Exosomes Derived from Bone Marrow-Mesenchymal Stem Cells Attenuates Cisplatin-Induced Ototoxicity in a Mouse Model
Abstract
:1. Introduction
2. Methods and Materials
2.1. BMSCs Isolation and Hypoxia Precondition
2.2. Exosome Isolation and Determination
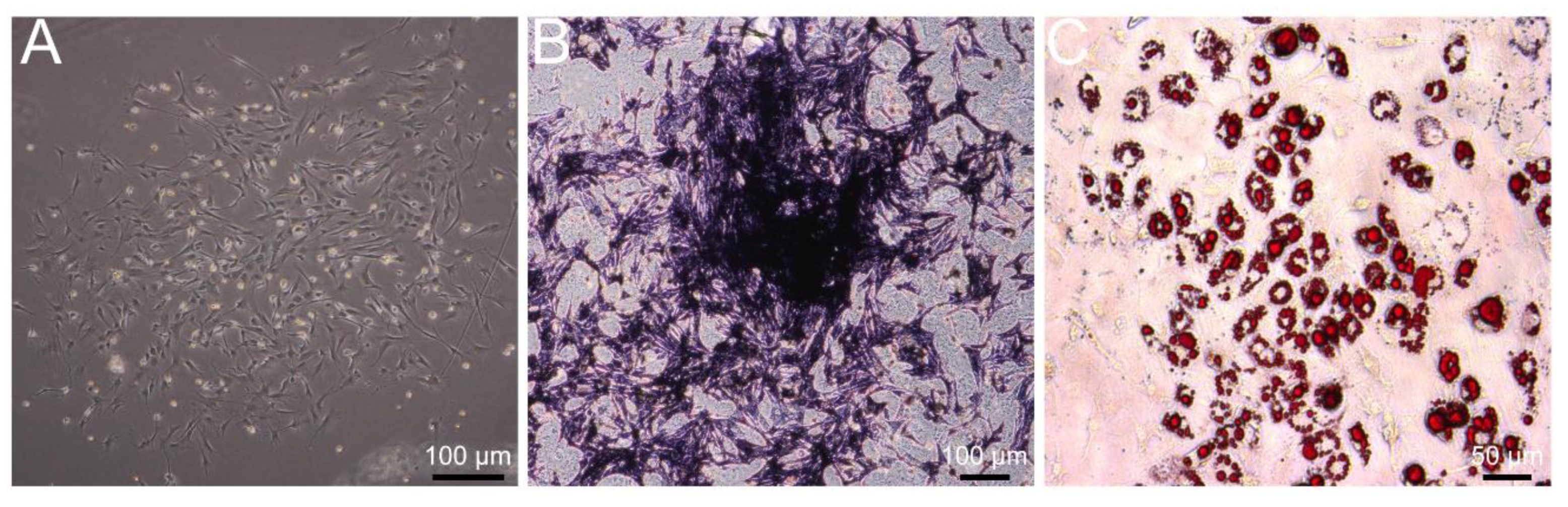
2.3. Adipogenic and Osteogenic Differentiation of BMSCs
2.4. Cisplatin-Exposed Mice and Exosomes Administration
2.5. Auditory Brainstem Response
2.6. Quantitative Real-Time RT-PCR (qRT-PCR)
2.7. Western Blotting
2.8. Myosin Staining
2.9. Oxidative Stress Assay
2.10. Statistical Analysis
3. Results
3.1. Identification of Primary BMSCs
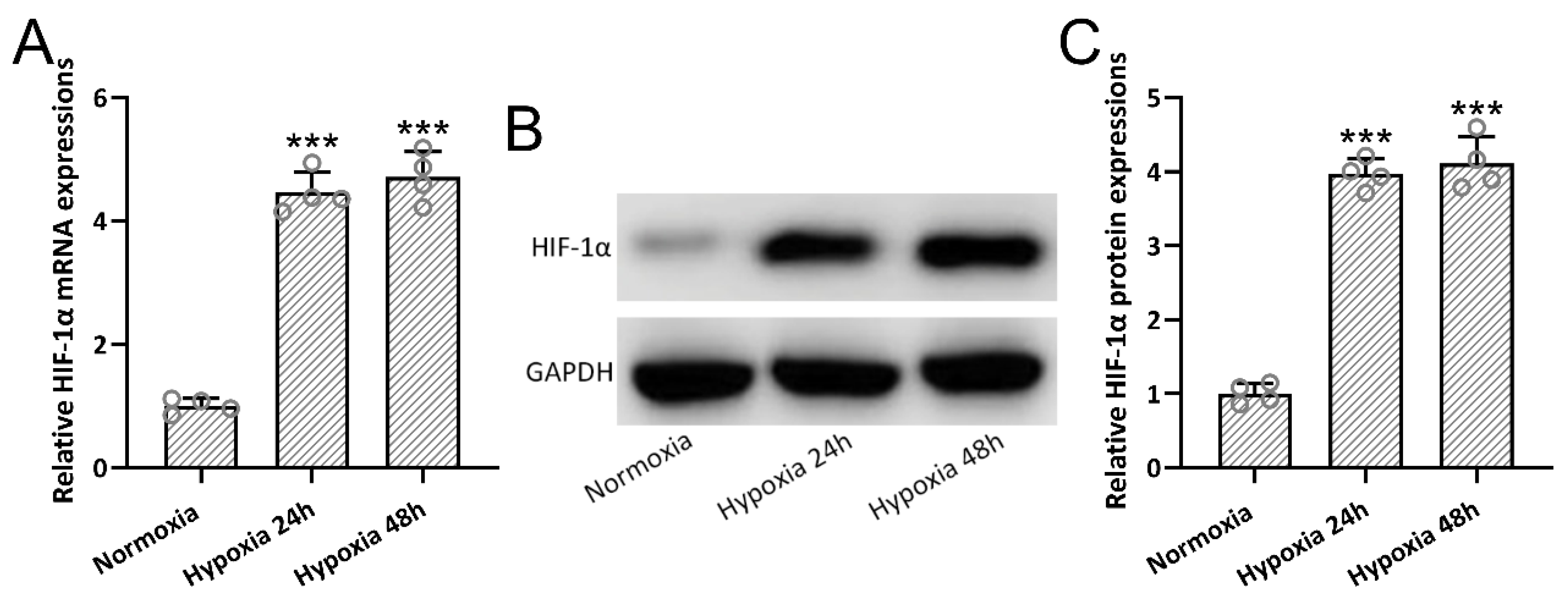
3.2. Hypoxia-Induced HIF-1α Expressions in BMSCs
3.3. Identification of Hypoxia-Preconditioned BMSC-Derived Exosomes
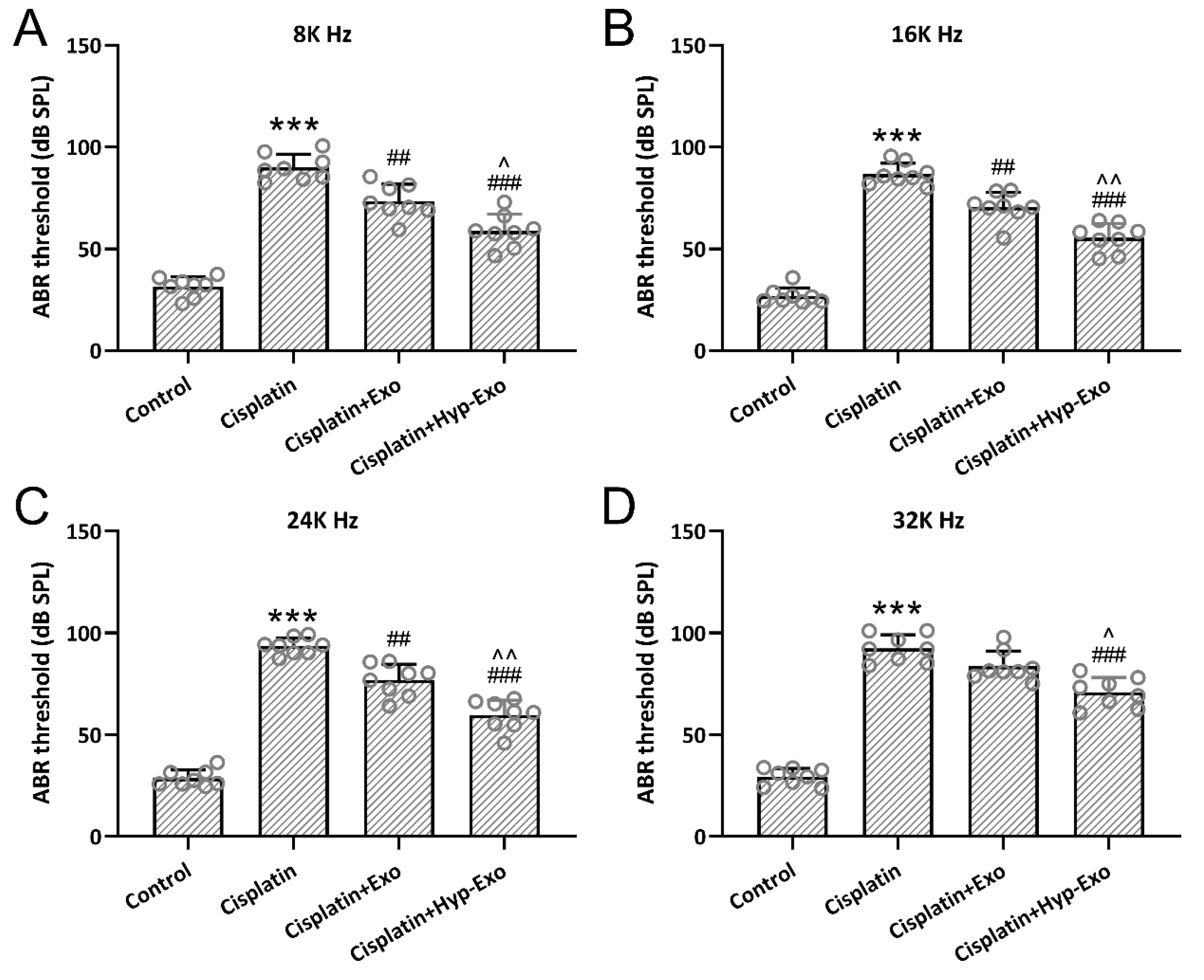
3.4. HypBMSC-Exo Ameliorated Auditory Sensitivity in Cisplatin-Exposed Mice
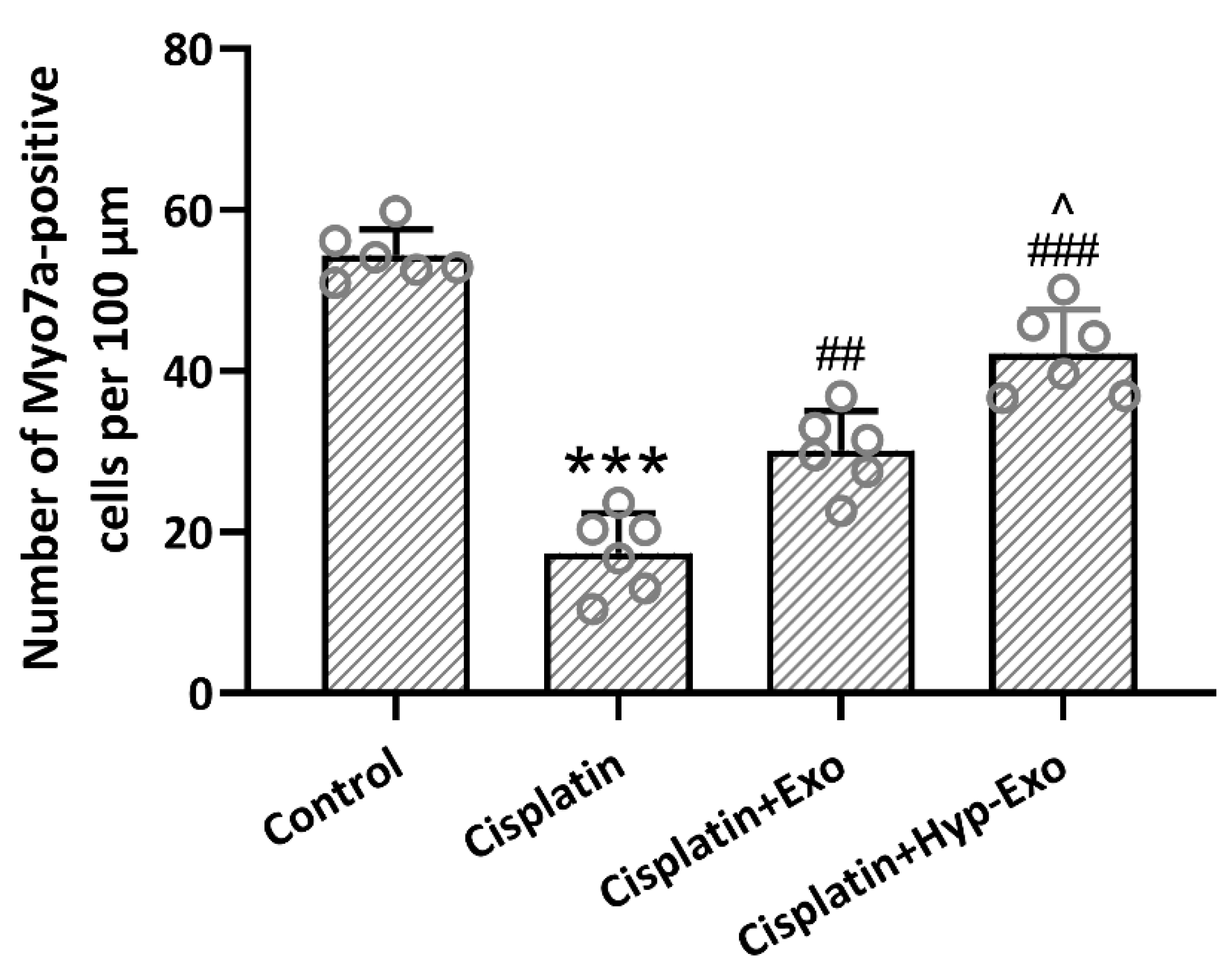
3.5. HypBMSC-Exo Ameliorated Hair Cell Loss in Cisplatin-Exposed Mice
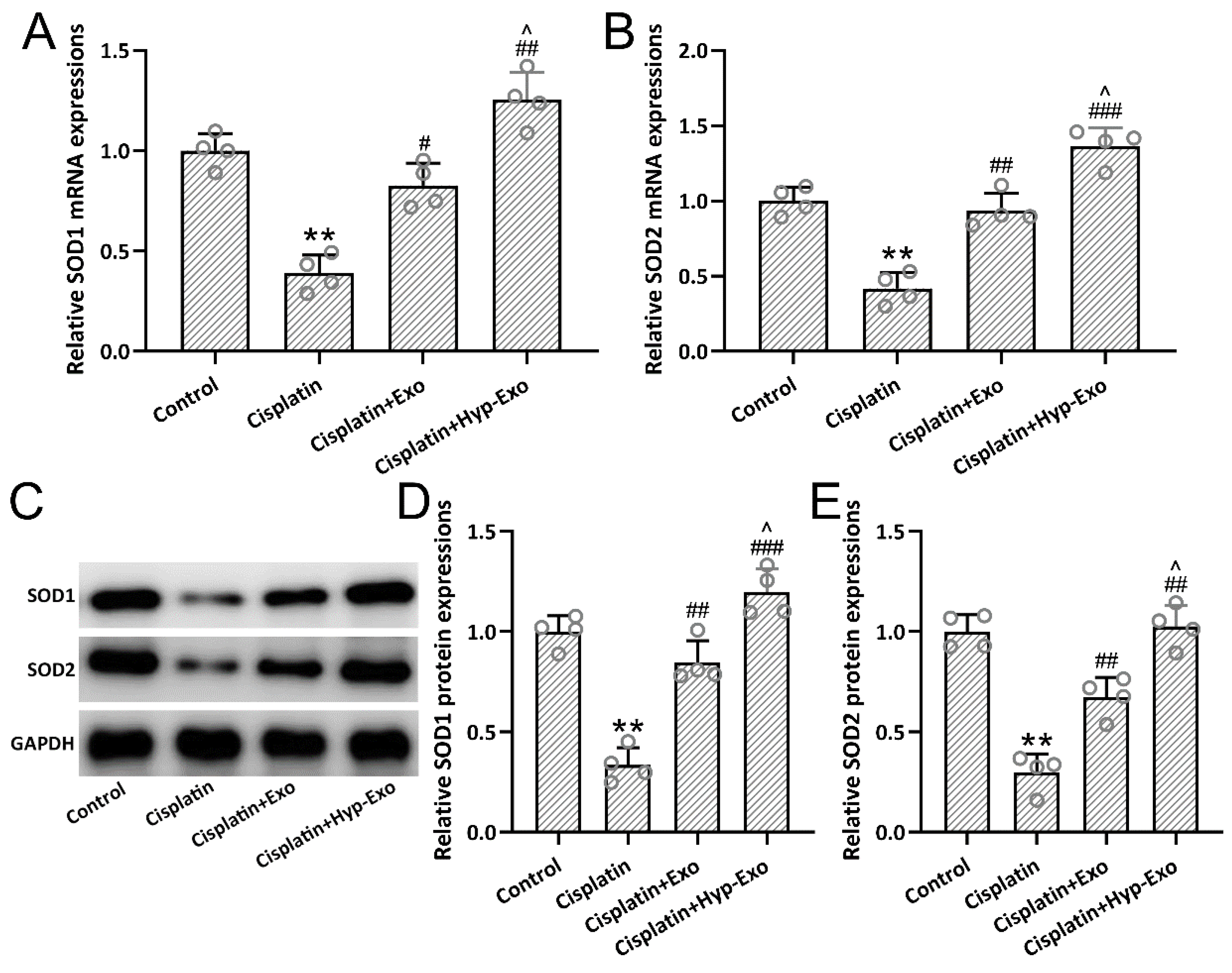
