009, 110, 697–706. [Google Scholar] [CrossRef]
- Wang, G.L.; Jiang, B.-H.; Rue, E.A.; Semenza, G.L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 1995, 92, 5510–5514. [Google Scholar] [CrossRef]
- Semenza, G.L. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu. Rev. Pathol. 2014, 9, 47–71. [Google Scholar] [CrossRef]
- Sharp, F.R.; Bernaudin, M. HIF1 and oxygen sensing in the brain. Nat. Rev. Neurosci. 2004, 5, 437–448. [Google Scholar] [CrossRef]
- Bulbarelli, A.; Lonati, E.; Brambilla, A.; Orlando, A.; Cazzaniga, E.; Piazza, F.; Ferrarese, C.; Masserini, M.; Sancini, G. Aβ42 production in brain capillary endothelial cells after oxygen and glucose deprivation. Mol. Cell. Neurosci. 2012, 49, 415–422. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, K.; Wang, R.; Cui, J.; Lipton, S.A.; Liao, F.F.; Xu, H.; Zhang, Y.W. Hypoxia-inducible factor 1alpha (HIF-1alpha)-mediated hypoxia increases BACE1 expression and beta-amyloid generation. J. Biol. Chem. 2007, 282, 10873–10880. [Google Scholar] [CrossRef]
- Garcia-Alloza, M.; Gregory, J.; Kuchibhotla, K.V.; Fine, S.; Wei, Y.; Ayata, C.; Frosch, M.P.; Greenberg, S.M.; Bacskai, B.J. Cerebrovascular lesions induce transient β-amyloid deposition. Brain A J. Neurol. 2011, 134, 3697–3707. [Google Scholar] [CrossRef]
- Tesco, G.; Koh, Y.H.; Kang, E.L.; Cameron, A.N.; Das, S.; Sena-Esteves, M.; Hiltunen, M.; Yang, S.H.; Zhong, Z.; Shen, Y.; et al. Depletion of GGA3 stabilizes BACE and enhances beta-secretase activity. Neuron 2007, 54, 721–737. [Google Scholar] [CrossRef]
- Hiltunen, M.; Mäkinen, P.; Peräniemi, S.; Sivenius, J.; van Groen, T.; Soininen, H.; Jolkkonen, J. Focal cerebral ischemia in rats alters APP processing and expression of Abeta peptide degrading enzymes in the thalamus. Neurobiol. Dis. 2009, 35, 103–113. [Google Scholar] [CrossRef]
- Park, L.; Zhou, P.; Pitstick, R.; Capone, C.; Anrather, J.; Norris, E.H.; Younkin, L.; Younkin, S.; Carlson, G.; McEwen, B.S.; et al. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc. Natl. Acad. Sci. USA 2008, 105, 1347–1352. [Google Scholar] [CrossRef]
- Manso, Y.; Holland, P.R.; Kitamura, A.; Szymkowiak, S.; Duncombe, J.; Hennessy, E.; Searcy, J.L.; Marangoni, M.; Randall, A.D.; Brown, J.T.; et al. Minocycline reduces microgliosis and improves subcortical white matter function in a model of cerebral vascular disease. Glia 2017, 66, 34–46. [Google Scholar] [CrossRef]
- Bhuvanendran, S.; Bakar, S.N.S.; Kumari, Y.; Othman, I.; Shaikh, M.F.; Hassan, Z. Embelin Improves the Spatial Memory and Hippocampal Long-Term Potentiation in a Rat Model of Chronic Cerebral Hypoperfusion. Sci. Rep. 2019, 9, 14507. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, W.; Lin, L.; Feng, J.; Chen, F.; Wei, W.; Zhao, X.; Guo, W.; Li, J.; Yin, W.; et al. Is endothelial dysfunction of cerebral small vessel responsible for white matter lesions after chronic cerebral hypoperfusion in rats? J. Neurol. Sci. 2010, 299, 72–80. [Google Scholar] [CrossRef]
- Ding, Y.; Li, J.; Rafols, J.A.; Phillis, J.W.; Diaz, F.G. Prereperfusion Saline Infusion Into Ischemic Territory Reduces Inflammatory Injury After Transient Middle Cerebral Artery Occlusion in Rats. Stroke 2002, 33, 2492–2498. [Google Scholar] [CrossRef]