3.6. HypBMSC-Exo Activated SOD Antioxidant Signal in Cisplatin-Exposed Mice
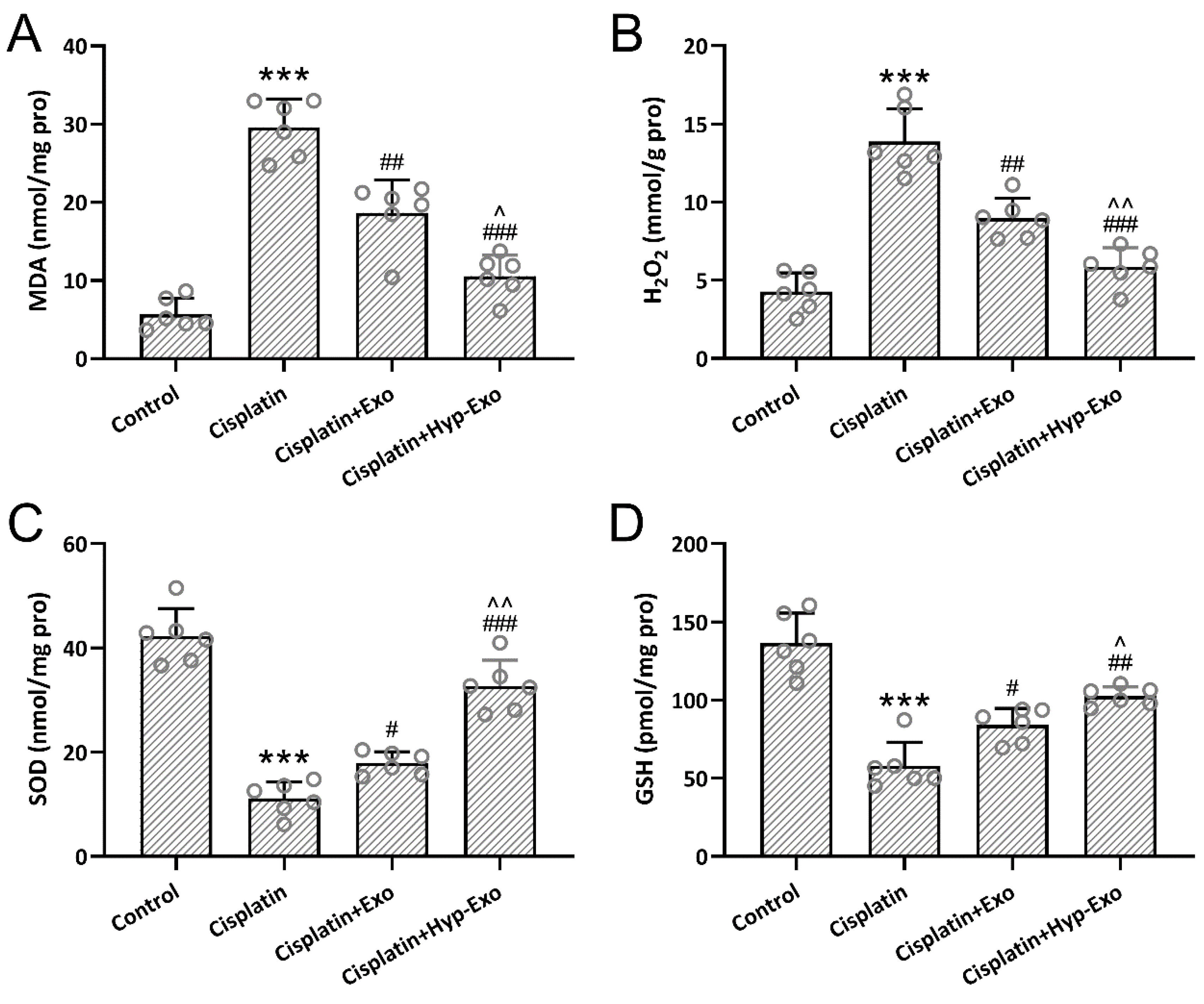
3.7. HypBMSC-Exo Ameliorated Cisplatin-Induced Oxidative Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clemens, E.; van den Heuvel-Eibrink, M.M.; Mulder, R.L.; Kremer, L.C.M.; Hudson, M.M.; Skinner, R.; Constine, L.S.; Bass, J.K.; Kuehni, C.E.; Langer, T.; et al. Recommendations for ototoxicity surveillance for childhood, adolescent, and young adult cancer survivors: A report from the international late effects of childhood cancer guideline harmonization group in collaboration with the pancare consortium. Lancet Oncol. 2019, 20, e29–e41. [Google Scholar] [CrossRef]
- Callejo, A.; Sedó-Cabezón, L.; Juan, I.D.; Llorens, J. Cisplatin-induced ototoxicity: Effects, mechanisms and protection strategies. Toxics 2015, 3, 268–293. [Google Scholar] [CrossRef]
- Mueller, R.S. Chapter 24—Topical dermatological therapy. In Small Animal Clinical Pharmacology, 2nd ed.; Maddison, J.E., Page, S.W., Church, D.B., Eds.; W.B. Saunders: Edinburgh, UK, 2008; pp. 546–556. [Google Scholar]