- Matsuyama, H.; Shindo, A.; Shimada, T.; Yata, K.; Wakita, H.; Takahashi, R.; Tomimoto, H. Chronic cerebral hypoperfusion activates AIM2 and NLRP3 inflammasome. Brain Res. 2020, 1736, 146779. [Google Scholar] [CrossRef]
- Poh, L.; Razak, S.M.B.A.; Lim, H.M.; Lai, M.K.; Chen, C.L.-H.; Lim, L.H.; Arumugam, T.V.; Fann, D.Y. AIM2 inflammasome mediates apoptotic and pyroptotic death in the cerebellum following chronic hypoperfusion. Exp. Neurol. 2021, 346, 113856. [Google Scholar] [CrossRef]
- Fan, H.; Wu, P.F.; Zhang, L.; Hu, Z.L.; Wang, W.; Guan, X.L.; Luo, H.; Ni, M.; Yang, J.; Wang, F.; et al. Methionine sulfoxide reductase A negatively controls microglia-mediated neuroinflammation via inhibiting ROS/MAPKs/NF-κB signaling pathways through a catalytic antioxidant function. Antioxid. Redox. Signal. 2015, 22, 832–847. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, Y.; Luo, Y.; Du, Y.; Zhang, X.; Fu, J. Curcumin inhibits LPS-induced neuroinflammation by promoting microglial M2 polarization via TREM2/TLR4/NF-κB pathways in BV2 cells. Mol. Immunol. 2019, 116, 29–37. [Google Scholar] [CrossRef]
- Poh, L.; Fann, D.Y.; Wong, P.; Lim, H.M.; Foo, S.L.; Kang, S.-W.; Rajeev, V.; Selvaraji, S.; Iyer, V.R.; Parathy, N.; et al. AIM2 inflammasome mediates hallmark neuropathological alterations and cognitive impairment in a mouse model of vascular dementia. Mol. Psychiatry 2020, 26, 4544–4560. [Google Scholar] [CrossRef]
- Frosch, O.H.; Yau, P.L.; Osorio, R.S.; Rusinek, H.; Storey, P.; Convit, A. Insulin resistance among obese middle-aged is associated with decreased cerebrovascular reactivity. Neurology 2017, 89, 249–255. [Google Scholar] [CrossRef]
- Hoffmann, A.; Baltimore, D. Circuitry of nuclear factor kappaB signaling. Immunol. Rev. 2006, 210, 171–186. [Google Scholar] [CrossRef]
- Husain, J.; Juurlink, B.H. Oligodendroglial precursor cell susceptibility to hypoxia is related to poor ability to cope with reactive oxygen species. Brain Res. 1995, 698, 86–94. [Google Scholar] [CrossRef]
- Huang, S.H.; Liu, G.W.; Li, J.H.; Xu, J.H.; Xu, D.W.; Zhang, W.Q.; Huang, J.R. Expression of TREM-2 and its inhibitory effects on TNF-α induced inflammation in fibroblast-like synoviocytes via inhibiting p38 pathway activation. Clin. Exp. Rheumatol. 2017, 36, 185–194. [Google Scholar]
- Wolf, G.; Lotan, A.; Lifschytz, T.; Ben-Ari, H.; Merzel, T.K.; Tatarskyy, P.; Valitzky, M.; Mernick, B.; Avidan, E.; Koroukhov, N.; et al. Differentially Severe Cognitive Effects of Compromised Cerebral Blood Flow in Aged Mice: Association with Myelin Degradation and Microglia Activation. Front. Aging Neurosci. 2017, 9, 191. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, J. Cerebral Hypoperfusion and Cognitive Impairment: The Pathogenic Role of Vascular Oxidative Stress. Int. J. Neurosci. 2012, 122, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Mracskó, E.; Hugyecz, M.; Institóris, A.; Farkas, E.; Bari, F. Changes in pro-oxidant and antioxidant enzyme levels during cerebral hypoperfusion in rats. Brain Res. 2010, 1321, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.-H.; Lee, K.-H.; Kim, J.-H.; Seo, J.-H.; Kim, H.Y.; Shin, C.Y.; Han, J.-S.; Han, S.-H.; Kim, Y.-S.; Lee, J. NADPH Oxidase 1, a Novel Molecular Source of ROS in Hippocampal Neuronal Death in Vascular Dementia. Antioxidants Redox Signal. 2014, 21, 533–550. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.-X.; Wang, X.-R.; Yan, C.-Q.; He, T.; Yang, J.-W.; Zeng, X.-H.; Xu, Q.; Zhu, W.; Du, S.-Q.; Liu, C.-Z. RETRACTED ARTICLE: Acupuncture elicits neuroprotective effect by inhibiting NAPDH oxidase-mediated reactive oxygen species production in cerebral ischaemia. Sci. Rep. 2015, 5, 17981. [Google Scholar] [CrossRef]
- Wang, X.-R.; Shi, G.-X.; Yang, J.-W.; Yan, C.-Q.; Lin, L.-T.; Du, S.-Q.; Zhu, W.; He, T.; Zeng, X.-H.; Xu, Q.; et al. Acupuncture ameliorates cognitive impairment and hippocampus neuronal loss in experimental vascular dementia through Nrf2-mediated antioxidant response. Free Radic. Biol. Med. 2015, 89, 1077–1084. [Google Scholar] [CrossRef]
- Freeman, L.R.; Keller, J.N. Oxidative stress and cerebral endothelial cells: Regulation of the blood–brain-barrier and antioxidant based interventions. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2012, 1822, 822–829. [Google Scholar] [CrossRef]
- Snapyan, M.; Lemasson, M.; Brill, M.S.; Blais, M.; Massouh, M.; Ninkovic, J.; Gravel, C.; Berthod, F.; Götz, M.; Barker, P.A.; et al. Vasculature Guides Migrating Neuronal Precursors in the Adult Mammalian Forebrain via Brain-Derived Neurotrophic Factor Signaling. J. Neurosci. 2009, 29, 4172–4188. [Google Scholar] [CrossRef]
- Arai, K.; Lo, E.H. An Oligovascular Niche: Cerebral Endothelial Cells Promote the Survival and Proliferation of Oligodendrocyte Precursor Cells. J. Neurosci. 2009, 29, 4351–4355. [Google Scholar] [CrossRef]
- Guo, S.; Kim, W.J.; Lok, J.; Lee, S.-R.; Besancon, E.; Luo, B.-H.; Stins, M.F.; Wang, X.; Dedhar, S.; Lo, E.H. Neuroprotection via matrix-trophic coupling between cerebral endothelial cells and neurons. Proc. Natl. Acad. Sci. USA 2008, 105, 7582–7587. [Google Scholar] [CrossRef]
- Wolburg, H.; Noell, S.; Mack, A.; Wolburg-Buchholz, K.; Fallier-Becker, P. Brain endothelial cells and the glio-vascular complex. Cell Tissue Res. 2008, 335, 75–96. [Google Scholar] [CrossRef]
- Park, Y.-K.; Oh, T.W.; Jung, H.W. Effect of modified Bo-yang-Hwan-o-Tang, a polyherbal medicine on the hippocampal neuronal damage in a rat model of global ischemia. Pharmacogn. Mag. 2015, 11, 665–673. [Google Scholar] [CrossRef]
- Shi, X.; Ohta, Y.; Shang, J.; Morihara, R.; Nakano, Y.; Fukui, Y.; Liu, X.; Feng, T.; Huang, Y.; Sato, K.; et al. Neuroprotective effects of SMTP-44D in mice stroke model in relation to neurovascular unit and trophic coupling. J. Neurosci. Res. 2018, 96, 1887–1899. [Google Scholar] [CrossRef]
- Shang, J.; Yamashita, T.; Zhai, Y.; Nakano, Y.; Morihara, R.; Fukui, Y.; Hishikawa, N.; Ohta, Y.; Abe, K. Strong Impact of Chronic Cerebral Hypoperfusion on Neurovascular Unit, Cerebrovascular Remodeling, and Neurovascular Trophic Coupling in Alzheimer’s Disease Model Mouse. J. Alzheimer’s Dis. 2016, 52, 113–126. [Google Scholar] [CrossRef]