- Tang, Q.; Wang, X.; Jin, H.; Mi, Y.; Liu, L.; Dong, M.; Chen, Y.; Zou, Z. Cisplatin-induced ototoxicity: Updates on molecular mechanisms and otoprotective strategies. Eur. J. Pharm. Biopharm. 2021, 163, 60–71. [Google Scholar] [CrossRef]
- Rybak, L.P.; Mukherjea, D.; Ramkumar, V. Mechanisms of cisplatin-induced ototoxicity and prevention. Semin Hear 2019, 40, 197–204. [Google Scholar] [CrossRef]
- Yu, D.; Gu, J.; Chen, Y.; Kang, W.; Wang, X.; Wu, H. Current strategies to combat cisplatin-induced ototoxicity. Front. Pharmacol. 2020, 11, 999. [Google Scholar] [CrossRef]
- Feng, W.; Jin, Q.; Yang, M.-Y.; Yang, H.; Xu, T.; Shi, Y.-X.; Bian, X.-T.; Wan, C.; Wang, Y.-J.; Huan, W.; et al. Mir-6924-5p-rich exosomes derived from genetically modified scleraxis-overexpressing PDGFRα(+) bmmscs as novel nanotherapeutics for treating osteolysis during tendon-bone healing and improving healing strength. Biomaterials 2021, 279, 121242. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Yu, X.; Bao, Y.; An, L.; Wei, X.; Yu, W.; Liu, B.; Li, J.; Yang, J.; et al. Bone marrow mesenchymal stem cell-derived exosomes promote plasminogen activator inhibitor 1 expression in vascular cells in the local microenvironment during rabbit osteonecrosis of the femoral head. Stem Cell Res. Ther. 2020, 11, 480. [Google Scholar] [CrossRef]
- Pak, J.H.; Kim, Y.; Yi, J.; Chung, J.W. Antioxidant therapy against oxidative damage of the inner ear: Protection and preconditioning. Antioxidants 2020, 9, 1076. [Google Scholar] [CrossRef]
- Luo, Z.; Wu, F.; Xue, E.; Huang, L.; Yan, P.; Pan, X.; Zhou, Y. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival by inducing hif-1α in injured neuronal cells derived exosomes culture system. Cell Death Dis. 2019, 10, 134. [Google Scholar] [CrossRef]
- Das, R.; Jahr, H.; van Osch, G.J.; Farrell, E. The role of hypoxia in bone marrow-derived mesenchymal stem cells: Considerations for regenerative medicine approaches. Tissue Eng. Part B Rev. 2010, 16, 159–168. [Google Scholar] [CrossRef]
- Yang, Y.; Lee, E.H.; Yang, Z. Hypoxia-conditioned mesenchymal stem cells in tissue regeneration application. Tissue Eng. Part B Rev. 2022; ahead of print. [Google Scholar] [CrossRef]
- Lai, R.; Cai, C.; Wu, W.; Hu, P.; Wang, Q. Exosomes derived from mouse inner ear stem cells attenuate gentamicin-induced ototoxicity in vitro through the mir-182-5p/foxo3 axis. J. Tissue Eng. Regen. Med. 2020, 14, 1149–1156. [Google Scholar] [CrossRef]
- Yang, T.; Cai, C.; Peng, A.; Liu, J.; Wang, Q. Exosomes derived from cochlear spiral ganglion progenitor cells prevent cochlea damage from ischemia-reperfusion injury via inhibiting the inflammatory process. Cell Tissue Res. 2021, 386, 239–247. [Google Scholar] [CrossRef]
- Yuan, N.; Ge, Z.; Ji, W.; Li, J. Exosomes secreted from hypoxia-preconditioned mesenchymal stem cells prevent steroid-induced osteonecrosis of the femoral head by promoting angiogenesis in rats. BioMed Res. Int. 2021, 2021, 6655225. [Google Scholar] [CrossRef]