- Kim, K.J.; Diaz, J.R.; Presa, J.L.; Muller, P.R.; Brands, M.W.; Khan, M.B.; Hess, D.C.; Althammer, F.; Stern, J.E.; Filosa, J.A. Decreased parenchymal arteriolar tone uncouples vessel-to-neuronal communication in a mouse model of vascular cognitive impairment. Geroscience 2021, 43, 1405–1422. [Google Scholar] [CrossRef]
- Lüscher, C.; Jan, L.Y.; Stoffel, M.; Malenka, R.C.; Nicoll, R.A. G Protein-Coupled Inwardly Rectifying K+ Channels (GIRKs) Mediate Postsynaptic but Not Presynaptic Transmitter Actions in Hippocampal Neurons. Neuron 1997, 19, 687–695. [Google Scholar] [CrossRef]
- Saggu, R.; Schumacher, T.; Gerich, F.; Rakers, C.; Tai, K.; Delekate, A.; Petzold, G.C. Astroglial NF-kB contributes to white matter damage and cognitive impairment in a mouse model of vascular dementia. Acta Neuropathol. Commun. 2016, 4, 76. [Google Scholar] [CrossRef]
- Holland, P.R.; Searcy, J.L.; Salvadores, N.; Scullion, G.; Chen, G.; Lawson, G.; Scott, F.; Bastin, M.E.; Ihara, M.; Horsburgh, K.; et al. Gliovascular disruption and cognitive deficits in a mouse model with features of small vessel disease. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2015, 35, 1005–1014. [Google Scholar] [CrossRef]
- Wang, L.; Du, Y.; Wang, K.; Xu, G.; Luo, S.; He, G. Chronic cerebral hypoperfusion induces memory deficits and facilitates Aβ generation in C57BL/6J mice. Exp. Neurol. 2016, 283, 353–364. [Google Scholar] [CrossRef]
- Miyamoto, N.; Maki, T.; Shindo, A.; Liang, A.C.; Maeda, M.; Egawa, N.; Itoh, K.; Lo, E.K.; Lok, J.; Ihara, M.; et al. Astrocytes Promote Oligodendrogenesis after White Matter Damage via Brain-Derived Neurotrophic Factor. J. Neurosci. 2015, 35, 14002–14008. [Google Scholar] [CrossRef]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Haseloff, R.F.; Dithmer, S.; Winkler, L.; Wolburg, H.; Blasig, I.E. Transmembrane proteins of the tight junctions at the blood-brain barrier: Structural and functional aspects. Semin. Cell Dev. Biol. 2015, 38, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, J.; Deng, M. Furin mediates brain-derived neurotrophic factor upregulation in cultured rat astrocytes exposed to oxygen-glucose deprivation. J. Neurosci. Res. 2014, 93, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Gao, C.; Gao, D.; Sun, R.; Li, W.; Wang, F.; Wang, Y.; Cao, H.; Zhou, G.; Zhang, J.; et al. Reduction in pericyte coverage leads to blood-brain barrier dysfunction via endothelial transcytosis following chronic cerebral hypoperfusion. Fluids Barriers CNS 2021, 18, 21. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Tomimoto, H.; Akiguchi, I.; Wakita, H.; Sakamoto, H. Blood-brain barrier disruption in white matter lesions in a rat model of chronic cerebral hypoperfusion. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2002, 22, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.M.; Maniskas, M.E.; Bix, G.J. Bilateral carotid artery stenosis causes unexpected early changes in brain extracellular matrix and blood-brain barrier integrity in mice. PLoS ONE 2018, 13, e0195765. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.M.; Jansen, J.F.; Zhang, C.E.; Hoff, E.I.; Staals, J.; van Oostenbrugge, R.J.; Backes, W.H. Blood-brain barrier impairment and hypoperfusion are linked in cerebral small vessel disease. Neurology 2019, 92, e1669–e1677. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.H.; Miyamoto, N.; Hayakawa, K.; Pham, L.-D.D.; Maki, T.; Ayata, C.; Kim, K.-W.; Lo, E.H.; Arai, K. Oligodendrocyte precursors induce early blood-brain barrier opening after white matter injury. J. Clin. Investig. 2013, 123, 782–786. [Google Scholar] [CrossRef]