- Nam, G.H.; Choi, Y.; Kim, G.B.; Kim, S.; Kim, S.A.; Kim, I.S. Emerging prospects of exosomes for cancer treatment: From conventional therapy to immunotherapy. Adv. Mater 2020, 32, e2002440. [Google Scholar] [CrossRef]
- Isaac, R.; Reis, F.C.G.; Ying, W.; Olefsky, J.M. Exosomes as mediators of intercellular crosstalk in metabolism. Cell Metab. 2021, 33, 1744–1762. [Google Scholar] [CrossRef]
- Babaei, G.; Zare, N.; Mihanfar, A.; Ansari, M.H.K. Exosomes and COVID-19: Challenges and opportunities. Comp. Clin. Pathol. 2022, 31, 347–354. [Google Scholar] [CrossRef]
- Ramkumar, V.; Mukherjea, D.; Dhukhwa, A.; Rybak, L.P. Oxidative stress and inflammation caused by cisplatin ototoxicity. Antioxidants 2021, 10, 1919. [Google Scholar] [CrossRef]
- Colakoglu, H.E.; Yazlik, M.O.; Kaya, U.; Colakoglu, E.C.; Kurt, S.; Oz, B.; Bayramoglu, R.; Vural, M.R.; Kuplulu, S. Mda and gsh-px activity in transition dairy cows under seasonal variations and their relationship with reproductive performance. J. Vet. Res. 2017, 61, 497–502. [Google Scholar] [CrossRef]