- Nakaji, K.; Ihara, M.; Takahashi, C.; Itohara, S.; Noda, M.; Takahashi, R.; Tomimoto, H. Matrix Metalloproteinase-2 Plays a Critical Role in the Pathogenesis of White Matter Lesions After Chronic Cerebral Hypoperfusion in Rodents. Stroke 2006, 37, 2816–2823. [Google Scholar] [CrossRef]
- Chandler, S.; Coates, R.; Gearing, A.; Lury, J.; Wells, G.; Bone, E. Matrix metalloproteinases degrade myelin basic protein. Neurosci. Lett. 1995, 201, 223–226. [Google Scholar] [CrossRef]
- Ben-Zvi, A.; Lacoste, B.; Kur, E.; Andreone, B.J.; Mayshar, Y.; Yan, H.; Gu, C. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature 2014, 509, 507–511. [Google Scholar] [CrossRef]
- Andreone, B.J.; Chow, B.W.; Tata, A.; Lacoste, B.; Ben-Zvi, A.; Bullock, K.; Deik, A.A.; Ginty, D.D.; Clish, C.B.; Gu, C. Blood-Brain Barrier Permeability Is Regulated by Lipid Transport-Dependent Suppression of Caveolae-Mediated Transcytosis. Neuron 2017, 94, 581–594.e5. [Google Scholar] [CrossRef]
- Qu, C.; Song, H.; Shen, J.; Xu, L.; Li, Y.; Qu, C.; Li, T.; Zhang, J. Mfsd2a Reverses Spatial Learning and Memory Impairment Caused by Chronic Cerebral Hypoperfusion via Protection of the blood-brain barrier. Front. Neurosci. 2020, 14, 461. [Google Scholar] [CrossRef]
- Dalkara, T.; Gursoy-Ozdemir, Y.; Yemisci, M. Brain microvascular pericytes in health and disease. Acta Neuropathol. 2011, 122, 1–9. [Google Scholar] [CrossRef]
- Bell, R.D.; Winkler, E.A.; Sagare, A.P.; Singh, I.; LaRue, B.; Deane, R.; Zlokovic, B.V. Pericytes Control Key Neurovascular Functions and Neuronal Phenotype in the Adult Brain and during Brain Aging. Neuron 2010, 68, 409–427. [Google Scholar] [CrossRef]
- Lee, J.M.; Lee, J.H.; Song, M.K.; Kim, Y.J. NXP031 Improves Cognitive Impairment in a Chronic Cerebral Hypoperfusion-Induced Vascular Dementia Rat Model through Nrf2 Signaling. Int. J. Mol. Sci. 2021, 22, 6285. [Google Scholar] [CrossRef]
- Armulik, A.; Genové, G.; Mäe, M.; Nisancioglu, M.H.; Wallgard, E.; Niaudet, C.; He, L.; Norlin, J.; Lindblom, P.; Strittmatter, K.; et al. Pericytes regulate the blood-brain barrier. Nature 2010, 468, 557–561. [Google Scholar] [CrossRef]
- Filley, C.M.; Fields, R.D. White matter and cognition: Making the connection. J. Neurophysiol. 2016, 116, 2093–2104. [Google Scholar] [CrossRef]
- Blinder, P.; Tsai, P.S.; Kaufhold, J.; Knutsen, P.M.; Suhl, H.; Kleinfeld, D. The cortical angiome: An interconnected vascular network with noncolumnar patterns of blood flow. Nat. Neurosci. 2013, 16, 889–897. [Google Scholar] [CrossRef]
- Scuteri, A.; Nilsson, P.M.; Tzourio, C.; Redon, J.; Laurent, S. Microvascular brain damage with aging and hypertension: Pathophysiological consideration and clinical implications. J. Hypertens. 2011, 29, 1469–1477. [Google Scholar] [CrossRef]