- Qu, J.; Li, X.; Wang, J.; Mi, W.; Xie, K.; Qiu, J. Inhalation of hydrogen gas attenuates cisplatin-induced ototoxicity via reducing oxidative stress. Int. J. Pediatric Otorhinolaryngol. 2012, 76, 111–115. [Google Scholar] [CrossRef]
- Roychowdhury, R.; Khan, M.H.; Choudhury, S. Chapter 16—Physiological and molecular responses for metalloid stress in rice—A comprehensive overview. In Advances in Rice Research for Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Nahar, K., Biswas, J.K., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 341–369. [Google Scholar]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef]
- Iskusnykh, I.Y.; Zakharova, A.A.; Pathak, D. Glutathione in brain disorders and aging. Molecules 2022, 27, 324. [Google Scholar] [CrossRef]
- Benedikter, B.J.; Bouwman, F.G.; Vajen, T.; Heinzmann, A.C.A.; Grauls, G.; Mariman, E.C.; Wouters, E.F.M.; Savelkoul, P.H.; Lopez-Iglesias, C.; Koenen, R.R.; et al. Ultrafiltration combined with size exclusion chromatography efficiently isolates extracellular vesicles from cell culture media for compositional and functional studies. Sci. Rep. 2017, 7, 15297. [Google Scholar] [CrossRef]