- Ihara, M.; Polvikoski, T.M.; Hall, R.; Slade, J.Y.; Perry, R.H.; Oakley, A.E.; Englund, E.; O’Brien, J.T.; Ince, P.G.; Kalaria, R.N. Quantification of myelin loss in frontal lobe white matter in vascular dementia, Alzheimer’s disease, and dementia with Lewy bodies. Acta Neuropathol. 2010, 119, 579–589. [Google Scholar] [CrossRef]
- Joutel, A.; Chabriat, H. Pathogenesis of white matter changes in cerebral small vessel diseases: Beyond vessel-intrinsic mechanisms. Clin. Sci. 2017, 131, 635–651. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Smith, E.E.; Biessels, G.J.; Cordonnier, C.; Fazekas, F.; Frayne, R.; Lindley, R.I.; O’Brien, J.T.; Barkhof, F.; Benavente, O.R.; et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013, 12, 822–838. [Google Scholar] [CrossRef]
- Holland, P.R.; Bastin, M.E.; Jansen, M.A.; Merrifield, G.D.; Coltman, R.B.; Scott, F.; Nowers, H.; Khallout, K.; Marshall, I.; Wardlaw, J.M.; et al. MRI is a sensitive marker of subtle white matter pathology in hypoperfused mice. Neurobiol. Aging 2011, 32, 2325.e1–2325.e6. [Google Scholar] [CrossRef] [PubMed]
- Farkas, E.; Luiten, P.G.; Bari, F. Permanent, bilateral common carotid artery occlusion in the rat: A model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res. Rev. 2007, 54, 162–180. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.S.; Ludwin, S.K. Evidence for a ?dying-back? gliopathy in demyelinating disease. Ann. Neurol. 1981, 9, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, A.; Majed, H.; Layfield, R.; Compston, A.; Chandran, S. Oligodendrocytes Promote Neuronal Survival and Axonal Length by Distinct Intracellular Mechanisms: A Novel Role for Oligodendrocyte-Derived Glial Cell Line-Derived Neurotrophic Factor. J. Neurosci. 2003, 23, 4967–4974. [Google Scholar] [CrossRef]
- Fernando, M.S.; Simpson, J.E.; Matthews, F.; Brayne, C.; Lewis, C.E.; Barber, R.; Kalaria, R.N.; Forster, G.; Esteves, F.; Ince, P.G.; et al. White matter lesions in an unselected cohort of the elderly: Molecular pathology suggests origin from chronic hypoperfusion injury. Stroke 2006, 37, 1391–1398. [Google Scholar] [CrossRef]
- Reimer, M.M.; McQueen, J.; Searcy, L.; Scullion, G.; Zonta, B.; Desmazieres, A.; Holland, P.; Smith, J.; Gliddon, C.; Wood, E.R.; et al. Rapid Disruption of Axon-Glial Integrity in Response to Mild Cerebral Hypoperfusion. J. Neurosci. 2011, 31, 18185–18194. [Google Scholar] [CrossRef]
- Matute, C.; Ransom, B.R. Roles of White Matter in Central Nervous System Pathophysiologies. ASN Neuro 2012, 4, AN20110060. [Google Scholar] [CrossRef]
- Trapp, B.D.; Andrews, S.B.; Cootauco, C.; Quarles, R. The myelin-associated glycoprotein is enriched in multivesicular bodies and periaxonal membranes of actively myelinating oligodendrocytes. J. Cell Biol. 1989, 109, 2417–2426. [Google Scholar] [CrossRef]
- Hudson, L.D.; Friedrich, V.L.; Behar, T.; Dubois-Dalcq, M.; Lazzarini, R.A. The initial events in myelin synthesis: Orientation of proteolipid protein in the plasma membrane of cultured oligodendrocytes. J. Cell Biol. 1989, 109, 717–727. [Google Scholar] [CrossRef]
- Redwine, J.M.; Armstrong, R.C. In vivo proliferation of oligodendrocyte progenitors expressing PDGF?R during early remyelination. J. Neurobiol. 1998, 37, 413–428. [Google Scholar] [CrossRef]