- Gagnon, P.M.; Simmons, D.D.; Bao, J.; Lei, D.; Ortmann, A.J.; Ohlemiller, K.K. Temporal and genetic influences on protection against noise-induced hearing loss by hypoxic preconditioning in mice. Hear. Res. 2007, 226, 79–91. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.; Li, W.; Peng, A.; Liu, J.; Wang, Q. Exosomes Derived from Bone Marrow-Mesenchymal Stem Cells Attenuates Cisplatin-Induced Ototoxicity in a Mouse Model. J. Clin. Med. 2022, 11, 4743. https://doi.org/10.3390/jcm11164743
Yang T, Li W, Peng A, Liu J, Wang Q. Exosomes Derived from Bone Marrow-Mesenchymal Stem Cells Attenuates Cisplatin-Induced Ototoxicity in a Mouse Model. Journal of Clinical Medicine. 2022; 11(16):4743. https://doi.org/10.3390/jcm11164743
Chicago/Turabian StyleYang, Tao, Wei Li, Anquan Peng, Jia Liu, and Qin Wang. 2022. "Exosomes Derived from Bone Marrow-Mesenchymal Stem Cells Attenuates Cisplatin-Induced Ototoxicity in a Mouse Model" Journal of Clinical Medicine 11, no. 16: 4743. https://doi.org/10.3390/jcm11164743