- Miyamoto, N.; Tanaka, R.; Shimura, H.; Watanabe, T.; Mori, H.; Onodera, M.; Mochizuki, H.; Hattori, N.; Urabe, T. Phosphodiesterase III Inhibition Promotes Differentiation and Survival of Oligodendrocyte Progenitors and Enhances Regeneration of Ischemic White Matter Lesions in the Adult Mammalian Brain. J. Cereb. Blood Flow Metab. 2010, 30, 299–310. [Google Scholar] [CrossRef]
- Back, S.A.; Han, B.H.; Luo, N.L.; Chricton, C.A.; Xanthoudakis, S.; Tam, J.; Arvin, K.L.; Holtzman, D.M. Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J. Neurosci. Off. J. Soc. Neurosci. 2002, 22, 455–463. [Google Scholar] [CrossRef]
- Arai, K.; Lo, E.H. Astrocytes protect oligodendrocyte precursor cells via MEK/ERK and PI3K/Akt signaling. J. Neurosci. Res. 2010, 88, 758–763. [Google Scholar] [CrossRef]
- Skrobot, O.A.; Attems, J.; Esiri, M.; Hortobágyi, T.; Ironside, J.; Kalaria, R.N.; King, A.; Lammie, G.A.; Mann, D.; Neal, J.; et al. Vascular cognitive impairment neuropathology guidelines (VCING): The contribution of cerebrovascular pathology to cognitive impairment. Brain 2016, 139, 2957–2969. [Google Scholar] [CrossRef]
- Marchesi, C.; Paradis, P.; Schiffrin, E.L. Role of the renin-angiotensin system in vascular inflammation. Trends Pharmacol. Sci. 2008, 29, 367–374. [Google Scholar] [CrossRef]
- Gill, R.; Tsung, A.; Billiar, T.R. Linking oxidative stress to inflammation: Toll-like receptors. Free Radic. Biol. Med. 2010, 48, 1121–1132. [Google Scholar] [CrossRef]
- Yuan, B.; Shi, H.; Zheng, K.; Su, Z.; Su, H.; Zhong, M.; He, X.; Zhou, C.; Chen, H.; Xiong, Q.; et al. MCP-1-mediated activation of microglia promotes white matter lesions and cognitive deficits by chronic cerebral hypoperfusion in mice. Mol. Cell. Neurosci. 2016, 78, 52–58. [Google Scholar] [CrossRef]
- Honjo, K.; Black, S.E.; Verhoeff, N.P. Alzheimer’s disease, cerebrovascular disease, and the β-amyloid cascade. Can. J. Neurol. Sci. 2012, 39, 712–728. [Google Scholar] [CrossRef]
- Gao, Y.-Z.; Zhang, J.-J.; Liu, H.; Wu, G.-Y.; Xiong, L.; Shu, M. Regional Cerebral Blood Flow and Cerebrovascular Reactivity in Alzheimer’s Disease and Vascular Dementia Assessed by Arterial Spinlabeling Magnetic Resonance Imaging. Curr. Neurovascular Res. 2012, 10, 49–53. [Google Scholar] [CrossRef]
- Richardson, K.; Stephan, B.C.; Ince, P.G.; Brayne, C.; Matthews, F.E. The Neuropathology of Vascular Disease in the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS). Curr. Alzheimer Res. 2012, 9, 687–696. [Google Scholar] [CrossRef]
- De Jong, D.; Kremer, B.P.; Olde Rikkert, M.G.; Verbeek, M.M. Current state and future directions of neurochemical biomarkers for Alzheimer’s disease. Clin. Chem. Lab. Med. 2007, 45, 1421–1434. [Google Scholar] [CrossRef]
- Love, S.; Miners, J.S. Cerebral Hypoperfusion and the Energy Deficit in Alzheimer’s Disease. Brain Pathol. 2016, 26, 607–617. [Google Scholar] [CrossRef]
- Tayler, H.; Miners, J.S.; Güzel, Ö.; MacLachlan, R.; Love, S. Mediators of cerebral hypoperfusion and blood-brain barrier leakiness in Alzheimer’s disease, vascular dementia and mixed dementia. Brain Pathol. 2021, 31, e12935. [Google Scholar] [CrossRef]
- Chabriat, H.; Joutel, A.; Dichgans, M.; Tournier-Lasserve, E.; Bousser, M.G. Cadasil. Lancet Neurol. 2009, 8, 643–653. [Google Scholar] [CrossRef]
- Viswanathan, A.; Godin, O.; Jouvent, E.; O’Sullivan, M.; Gschwendtner, A.; Peters, N.; Duering, M.; Guichard, J.-P.; Holtmannspötter, M.; Dufouil, C.; et al. Impact of MRI markers in subcortical vascular dementia: A multi-modal analysis in CADASIL. Neurobiol. Aging 2010, 31, 1629–1636. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Craggs, L.; Watanabe, A.; Booth, T.; Attems, J.; Low, R.W.C.; Oakley, A.E.; Kalaria, R.N. Brain Microvascular Accumulation and Distribution of the NOTCH3 Ectodomain and Granular Osmiophilic Material in CADASIL. J. Neuropathol. Exp. Neurol. 2013, 72, 416–431. [Google Scholar] [CrossRef]
- Verghese, P.B.; Castellano, J.M.; Holtzman, D.M. Apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neurol. 2011, 10, 241–252. [Google Scholar] [CrossRef]
- Hara, K.; Shiga, A.; Fukutake, T.; Nozaki, H.; Miyashita, A.; Yokoseki, A.; Kawata, H.; Koyama, A.; Arima, K.; Takahashi, T.; et al. Association of HTRA1 Mutations and Familial Ischemic Cerebral Small-Vessel Disease. N. Engl. J. Med. 2009, 360, 1729–1739. [Google Scholar] [CrossRef] [PubMed]
- Richards, A.; Maagdenberg, A.M.J.M.V.D.; Jen, J.C.; Kavanagh, D.; Bertram, P.; Spitzer, D.; Liszewski, M.K.; Barilla-LaBarca, M.-L.; Terwindt, G.M.; Kasai, Y.; et al. C-terminal truncations in human 3′-5′ DNA exonuclease TREX1 cause autosomal dominant retinal vasculopathy with cerebral leukodystrophy. Nat. Genet. 2007, 39, 1068–1070. [Google Scholar] [CrossRef] [PubMed]
- Gould, D.B.; Phalan, F.C.; van Mil, S.E.; Sundberg, J.P.; Vahedi, K.; Massin, P.; Bousser, M.G.; Heutink, P.; Miner, J.H.; Tournier-Lasserve, E.; et al. Role of COL4A1 in Small-Vessel Disease and Hemorrhagic Stroke. N. Engl. J. Med. 2006, 354, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, J.M.; Smith, C.; Dichgans, M. Mechanisms of sporadic cerebral small vessel disease: Insights from neuroimaging. Lancet Neurol. 2013, 12, 483–497. [Google Scholar] [CrossRef]
- Fern, R.; Matute, C. Glutamate receptors and white matter stroke. Neurosci. Lett. 2018, 694, 86–92. [Google Scholar] [CrossRef]
- Ndung’U, M.; Härtig, W.; Wegner, F.; Mwenda, J.M.; Low, R.W.C.; Akinyemi, R.O.; Kalaria, R.N. Cerebral amyloid β(42) deposits and microvascular pathology in ageing baboons. Neuropathol. Appl. Neurobiol. 2012, 38, 487–499. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, W.; Li, Y.; Hu, J.; Wu, J.; Huang, Y. A Study on the Pathogenesis of Vascular Cognitive Impairment and Dementia: The Chronic Cerebral Hypoperfusion Hypothesis. J. Clin. Med. 2022, 11, 4742. https://doi.org/10.3390/jcm11164742
Yu W, Li Y, Hu J, Wu J, Huang Y. A Study on the Pathogenesis of Vascular Cognitive Impairment and Dementia: The Chronic Cerebral Hypoperfusion Hypothesis. Journal of Clinical Medicine. 2022; 11(16):4742. https://doi.org/10.3390/jcm11164742
Chicago/Turabian StyleYu, Weiwei, Yao Li, Jun Hu, Jun Wu, and Yining Huang. 2022. "A Study on the Pathogenesis of Vascular Cognitive Impairment and Dementia: The Chronic Cerebral Hypoperfusion Hypothesis" Journal of Clinical Medicine 11, no. 16: 4742. https://doi.org/10.3390/jcm11164742





